Figure 1.
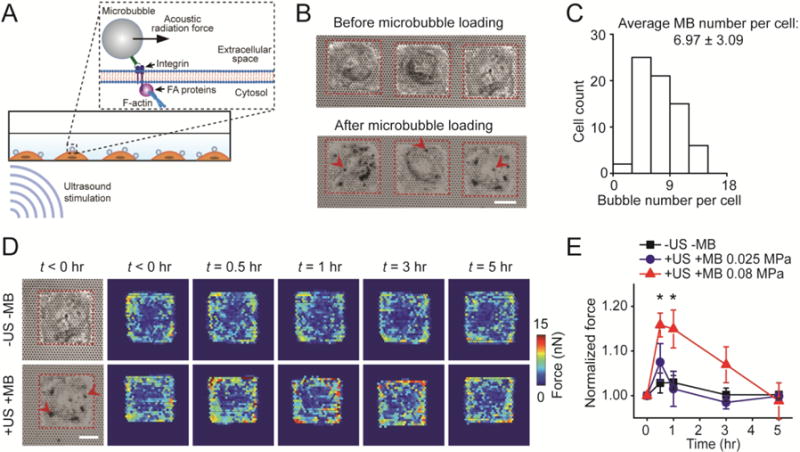
Acoustic tweezing cytometry (ATC) modulates cytoskeletal contractility of human mesenchymal stem cells (hMSCs). (A) Schematic showing ultrasound (US) excitation of functionalized microbubbles (MBs) attached to a cell via RGD-integrin binding. US excitation of MBs led to an acoustic radiation force acting on the MBs, resulting in lateral displacement of MBs and thus mechanical stimulation of the MB-integrin-F-actin linkage. (B) Bright field images showing micropatterned single hMSCs seeded on the PDMS micropost array (PMA) before and after loading MBs on hMSCs. Arrow heads mark representative MBs. Red rectangles in micrographs highlight cell boundaries. Scale bar, 30 μm. (C) Distribution of MB number per cell. The average MB number per cell was 6.97 ± 3.09 (mean ± standard deviation). The MB number per cell was counted based on bright field images shown in Fig. 1B. (D) Subcellular contractile force distribution for micropatterned single hMSCs loaded with MBs before (t < 0 hr) and after (t > 0 hr) US stimulation (+US +MB; bottom). Dynamic evolution of subcellular contractile forces of single hMSCs without MBs and US stimulation (−US − MB; top) was also shown for comparison. Scale bar, 30 μm. (E) Change in total cytoskeletal contractile force of micropatterned single hMSCs (relative to pre-US application values) as a function of time. Error bar denotes SEM, with n = 7 for acoustic pressure of 0.025 MPa and n = 6 for acoustic pressure of 0.08 MPa. Control group without MBs and US stimulation (−US −MB, n = 7) was included for comparison. P-values were calculated using two group t-test with respect to control. *, P < 0.05. The post height of the PMA used for cytoskeletal contractility measurements was 7.1 μm. US parameters: frequency 1 MHz, pulse repetition frequency 10 Hz, pulse duration 50 ms, treatment duration 30 s, and acoustic pressure 0.08 MPa or as indicated.
