Abstract
Purpose
Rapid infant and childhood growth has been associated with chronic disease later in life, including breast cancer. Early life socioeconomic status (SES) influences childhood growth, but few studies have prospective measures from birth to consider the effects of early life growth and SES on breast cancer risk.
Methods
We used prospectively measured early life SES and growth (percentile weight change in height and weight between each pair of consecutive time points at birth, 4 months, 1 and 7 years). We performed linear regression models to obtain standardized estimates of the association between 1 standard deviation increase in early life SES and growth and adult mammographic density, a strong risk factor for breast cancer, in a diverse birth cohort (n=151; 37% White, 38% Black, 25% Puerto Rican; average age=42.1).
Results
In models adjusted for race, prenatal factors, birthweight, infant and childhood growth and adult body mass index (BMI), percentile weight change from 1 year to 7 years was inversely associated with percent mammographic density (%MD) (standardized coefficient (Stdβ) =−0.28, 95% CI:−0.55, −0.01), and higher early life SES was positively associated with %MD (Stdβ =0.24, 95% CI: 0.04, 0.43). Similar associations were observed for dense area, but those estimates were not statistically significance.
Conclusions
These results suggest opposite and independent effects of early life SES and growth on mammographic density.
INTRODUCTION
Emerging evidence supports the role of early life growth in risk of breast cancer (1–7). Notably, rapid growth in utero reflected through birthweight may influence breast cancer risk, as this is a critical life period when the mammary tissue is rapidly developing (8, 9). Birthweight is affected by genetics as well as environmental factors during the intrauterine period, and has been positively associated with breast cancer. The influence of birthweight on breast cancer risk may depend on the rate of growth after birth, including rapid growth in childhood(7, 10, 11). However, results of studies evaluating these associations have been mixed, likely due to lack of prospective measures of growth during infancy and childhood in addition to birth characteristics.
Birth and childhood growth, as well as breast cancer risk, are influenced by parental socioeconomic status (SES) (12, 13). Although babies born to lower SES parents have on average lower birthweights than babies of higher SES parents, they experience rapid childhood growth and are more likely to be overweight in childhood (14, 15). The association between early life SES, measured through parental education and/or income, and breast cancer incidence later in life has been examined in a few studies (16, 17). In these studies, higher early life SES was significantly associated with increased breast cancer incidence after adjusting for adult reproductive and lifestyle characteristics, providing evidence for a direct role of early life SES on breast cancer risk (16, 17). Early life SES may increase breast cancer risk through altering the environment during the development and differentiation of mammary tissue in the critical window of early life, which may involve early life body size and growth (17). Most studies to date have lacked data on prospective early life SES and growth measures to tease apart the independent effects of these factors on breast cancer risk.
Mammographic density (MD), one of the strongest risk factors for breast cancer, reflects the amount of dense fibroglandular tissue present in the breast as seen on mammograms (18–21). As prospective collection of early life data in breast cancer studies is difficult and costly, researchers interested in examining early life determinants of breast cancer risk have often examined MD as a marker of risk that can be assessed in all women through mammography. Yochum et al (22) reviewed the results of 9 studies examining birthweight (23–31) and 9 studies examining body size in childhood through adolescence in relation to adult MD (23–26, 32–36). Three studies found positive associations between birthweight and MD (27, 29, 37) and seven studies found inverse associations between childhood body size and MD (23–26, 33–35), with the remainder of the studies observing mostly null associations. Only a few studies have simultaneously assessed birthweight, childhood growth and early life SES in relation to MD (23, 24), and none has used prospective data on all of these factors.
We address these limitations by using comprehensive and prospectively collected early life data to measure growth from the prenatal period to age 7, and multidimensional SES based on parental income, education and occupation at birth. We focused on a cohort of racially/ethnically diverse population of pre- and perimenopausal women to investigate the association between prospectively assessed early life growth in relation to MD, and whether the observed associations were independent of early life SES.
MATERIALS AND METHODS
Study Population
We used data from the New York Women’s Birth Cohort, a follow-up study of women enrolled at birth in the New York City site of the National Collaborative Perinatal Project (NYC NCPP). Study details have been published elsewhere (38–40). Briefly, the NYC NCPP study included 1,026 female children born at Columbia University Medical Center between 1959 and 1963. Of the 841 (82%) of the infants who were followed until age 7 and eligible for the adult follow-up, we traced 44% as adults and enrolled 70% of the traced participants (n=262). We obtained IRB approval from the Columbia University Medical Center.
Perinatal and Childhood Data
The NCPP recruited mothers into the study during their second and third trimesters (38, 41). At the initial study visit, clinical coordinators prospectively recorded maternal information including current age, age at menarche, race, and smoking behavior, pregnancy conditions and anthropometric factors. We calculated maternal weight gain using maternal pre-pregnancy weight and maternal weight just before birth, and calculated maternal body mass index (BMI) using self-reported pre-pregnancy weight and height. We used a validated continuous index for measuring parental SES, which uses a combination of parental education, income and occupation data for the head of household or main wage earner. The SES index ranged from 0 to 100 with higher scores indicated higher SES (see for detailed background information on this measure (42)). We obtained prospective height and weight measures at birth and ages 4 months, 1 year and 7 years at clinical visits using a standardized protocol. Due to differences in participant’s age for each height and weight follow-up measures (i.e., not all participants attended the clinic at exactly 4 months, 1 year and 7 years of age), we used individual cubic splines to interpolate the data for each participant at the target times (4 months, 1 year, 7 years). We did this for all measures except for birth measurements, which were measured for all participants at the time of their birth. To measure growth, we calculated the differences in height and in weight between measures taken at each pair of consecutive time points; i.e., between birth and 4 months, between 4 months and 1 year, and between 1 year and 7 years.
Adult Follow-Up and Mammogram data
Between 2001 and 2006, 262 women enrolled in the adult follow up study (New York Women’s Study) provided detailed epidemiologic data, including data on adult body size (current height and weight) and availability of mammogram. Of this sample, 228 (87%) women had received a prior mammogram, and 166 (73%) provided a medical release form for accessing their mammograms. We requested recent mammograms from the radiological facilities where participants had been screened, and obtained films on 163 women. We excluded data from 11 participants due to poor mammogram quality and for 1 participant whose mammogram was taken after breast cancer diagnosis (40).
For each participant, we selected the mammogram that was taken closest to the date of completion of adult follow-up questionnaire. We used the cranio-caudal mammogram views of the left breast for all participants, as breast density in left and right breasts are strongly correlated (43, 44). We digitized mammograms using a Kodak Lumisys Film Digitizer (Kodak LS85), and assessed MD using Cumulus, a computer-assisted thresholding program that allows readers to measure the size of the total breast area and dense areas and identify the number of pixels within the areas. We calculated the size of breast area and dense area by converting the number of pixels to cm2, and calculated percent MD (%MD) by taking the ratio of the dense area to the breast area and multiplying that by 100. A single reader, trained in the MD assessment using Cumulus software, read all mammograms arranged in random order, while blinded to exposure status, and repeated reading for a random 10% of all films. We obtained a Pearson correlation coefficient of 0.93 for dense area, and 0.90 for %MD for the repeated readings.
Statistical Analyses
We examined the associations of prenatal, birth and childhood characteristics and early life SES with %MD and dense area. We further investigated the same associations with non-dense area as the outcome. We modeled postnatal growth as standard deviation (SD) increases in percentile-rank changes in height and weight during three growth periods: birth to 4 months, 4 months to 1 year, 1 year to 7 years. The use of percentile rank changes allows for the assessment of growth rates without adjustment for age-dependent measurement scales(45). For example, a participant at 20th percentile for birth weight and 35th percentile for weight at 4 months would have a value of 0.15 for percentile weight change from birth to 4 months growth period (45–47). We used partial regression plots to examine for the linearity of associations between the main exposures and MD. We generated standardized (Std) beta estimates of the associations between early life growth and SES and MD from linear regression models; this allowed us to compare the magnitude of the association for variables with different units in multivariable analysis (Supplemental Tables 2–3 present results of unstandardized estimates and supplemental table 1 presents descriptive statistics for early life growth and SES and MD measures). We assessed these associations for confounding by prenatal and birth variables selecting those that changed the estimates of the associations between childhood growth or SES at birth and MD by >10%. Next, we conducted linear regression models to examine the associations between early life SES, early life height and weight changes at ages 4 months, 1 year and 7 years and MD. All models were adjusted for age at mammogram and race/ethnicity. Model 1 included all birth and growth variables, model 2 included SES at birth, model 3 included SES at birth, and birth and growth variables, and model 4 included all the previous variables in addition to adult BMI to account for the strong correlation of this variable and non-dense (fat) breast tissue. We did not consider factors occurring at later life periods (e.g., age at menarche, parity, adult SES), as we did not assess mediation of associations of early life growth and SES with MD by these variables, and these variables would not be confounders of these associations due to their timing in relation to early life factors. We tested for the presence of statistical interactions on the additive scale by race/ethnicity or SES at birth of the association between early life growth and MD.
RESULTS
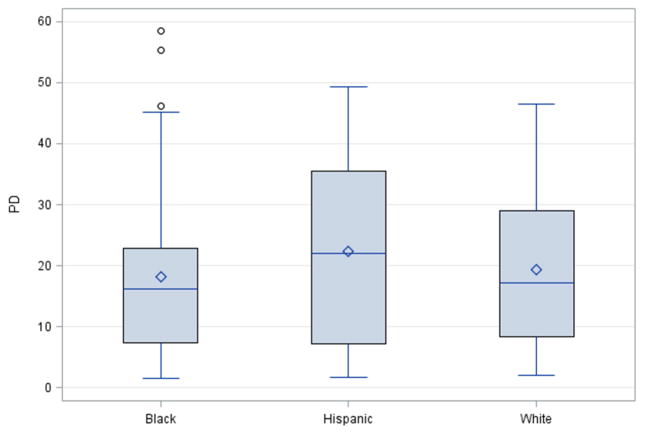
A total of 151 women participating in this study had available mammogram (37% White, 38% Black and 25% Puerto Rican) (Table 1). Participants with mammograms were older than participants without mammograms at the time of adult follow-up interview, but there were no substantial differences with respect to any prenatal, birth, childhood, and adult variables (data not shown). There were no significant differences in %MD and dense area between black, white and Puerto Rican women (Figure 1).
Table 1.
Distribution of Prenatal, Birth and Postnatal Growth Characteristics, New York Women’s Birth Cohort (Born 1959–1965), n=151
| N | Mean/% | SD | |
|---|---|---|---|
|
| |||
| Prenatal | |||
|
| |||
| Socioeconomic status at birth | 144 | 51.7 | 17.1 |
|
| |||
| Maternal smoking status | |||
| Ever | 71 | 47.3 | |
| Never | 79 | 52.7 | |
|
| |||
| Maternal Race | |||
| White | 56 | 37.1 | |
| Black | 57 | 37.8 | |
| Puerto Rican | 38 | 25.2 | |
|
| |||
| Maternal Pre-eclampsia | |||
| None | 125 | 83.3 | |
| Possible Pre-eclampsia | 11 | 7.3 | |
| Pre-eclampsia | 14 | 9.3 | |
|
| |||
| Maternal Pre-pregnancy BMI (kg/m2) | 139 | 22.9 | 4.0 |
|
| |||
| Maternal Age at Registration (years) | 151 | 26.6 | 6.2 |
|
| |||
| Maternal Pregnancy Weight Gain (lbs) | 143 | 23.0 | 9.9 |
|
| |||
| Birth | |||
|
| |||
| Birth weight (kg) | 151 | 3.2 | 0.5 |
|
| |||
| Length at Birth (cm) | 148 | 50.3 | 2.4 |
|
| |||
| Gestational Age (weeks) | 151 | 39.5 | 2.6 |
|
| |||
| Postnatal | |||
|
| |||
| Weight at 4 months (kg) | 149 | 6.16 | 0.8 |
|
| |||
| Length at 4 months (cm) | 149 | 61.6 | 2.9 |
|
| |||
| Weight at 1 year (kg) | 147 | 9.5 | 1.1 |
|
| |||
| Height at 1 year (cm) | 148 | 73.7 | 3.1 |
|
| |||
| Weight at 7 years (kg) | 151 | 23.5 | 4.7 |
|
| |||
| Height at 7 years (cm) | 149 | 121.4 | 4.9 |
|
| |||
| Percentile Weight change: Birth to 4 months | 150 | −1.5 | 28.6 |
|
| |||
| Percentile Weight change: 4 months to 1 year | 150 | −1.3 | 20.3 |
|
| |||
| Percentile Weight Change: 1 year to 7 years | 150 | −0.4 | 27.4 |
|
| |||
| Percentile Height change: Birth to 4 months | 150 | −5 | 28.5 |
|
| |||
| Percentile Height change: 4 months to 1 year | 150 | −0.4 | 27 |
|
| |||
| Percentile Height change: 1 year to 7 years | 150 | 2.3 | 29.3 |
|
| |||
| Adult | |||
|
| |||
| Age at interview (years) | 151 | 42.1 | 1.8 |
|
| |||
| Adult BMI (kg/m2) | 147 | 27.6 | 6.5 |
Figure 1.
Distribution of Percent Mammographic Density by Race/Ethnicity, New York NCPP
In Table 2, we present the multivariable regression analysis results of the association between early life growth and SES, and %MD. In model 1, after adjusting for age at mammogram, race/ethnicity, prenatal and birth variables, there was a significant inverse association between percentile weight change from birth to 4 months and %MD (Std β= −0.37, 95% CI: −0.63, −0.12), and between percentile weight change from age 1 year to 7 years and %MD (Std β= −0.37, 95% CI: −0.61, −0.12). In model 2, after adjusting for age at mammogram and race/ethnicity, SES at birth was positively associated with %MD (Std β =0.20, 95% CI: 0.02, 0.39). In model 3, the positive association between SES and %MD remained after adjusting for all birth and growth variables (Std β= 0.23, 95% CI: 0.04, 0.43), as did the inverse association between percentile weight change from birth to 4 months (Std β= −0.34, 95% CI: −0.59, −0.10), and from 1 year to 7 years (Std β =−0.39, 95% CI: −0.63, −0.15). In the final model with further adjustment for adult BMI (model 4), the associations of percentile weight change from birth to 4 months and percentile weight change from age 1 year to 7 years with %MD were attenuated, and the percentile weight change from birth to 4 months became statistically non-significant.
Table 2.
Multivariable Associations between Prenatal, Birth and Growth Characteristics and Percent Mammographic Density (Standardized β)*
| Model 1 β (95% CI) |
Model 2 β (95% CI) |
Model 3 β (95% CI) |
Model 4 β (95% CI) |
|
|---|---|---|---|---|
| SES at Enrollment | 0.20 (0.02, 0.39) | 0.23 (0.04, 0.43) | 0.24 (0.04, 0.43) | |
| Age at Mammogram | −0.06 (−0.24, 0.13) | −0.08 (−0.26, 0.11) | −0.06 (−0.24, 0.13) | −0.06 (−0.25, 0.13) |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | −0.03 (−0.26, 0.19) | 0.06 (−0.15, 0.26) | 0.01 (−0.22, 0.24) | 0.03 (−0.20, 0.27) |
| Hispanic | −0.01 (−0.22, 0.20) | 0.16 (−0.04, 0.36) | 0.08 (−0.14, 0.30) | 0.08 (−0.14, 0.30) |
| Birth Weight (kg) | −0.38 (−0.78, 0.01) | −0.28 (−0.68, 0.11) | −0.20 (−0.61, 0.22) | |
| Birth Length (cm) | 0.03 (−0.37, 0.43) | −0.05 (−0.45, 0.36) | −0.12 (−0.53, 0.28) | |
| Gestation at Delivery (weeks) | −0.03 (−0.22, 0.16) | 0.001 (−0.19, 0.19) | 0.02 (−0.18, 0.22) | |
| Weight change: Birth to 4 months | −0.37 (−0.63, −0.12) | −0.34 (−0.59, −0.10) | −0.24 (−0.51, 0.02) | |
| Weight change: 4 months to 1 year | −0.15 (−0.38, 0.07) | −0.19 (−0.42, 0.04) | −0.15 (−0.40, 0.09) | |
| Weight Change: 1 year to 7 years | −0.37 (−0.61, −0.12) | −0.39 (−0.63, −0.15) | −0.28 (−0.55, −0.01) | |
| Height change: Birth to 4 months | −0.02 (−0.30, 0.27) | −0.01 (−0.30, 0.27) | −0.06 (−0.36, 0.23) | |
| Height change: 4 months to 1 year | −0.04 (−0.32, 0.23) | −0.08 (−0.36, 0.20) | −0.14 (−0.43, 0.15) | |
| Height change: 1 year to 7 years | −0.06 (−0.31, 0.19) | 0.04 (−0.22, 0.30) | 0.03 (−0.24, 0.29) | |
| Adult BMI (kg/m2) | −0.21 (−0.42, −0.01) | |||
Model 1 includes age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change birth-4 months, 4 months-1 year, and 1 year–7 years.
Model 2 includes SES at birth, age at mammogram, and race/ethnicity
Model 3 includes SES at birth, age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change from birth-4 months, 4 months–1 year, and 1 year–7 years
Model 4 includes SES at birth, age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change from birth-4 months, 4 months–1 year, 1 year–7 years and adult BMI
All models additionally adjusted for maternal pre-pregnancy BMI, maternal weight gain, pre-eclampsia, and maternal smoking
In the same model, SES at birth remained positively associated with %MD of equivalent magnitude as the association for percentile weight change from 1 to 7 years and for adult BMI. Specifically, %MD decreased by 0.28 percentage points (95% CI: −0.55, −0.01) for every standard deviation increase in percentile weight change from age 1 to 7 years, decreased by 0.21 percentage points (95% CI: −0.42, −0.01) for every standard deviation increase in adult BMI, and increased by 0.24 percentage points (95% CI: 0.04, 0.43) for every standard deviation increase in SES at birth. The unstandardized estimates of the associations showed 0.18 higher %MD (95% CI: 0.03, 0.33) for each unit increase in early life SES index and 0.13 lower %MD (−0.26, 0.00) for a unit increase in weight change from 1 year to 7 years (Supplemental Table 2). We did not detect an additive interaction between race/ethnicity or SES and early life growth in the associations with %MD in the fully adjusted models (all p values for interaction terms >0.05); however, the small sample size in each racial group may have limited the statistical power to detect interactions.
In Table 3, we present the results of the associations between early life growth and SES variables, and mammographic dense area. Although the estimates were in the same direction as those from the analysis of %MD, that is, inverse associations between early life growth variables and positive associations with early life SES, none of these estimates were statistically significant at the 5% significance level.
Table 3.
Multivariable Associations between Prenatal, Birth and Growth Characteristics and Mammographic Dense Area (cm2) (Standardized β)*
| Model 1 β (95% CI) |
Model 2 β (95% CI) |
Model 3 β (95% CI) |
Model 4 β (95% CI) |
|
|---|---|---|---|---|
| SES at Enrollment | 0.16 (−0.03, 0.35) | 0.20 (−0.01, 0.40) | 0.20 (−0.01, 0.41) | |
| Age at Mammogram | −0.14 (−0.34, 0.06) | −0.10 (−0.29, 0.08) | −0.12 (−0.32, 0.08) | −0.13 (−0.34, 0.07) |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | −0.04 (−0.27, 0.20) | 0.02 (−0.19, 0.23) | −0.02 (−0.26, 0.23) | −0.06 (−0.31, 0.18) |
| Hispanic | −0.07 (−0.30, 0.15) | 0.04 (−0.17, 0.24) | −0.03 (−0.26, 0.21) | −0.02 (−0.26, 0.22) |
| Birth Weight (kg) | −0.11 (−0.53, 0.31) | −0.02 (−0.45, 0.40) | −0.15 (−0.60, 0.30) | |
| Birth Length (cm) | −0.14 (−0.57, 0.28) | −0.21 (−0.64, 0.23) | −0.14 (−0.58, 0.30) | |
| Gestation at Delivery (weeks) | −0.10 (−0.30, 0.10) | −0.09 (−0.30, 0.11) | −0.08 (−0.29, 0.14) | |
| Weight change: Birth to 4 months | −0.18 (−0.45, 0.09) | −0.14 (−0.41, 0.12) | −0.20 (−0.49, 0.08) | |
| Weight change: 4 months to 1 year | −0.04 (−0.28, 0.20) | −0.07 (−0.32, 0.17) | −0.13 (−0.39, 0.14) | |
| Weight Change: 1 year to 7 years | −0.05 (−0.31, 0.20) | −0.07 (−0.33, 0.19) | −0.15 (−0.45, 0.14) | |
| Height change: Birth to 4 months | −0.16 (−0.46, 0.14) | −0.16 (−0.46, 0.15) | −0.09 (−0.41, 0.23) | |
| Height change: 4 months to 1 year | −0.16 (−0.46, 0.13) | −0.19 (−0.49, 0.11) | −0.11 (−0.42, 0.21) | |
| Height change: 1 year to 7 years | −0.16 (−0.43, 0.10) | −0.10 (−0.38, 0.18) | −0.05 (−0.34, 0.23) | |
| Adult BMI (kg/m2) | 0.18 (−0.05, 0.40) | |||
Model 1 includes age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change birth-4 months, 4 months–1 year, and 1 year–7 years.
Model 2 includes SES at birth, age at mammogram, and race/ethnicity
Model 3 includes SES at birth, age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change from birth-4 months, 4 months–1 year, and 1 year–7 years
Model 4 includes SES at birth, age at mammogram, race/ethnicity, birth weight, birth length, gestational age, percentile weight and height change from birth-4 months, 4 months–1 year, 1 year–7 years and adult BMI
All models additionally adjusted for maternal pre-pregnancy BMI, maternal weight gain, pre-eclampsia, and maternal smoking
Results from models using non-dense area as the outcome showed positive associations between birthweight, percentile weight change from 4 months to 1 year and from 1 year to 7 years with percent density, but these associations were attenuated and statistically nonsignificant after adjusting for adult BMI. Early life SES was not associated with non-dense area (data not shown).
DISCUSSION
In a racially diverse and predominantly premenopausal cohort of women, we observed an inverse association between early life growth and %MD, but we observed no significant associations between early life growth and dense area. %MD represents the extent of dense tissue comprised primarily of fibroglandular tissue relative to the total breast tissue comprised primarily of adipose or nondense tissue, and is strongly correlated with BMI. In contrast, dense area measures the absolute amount of dense breast tissue and is less affected by BMI. In our study, the associations of percentile weight change from ages 1 to 7 years with %MD, but not with non-dense area, remained after adjusting for birthweight, other measures of early life growth and adult BMI. Several studies have shown inverse associations between different indicators of early life growth, including childhood body size, weight and BMI velocities, and different measurement of MD, including qualitative, semi-quantitative and quantitative assessments (24–26, 33, 34), while other studies have reported mostly null or nonsignificant results (23, 27, 31, 37, 48, 49). We found no evidence of an association between %MD and birthweight, birth length and gestational age, which is consistent with results of some studies (23–25, 31, 48), but differs from results of other studies that have reported positive associations between birthweight and MD (27, 29, 37). The considerable heterogeneity in the study populations and in the measurement of early life growth and of MD across these studies likely to contributes to the observed mixed results. More importantly, most studies have lacked detailed prospectively ascertained early life growth data, which presents a particular challenge in detecting complex associations of modest magnitude.
We observed a significant association between a composite measure of early childhood SES and %MD, even after adjusting for early life body size as well as adult BMI, suggesting an independent effect of SES on density. We found a similar but statistically non-significant association between early life SES and dense area. The positive association between SES at birth and %MD that we observed parallel the well-established positive association between adult SES and breast cancer risk, and the more limited research on adult SES and MD (50–53). For example, Aitken et al reported 6–7% higher %MD in women in the highest adult SES category as compared to those in the lowest adult SES category (53). None of the few studies that assessed the association between early life SES and MD in addition to other early life growth data have observed an independent association (23, 24, 29, 30). However, unlike our prospective data on multiple dimensions of SES, these studies used data for a single dimension of SES (29, 30), and from adult participants’ recall of parental SES (23, 24). Our findings suggest that the influence of SES on %MD may begin as early as at birth and may not be mediated by early life growth and adult BMI. Importantly, the difference in %MD associated with a unit increase in SES (Stdβ= 0.24) was comparable to the magnitude of the difference associated with adult BMI (Stdβ= −0.21) and early life growth (Stdβ= −0.28 for weight change from ages 1–7 years), although in the opposite direction. These intriguing results merit further confirmation in other prospective studies.
Early life growth measures are thought to influence MD through hormonal pathways influencing the development of mammary tissue (8, 9). These changes are believed to occur as early as in utero, a period characterized by high levels of circulating endogenous estrogen. Early childhood also constitutes a critical period in mammary tissue development during which increases in adipocytes associated with weight gain increases circulatory estrogen levels (54–56). These discoveries are especially critical to understanding the mechanisms underlying the development of breast cancer from a life course perspective. Our observation that the influence of early childhood characteristics, in this case childhood growth rates and SES, on %MD persist into adulthood highlights the importance of (1) understanding the etiology and pathways involved in the development of breast cancer, and (2) using a life-course approach to understand changes that occur early in life and may potentially be modifiable. Primary prevention efforts for breast cancer are more likely to succeed if modifiable risk factors occurring early in life are understood and altered (57). In addition, risk stratification strategies may benefit from better understanding of the mechanistic pathways that places certain population groups at higher risk, and which can potentially inform routine screening guidelines and clinical practice.
A major strength of our study includes the prospective study design. All growth and SES measurements were taken without participant and staff knowledge of the outcome. Furthermore, early life data were collected prospectively using a uniform, standardized protocol, limiting the role of information bias in the study. Therefore, any potential measurement error would be non-differential and most likely would have attenuated the observed associations. The composite SES measure used in our analysis captured different dimensions of parental income, education and occupation at birth, providing a more comprehensive and accurate measure of early life SES. Although women who participated in this study had somewhat higher SES at birth compared with non-participants, participants and non-participants were comparable on maternal, birth, prenatal and growth characteristics, reducing the likelihood of serious selection bias (58). We were able to assess confounding by several important variables, and adjust for other early life events such as maternal BMI and smoking. However, there is potential for some residual confounding due to neighborhood SES, which is also implicated in multiple health outcomes in adulthood (59). The multi-ethnic nature of our cohort also allowed us to account for racial/ethnic differences, something that has been lacking in most of the prior studies. We obtained a highly reliable measure of MD, and confirmed this with high correlation coefficients for double-read films.
Our study suggests that early life factors are important predictors of adult MD. Even after adjusting for other early life events including maternal and prenatal characteristics, SES at birth and percentile weight change in childhood were associated with MD in midlife. The association between adult BMI and MD has been well established. Our study shows that the influence of weight or body fat, which modulates hormonal levels, on MD begins as early as in childhood and persists into adulthood even after accounting for differences in adult BMI. The influence of early life SES on MD in our study was relatively large, and independent of birth, childhood and adult body size. The direction of the associations of early life SES and body size with MD are consistent with the associations of these factors with breast cancer. If replicated in larger prospective birth cohorts, these results provide indirect evidence for the influence of early life factors on breast cancer risk through mammographic density.
Supplementary Material
Acknowledgments
We would like to thank the following individuals for their contributions to the New York Women’s Birth Cohort: Ezra Susser, Tara Kalra, Tamarra James-Todd, Lina Titievsky-Konikov, Dipal Shah, Shobana Ramachandran, Julia Meurling, Adey Tsega, Sujata Narayanan, Summer Wright and all of the participants in the New York Women’s Birth Cohort. This work was supported by the Department of Defense Breast Cancer Research Program [DAMD170210357]; and the National Cancer Institute [K07CA90685].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanderson M, Williams MA, Malone KE, et al. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7(1):34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 2.McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, Lithell HO. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. Bmj. 2003;326(7383):248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Stavola BL, dos Santos Silva I, Wadsworth MJ. Birth weight and breast cancer. The New England journal of medicine. 2005;352(3):304–306. author reply 304–306. [PubMed] [Google Scholar]
- 4.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. The New England journal of medicine. 2004;351(16):1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 5.Park SK, Kang D, McGlynn KA, et al. Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast cancer research: BCR. 2008;10(1):R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trichopoulos D, Adami HO, Ekbom A, Hsieh CC, Lagiou P. Early life events and conditions and breast cancer risk: from epidemiology to etiology. International journal of cancer Journal international du cancer. 2008;122(3):481–485. doi: 10.1002/ijc.23303. [DOI] [PubMed] [Google Scholar]
- 7.dos Santos Silva I, De Stavola BL, Hardy RJ, Kuh DJ, McCormack VA, Wadsworth ME. Is the association of birth weight with premenopausal breast cancer risk mediated through childhood growth? Br J Cancer. 2004;91(3):519–524. doi: 10.1038/sj.bjc.6601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruder EH, Dorgan JF, Kranz S, Kris-Etherton PM, Hartman TJ. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clinical breast cancer. 2008;8(4):334–342. doi: 10.3816/CBC.2008.n.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okasha M, McCarron P, Gunnell D, Smith GD. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast cancer research and treatment. 2003;78(2):223–276. doi: 10.1023/a:1022988918755. [DOI] [PubMed] [Google Scholar]
- 10.De Stavola BL, dos Santos Silva I, McCormack V, Hardy RJ, Kuh DJ, Wadsworth ME. Childhood growth and breast cancer. Am J Epidemiol. 2004;159(7):671–682. doi: 10.1093/aje/kwh097. [DOI] [PubMed] [Google Scholar]
- 11.Hilakivi-Clarke L, Forsen T, Eriksson JG, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85(11):1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan DP, Park JM. Family factors and social support in the developmental outcomes of very low-birth weight children. Clinics in perinatology. 2000;27(2):433–459. doi: 10.1016/s0095-5108(05)70030-0. [DOI] [PubMed] [Google Scholar]
- 13.Keane E, Layte R, Harrington J, Kearney PM, Perry IJ. Measured parental weight status and familial socio-economic status correlates with childhood overweight and obesity at age 9. PloS one. 2012;7(8):e43503. doi: 10.1371/journal.pone.0043503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wronka I. Body height and socioeconomic status of females at different life stages. Journal of biosocial science. 2013;45(4):471–480. doi: 10.1017/S0021932012000600. [DOI] [PubMed] [Google Scholar]
- 15.Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990–2005. Obesity (Silver Spring) 2008;16(2):275–284. doi: 10.1038/oby.2007.35. [DOI] [PubMed] [Google Scholar]
- 16.de Kok IM, van Lenthe FJ, Avendano M, Louwman M, Coebergh JW, Mackenbach JP. Childhood social class and cancer incidence: results of the globe study. Soc Sci Med. 2008;66(5):1131–1139. doi: 10.1016/j.socscimed.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Pudrovska T, Anikputa B. The role of early-life socioeconomic status in breast cancer incidence and mortality: unraveling life course mechanisms. Journal of aging and health. 2012;24(2):323–344. doi: 10.1177/0898264311422744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1998;7(12):1133–1144. [PubMed] [Google Scholar]
- 19.Bertrand KA, Tamimi RM, Scott CG, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast cancer research: BCR. 2013;15(6):R104. doi: 10.1186/bcr3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglietto L, Krishnan K, Stone J, et al. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. American journal of epidemiology. 2014;179(4):475–483. doi: 10.1093/aje/kwt260. [DOI] [PubMed] [Google Scholar]
- 21.Boyd NF, Rommens JM, Vogt K, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 22.Yochum L, Tamimi RM, Hankinson SE. Birthweight, early life body size and adult mammographic density: a review of epidemiologic studies. Cancer Causes Control. 2014;25(10):1247–1259. doi: 10.1007/s10552-014-0432-0. [DOI] [PubMed] [Google Scholar]
- 23.Jeffreys M, Warren R, Gunnell D, McCarron P, Smith GD. Life course breast cancer risk factors and adult breast density (United Kingdom) Cancer Causes Control. 2004;15(9):947–955. doi: 10.1007/s10522-004-2473-3. [DOI] [PubMed] [Google Scholar]
- 24.Lope V, Perez-Gomez B, Moreno MP, et al. Childhood factors associated with mammographic density in adult women. Breast Cancer Res Treat. 2011;130(3):965–974. doi: 10.1007/s10549-011-1664-2. [DOI] [PubMed] [Google Scholar]
- 25.McCormack VA, dos Santos Silva I, De Stavola BL, et al. Life-course body size and perimenopausal mammographic parenchymal patterns in the MRC 1946 British birth cohort. Br J Cancer. 2003;89(5):852–859. doi: 10.1038/sj.bjc.6601207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sorensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast cancer research: BCR. 2014;16(1):R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamimi RM, Eriksson L, Lagiou P, et al. Birth weight and mammographic density among postmenopausal women in Sweden. Int J Cancer. 2010;126(4):985–991. doi: 10.1002/ijc.24786. [DOI] [PubMed] [Google Scholar]
- 28.Cerhan JR, Sellers TAJC, Pankratz VS, Brandt KR, Vachon CM. Prenatal and perinatal correlates of adult mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1502–1508. doi: 10.1158/1055-9965.EPI-04-0762. [DOI] [PubMed] [Google Scholar]
- 29.Pearce MS, Tennant PW, Mann KD, et al. Lifecourse predictors of mammographic density: the Newcastle Thousand Families cohort Study. Breast Cancer Res Treat. 2011;131(1):187–195. doi: 10.1007/s10549-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 30.Ekbom A, Thurfjell E, Hsieh CC, Trichopoulos D, Adami HO. Perinatal characteristics and adult mammographic patterns. Int J Cancer. 1995;61:177–180. doi: 10.1002/ijc.2910610206. [DOI] [PubMed] [Google Scholar]
- 31.Lokate M, van Duijnhoven FJ, van den Berg SW, Peeters PH, van Gils CH. Early life factors and adult mammographic density. Cancer Causes Control. 2013;24(10):1771–1778. doi: 10.1007/s10552-013-0254-5. [DOI] [PubMed] [Google Scholar]
- 32.Rice MS, Bertrand KA, Lajous M, et al. Body size throughout the life course and mammographic density in Mexican women. Breast Cancer Res Treat. 2013;138(2):601–610. doi: 10.1007/s10549-013-2463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses’ Health Study. Breast Cancer Res Treat. 2008;109(1):113–122. doi: 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 34.Sellers TA, Vachon CM, Pankratz VS, et al. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–464. doi: 10.1093/aje/kwm112. [DOI] [PubMed] [Google Scholar]
- 35.Dorgan JF, Klifa C, Shepherd JA, et al. Height, adiposity and body fat distribution and breast density in young women. Breast cancer research: BCR. 2012;14(4):R107. doi: 10.1186/bcr3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng M, Olufade TO, Evers KA, Byrne C. Adolescent lifestyle factors and adult breast density in U.S. Chinese immigrant women. Nutr Cancer. 2011;63(3):342–349. doi: 10.1080/01635581.2011.535955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerhan JR, Sellers TA, Janney CA, Pankratz VS, Brandt KR, Vachon CM. Prenatal and perinatal correlates of adult mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1502–1508. doi: 10.1158/1055-9965.EPI-04-0762. [DOI] [PubMed] [Google Scholar]
- 38.Terry MB, Flom J, Tehranifar P, Susser E. The role of birth cohorts in studies of adult health: the New York women’s birth cohort. Paediatric and Perinatal Epidemiology. 2009;23(5):431–445. doi: 10.1111/j.1365-3016.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tehranifar P, Liao Y, Ferris JS, Terry MB. Life course socioeconomic conditions, passive tobacco exposures and cigarette smoking in a multiethnic birth cohort of U.S. women. Cancer Causes Control. 2009;20(6):867–876. doi: 10.1007/s10552-009-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flom JD, Ferris JS, Tehranifar P, Terry MB. Alcohol intake over the life course and mammographic density. Breast Cancer Research and Treatment. 2009;117(3):643–651. doi: 10.1007/s10549-008-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broman SH. The collaborative perinatal project: an overview. In: Mednick SA, Harway M, Finello KM, editors. Hanbook of Longitudinal Research. New York: Praeger; 1984. pp. 166–179. [Google Scholar]
- 42.Myrianthopoulos NC, French KS. An application of the U.S. Bureau of the Census socioeconomic index to a large, diversified patient population. Social science & medicine. 1968;2(3):283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 43.Byng JW, Boyd NF, Little L, et al. Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev. 1996;5(5):319–327. doi: 10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Kataoka M, Atkinson C, Warren R, et al. Mammographic density using two computer-based methods in an isoflavone trial. Maturitas. 2008;59(4):350–357. doi: 10.1016/j.maturitas.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. doi: 10.1093/aje/kwp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terry MB, Wei Y, Esserman D, McKeague IW, Susser E. Pre- and postnatal determinants of childhood body size: cohort and sibling analyses. Journal of developmental origins of health and disease. 2011;2(2):99–111. doi: 10.1017/S2040174411000067. [DOI] [PubMed] [Google Scholar]
- 47.Terry MB, Wei Y, Esserman D. Maternal, birth, and early-life influences on adult body size in women. Am J Epidemiol. 2007;166(1):5–13. doi: 10.1093/aje/kwm094. [DOI] [PubMed] [Google Scholar]
- 48.Ekbom A, Thurfjell E, Hsieh CC, Trichopoulos D, Adami HO. Perinatal characteristics and adult mammographic patterns. International journal of cancer Journal international du cancer. 1995;61(2):177–180. doi: 10.1002/ijc.2910610206. [DOI] [PubMed] [Google Scholar]
- 49.Pearce MS, Tennant PW, Mann KD, et al. Lifecourse predictors of mammographic density: the Newcastle Thousand Families cohort Study. Breast cancer research and treatment. 2012;131(1):187–195. doi: 10.1007/s10549-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 50.Keegan TH, John EM, Fish KM, Alfaro-Velcamp T, Clarke CA, Gomez SL. Breast cancer incidence patterns among California Hispanic women: differences by nativity and residence in an enclave. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1208–1218. doi: 10.1158/1055-9965.EPI-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds P, Hurley SE, Quach AT, et al. Regional variations in breast cancer incidence among California women, 1988–1997. Cancer causes & control: CCC. 2005;16(2):139–150. doi: 10.1007/s10552-004-2616-5. [DOI] [PubMed] [Google Scholar]
- 52.Robert SA, Strombom I, Trentham-Dietz A, et al. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15(4):442–450. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 53.Aitken Z, Walker K, Stegeman BH, et al. Mammographic density and markers of socioeconomic status: a cross-sectional study. BMC cancer. 10:35. doi: 10.1186/1471-2407-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucci LA, Lagiou P, Tamimi RM, Hsieh CC, Adami HO, Trichopoulos D. Pregnancy estriol, estradiol, progesterone and prolactin in relation to birth weight and other birth size variables (United States) Cancer causes & control: CCC. 2003;14(4):311–318. doi: 10.1023/a:1023966813330. [DOI] [PubMed] [Google Scholar]
- 55.Russo J, Russo IH. Differentiation and breast cancer. Medicina. 1997;57(Suppl 2):81–91. [PubMed] [Google Scholar]
- 56.McFadyen IR, Worth HG, Wright DJ, Notta SS. High oestrogen excretion in pregnancy. British journal of obstetrics and gynaecology. 1982;89(12):994–999. doi: 10.1111/j.1471-0528.1982.tb04653.x. [DOI] [PubMed] [Google Scholar]
- 57.Colditz GA, Bohlke K, Berkey CS. Breast cancer risk accumulation starts early: prevention must also. Breast Cancer Res Treat. 2014;145(3):567–579. doi: 10.1007/s10549-014-2993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terry MB, Flom J, Tehranifar P, Susser E. The role of birth cohorts in studies of adult health: the New York women’s birth cohort. Paediatric and perinatal epidemiology. 2009;23(5):431–445. doi: 10.1111/j.1365-3016.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akinyemiju TF, Genkinger JM, Farhat M, Wilson A, Gary-Webb TL, Tehranifar P. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC cancer. 2015;15:191. doi: 10.1186/s12885-015-1098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.