Abstract
Studies in mammals and Drosophila have demonstrated the existence and significance of secreted factors involved in communication between distal organs. In this review, primarily focusing on Drosophila, we examine the known interorgan communication factors and their functions, physiological inducers, and integration in regulating physiology. Moreover, we describe how organ-sensing screens in Drosophila can systematically identify novel conserved interorgan communication factors. Finally, we discuss how interorgan communication enabled and evolved as a result of specialization of organs. Together, we anticipate that future studies will establish a model for metazoan interorgan communication network (ICN) and how it is deregulated in disease.
Keywords: interorgan communication, interorgan communication network, secreted factor, myokine, adipokine, secretion
INTRODUCTION
Organ functions are highly specialized. For example, nutrients are taken up through the gut; sensed, processed, stored, and released by the liver and adipose tissues; and utilized by the peripheral organs. Nutrients are used by the skeletal muscle and heart for contraction, by the brain for behavior, by the kidney for water balance and waste disposal, by the gonads for reproduction, and by other tissues for growth (Figure 1) (136, 148). In addition, each organ depends on other organs. For instance, increased physiological demands such as exercise, brain activity, growth, and disease require increased nutrient uptake by the gut and nutrient release (sugars, fats, ketone bodies) by the liver and adipose tissues (136, 148). Because organ functions are interdependent, it is important to understand how organs communicate their states to each other. In this review, we focus on the identification of factors that mediate communication between organs. In particular, we focus on the fruit fly Drosophila melanogaster, which offers the advantages of having organ systems with similar functions to those of humans, and powerful genetic tools. Importantly, many of the Drosophila organ communication factors identified to date have human orthologs.
Figure 1.
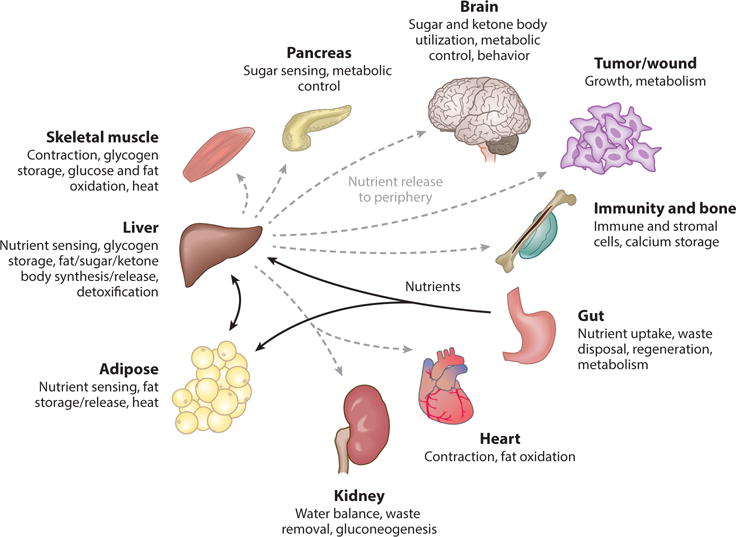
A simplified view of organ specialization and integration in mammals (136, 148). Nutrients are taken up by the gut (black arrows) and then sensed, processed, stored, and released to peripheral organs by the liver and adipose tissues (gray dashed arrows). Increased physiological demands enhance nutrient traffic to certain organs. For instance, during exercise, muscles require increased levels of fatty acids and glucose from the liver and adipose tissues.
IDENTIFICATION OF INTERORGAN COMMUNICATION FACTORS IN MAMMALS
Experiments in mammals and other vertebrates have identified important interorgan communication factors. As a number of recent reviews (34, 37, 65, 107, 117) have described mammalian systemic factors in detail, here we highlight only a few recent examples (see Figure 2, Table 1, and references therein for an overview). Adipose tissue– and muscle-derived growth factors and cytokines—adipokines and myokines, respectively—have been recently recognized for their roles in organ cross talk (107). For instance, regulated by stored nutrients, the adipokine leptin is secreted from adipose tissue and acts on neuroendocrine organs via the leptin receptor (2, 144, 158). Also, fibronectin type III domain–containing protein 5 (FNDC5) transmembrane protein cleavage product irisin is derived from muscle during exercise and regulates adipose tissue browning and metabolism (12). In addition, transforming growth factor (TGF)-β family member growth differentiation factor 11 (GDF-11) regulates the aging heart, muscle stem cells, and brain vasculature (67, 90, 113, 138, but see controversy in 39, 132). Interestingly, parathyroid hormone related protein (PTHrP) released systemically from tumors regulates adipose tissue wasting, or cachexia, through the G-protein–coupled PTH receptor (70).
Figure 2.
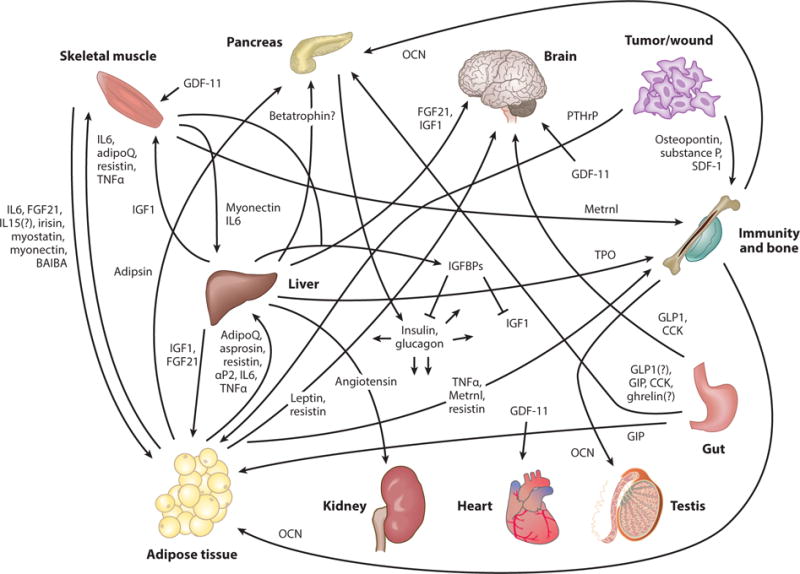
Selected recent examples of mammalian interorgan communication factors, their origins, and destinations; also see Table 1. Selected recent references for these factors: adipoQ (adiponectin) (154), adipsin (89), angiotensin (55), aP2 (19), asprosin (126), BAIBA (β-aminoisobutyric acid) (124), betatrophin (53, 157), CCK (cholecystokinin) (84, 88, 120), FGF21 (42), GDF-11 (growth differentiation factor 11; tissue source is unclear) (39, 67, 90, 113, 138), ghrelin (94, 97), GIP (gastric inhibitory polypeptide) (18), GLP1 (glucagon-like peptide-1) (36, 140), glucagon (136, 148), IGF1 (insulin growth factor 1) (13, 24), IGFBPs (insulin growth factor binding proteins) (61), IL6 (112, 128), IL15 (112), insulin (136, 148), irisin (12), leptin (2, 100, 144, 158), Metrnl (meterorin-like) (119), myonectin (133), myostatin (95), OCN (osteocalcin) (79, 106), osteopontin (93), PTHrP (parathyroid hormone related protein) (70), resistin (8, 64, 101, 130, 143), SDF-1 (116), substance P (59), TNFα (tumor necrosis factor α) (46, 60, 142), and TPO (thrombopoietin; also made by kidney) (31, 68).
Table 1.
Selected recent examples of mammalian interorgan communication factors, their origins, and their destinations (also see Figure 2)
| Factor | Communication axes | Reference(s) |
|---|---|---|
| Irisin | Muscle → adipose tissue | 12 |
| Myostatin | Muscle → adipose tissue | 95 |
| IL6 | Muscle → adipose tissue, liver | 112, 128 |
| IL15 (?) | Muscle → adipose tissue | 112 |
| Myonectin | Muscle → adipose tissue, liver | 133 |
| BAIBA (β-aminoisobutyric acid) | Muscle → adipose tissue | 124 |
| FGF21 | Muscle → adipose tissue | 42 |
| AdipoQ (adiponectin) | Adipose tissue → muscle, liver | 154 |
| Asprosin | Adipose tissue → liver | 126 |
| Resistin | Adipose tissue/blood monocytes → muscle, brain, liver | 8, 64, 101, 130, 143 |
| IGF1 | Liver → adipose tissue, muscle | 13, 24 |
| Adipsin | Adipose tissue → pancreas | 89 |
| aP2 | Adipose tissue → liver | 19 |
| Leptin | Adipose tissue → brain | 2, 100, 144, 158 |
| Betatrophin (?) | Liver? Adipose tissue? → pancreas? | 53, 157 |
| Angiotensin | Liver → kidney | 55 |
| Insulin | Pancreas → multiple organs | 136, 148 |
| Glucagon | Pancreas → multiple organs | 136, 148 |
| Insulin growth factor binding proteins (IGFBPs) | Liver, muscle → bind to insulin, insulin growth factors | 61 |
| Metrnl (meterorin-like) | Adipose tissue, muscle → immune system | 119 |
| TNFα | Adipose tissue → immune system, muscle, liver | 46, 60, 142 |
| GLP1 (glucagon-like peptide-1) | Gut → pancreas?, brain | 35, 136 |
| GIP (gastric inhibitory polypeptide) | Gut → pancreas | 18 |
| CCK (cholecystokinin) | Gut → pancreas, brain | 84, 88, 120 |
| Ghrelin | Gut → pancreas? | 94, 97 |
| PTHrP | Tumor → adipose tissue | 70 |
| GDF-11 | Unclear organ source → muscle, heart, brain | 39, 67, 90, 113, 132, 138 |
| TPO (thrombopoietin) | Kidney, liver → bone/hematopoiesis | 31, 68 |
| Osteopontin | Tumor → bone/immunity | 93 |
| Substance P | Tumor → bone | 59 |
| SDF-1 | Wound → bone | 116 |
| OCN (osteocalcin) | Bone → testis, adipose tissue | 79, 106 |
A number of questions for future studies remain. For instance, the nature of the irisin receptor (151) and the organ source of GDF-11 (90) are unclear. In addition, could systemic factors also have a local function? For example, leptin may be sensed by neurons within the adipose tissue (100), whereas the intestine-derived peptide cholecystokinin (CCK) might regulate insulin secretion (88) via local release from pancreatic neurons (120). Also, tissue-specific loss-of-function studies of the incretin glucagon-like peptide 1 (GLP-1) receptor suggest that postprandial gut-derived GLP-1 may increase insulin secretion not systemically through the circulation as previously proposed but instead through paracrine mechanisms (neurons near the gut, other pancreatic cells, etc.) (36, 140). Moreover, the physiological relevance of stomach-derived peptide ghrelin to food uptake, postprandial insulin secretion, and its systemic role (ghrelin can be secreted by the pancreatic islets themselves) has been questioned (94, 97). Finally, the question of conservation of a mouse-secreted factor and its mechanism of action to humans needs to be addressed. For example, cysteine-rich cytokine resistin is derived from adipocytes in the mouse, whereas in humans, resistin is found in blood monocytes but not in adipocytes (130).
Studies in nonprimate mammalian systems have been instrumental in identifying interorgan communication factors, and advantages include the availability of tissues and cells for biochemistry and proteomics and the likelihood of a given factor to be conserved to humans. Loss-of-function genetic studies are essential to show the function of a systemic factor. For example, overexpression of angiopoietin-like protein 8 (ANGPTL8) betatrophin was reported to induce β-cell proliferation (157); however, betatrophin knockouts showed no phenotype on β-cell proliferation (53). In addition, the roles of many secreted proteins in interorgan communication remain to be investigated. Human normal and diseased blood contains thousands of proteins (40, 87). Moreover, human adipocytes (80) and myocytes (57) secrete hundreds of proteins in vitro. A number of these proteins have poorly characterized functions, and we expect some to have signaling functions. However, large-scale systematic in vivo functional studies (genetic screens) are challenging in mammals. Thus, invertebrate genetic model systems such as Drosophila are expected to contribute significantly to discoveries in interorgan communication.
In this review, we describe Drosophila as a genetic model system for understanding principles of interorgan communication. We present the secreted factors, their physiological inducers, their mechanisms of action, and how they are integrated together to regulate physiology. Also, based on evolutionary considerations, we describe how interorgan communication enabled and evolved as a result of specialization of organs. Finally, we discuss how future studies in Drosophila may lead to an interorgan communication network (ICN) (37) conserved to humans and deregulated in disease.
DROSOPHILA AS A MODEL SYSTEM TO DISSECT INTERORGAN COMMUNICATION
Drosophila Has Functionally Equivalent Organ Systems to Mammals
Drosophila is an emerging model system for studying interorgan communication. Importantly, Drosophila has functionally similar organs to the human digestive tract, gonads, liver and adipose tissue [fat bodies (FBs) and oenocytes in flies], muscle, brain, and others (Figure 3) (4, 35, 81, 109). In addition, like in mammals, Drosophila organs are specialized and integrated. For instance, nutrients are taken up through the gut; sensed, processed, stored, and released by FBs and oenocytes; and utilized by the peripheral organs. Nutrients are used by the muscle and heart for contraction, by the brain for behavior, by the gonads for reproduction, and by developing organs (imaginal discs) and other tissues for growth (Figure 4). Moreover, increased physiological demands such as exercise, brain activity, growth, and disease require increased nutrient uptake by the gut and nutrient release by the FBs and oenocytes (4, 35, 81, 109).
Figure 3.

Drosophila melanogaster has functionally equivalent organ systems to the human liver/adipose tissues (fat bodies and oenocytes in Drosophila), gut, brain, muscle, gonads, and others (4, 17, 35, 81, 109). This allows the study of interorgan communication using the powerful genetic tools available in flies.
Figure 4.
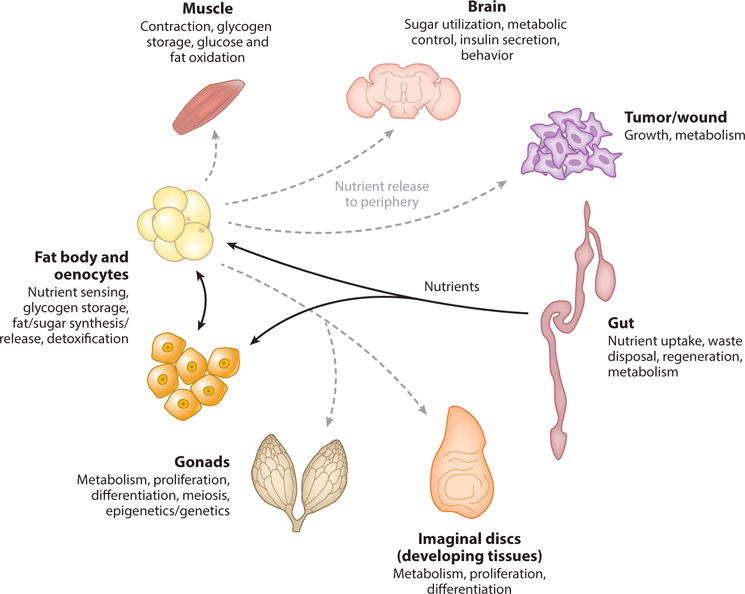
A simplified view of organ specialization and integration in Drosophila (4, 17, 35, 81, 109).
Drosophila proceeds through embryonic, larval, pupal, and adult life stages. Both larvae and adults have FBs, oenocytes, muscles, heart, brain, and gut (4, 35, 81, 109). However, the physiological and environmental differences between the stages lead to additional specialized organs. For instance, larvae have an actively developing hematopoietic system, dividing muscle precursors, a brain containing dividing neural precursors, and developing adult organs (imaginal discs), including wings, eyes, gonads, and others. By contrast, adults have a fully developed nondividing brain, hematopoietic cells, muscles, wings, eyes, and gonads, but have dividing stem cells in the gut and gonads (4, 17, 35, 81, 109). In sum, larvae are a model for growing, developing organisms, whereas adults are a model for homeostatic regulation of metabolism, reproduction, tissue homeostasis, and aging.
Drosophila Interorgan Communication Factors
A number of conserved interorgan communication factors have been identified over the years— many recently—in Drosophila larvae and adults using genetic approaches (summarized in Table 2 and Figures 5–9).
Table 2.
Drosophila interorgan communication factors
|
Drosophila factor |
Mammalian ortholog |
Developmental stage |
Physiological inducer |
Origin | Destination | Receptor | Effect | Questions | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Dilp2, 3, 5 | Insulin/IGF | Larvae and adults | Food uptake | IPCs | All organs | InR | Increased growth, nutrient storage, anabolism; regulation of germline stem cells, aging | Why are there so many insulins in Drosophila? | 15, 62, 75, 102, 127, 145, 152 |
| Dilp6 | Insulin/IGF | Larvae and adults | Cessation of feeding (pupariation, starvation) | FB | Oenocytes, imaginal discs, IPCs (?) | InR | Take up lipids (oenocytes), promote growth (imaginal discs), decrease Dilp secretion | Relationship to other insulins? Paracrine from local FB? |
7, 21, 105, 139 |
| ImpL2 | IGFBP7 | Larvae and adults | ISC overproliferation; muscle mitochondrial damage; starvation | Gut, muscle, FB | Blood, organs | Insulin | Binding to insulin (inhibits insulin) | Relationship to other insulin binding proteins? | 41, 74, 108 |
| SDR | InR (soluble) | Larvae | Unknown | Brain glia | Blood, organs | Insulin | Binding to insulin (inhibits insulin) | Relationship to other insulin binding proteins? Inducer? |
104 |
| Upd2 | Leptin | Larvae and adults | Dietary sugar/fat | FB | Neurons regulating IPCs | Dome | Increase insulin secretion | Presence in hemolymph? Crossing of BBB? Sensing by peripheral neurons? Additional FB-derived signals in response to other nutrients? Relationship to CCHa2 and GBPs? |
118 |
| Egr | TNF-α | Larvae and possibly adults | Low dietary protein (through TACE induction) | FB | IPCs, FB | Grnd | Decrease insulin secretion; decrease growth; and (in high sugar conditions) increase insulin resistance (larva). Increase TAG upon protein starvation (adult). | BBB crossing? Hemolymph interacting proteins? Is insulin affected in adults? How is TACE induced downstream of TOR? Functional homology to AdipoQ? |
1, 134 |
| Dilp8 | Insulin/relaxin family | Larvae | Injury/tumors | Injured organs (imaginal discs) | Lgr3 neurons | Lgr3? | Inhibition of insulin and PTTH/JH signaling to inhibit growth and pupariation | Is endogenous Dilp8 present in hemolymph? Crossing of BBB? Functions in adults? Is Lgr3 is the bona fide Dilp8 receptor? |
26, 27, 48, 49, 147 |
| Lst | NMU? | Adults | Food restriction | CC cells | IPCs | LstR (CG9918)? | Decrease insulin secretion | Presence in hemolymph? Other sites of limostatin action? Is LstR the bona fide Lst receptor? |
3 |
| AKH | Glucagon | Larvae and adults | Starvation | CC cells | All organs | AKHR | Metabolism; increased catabolism of sugar and fat in fat body; regulation of life span, food search, sleep, resistance to oxidative stress | Systemic factors regulating AKH? | 47, 51, 66, 69, 78, 96, 150 |
| Myo | Myostatin, GDF11 | Adults | ? | Muscle | FB | Babo? Wit? | Decrease FB nucleolar size and rRNA; increase life span | Physiological inducer? Presence in hemolymph? Relationship to Daw? |
32 |
| Daw | TGF-β | Larvae and adults | Dietary sugar | FB, muscle, others? | Gut, FB, muscle | Babo/Punt | Gut digestive enzymes repression; metabolism; pH equilibrium; muscle protein homeostasis | Local or systemic factor (present in many organs)? Relationship to myoglianin? |
6, 23, 50, 82 |
| Hh | Shh and other mammalian Hh proteins | Larvae | Starvation | Gut and possibly imaginal discs? | FB, prothoracic gland | Ptc | Growth and development; lipid release from FB | Local secretion from neurons on FB? | 92, 110, 125 |
| JH | ? | Larvae and adults | Mating (adult females), other | Corpora allata | Several organs | Gce/Met | Increased growth, metabolism; increased proliferation of intestinal stem cells; regulation of insulin signaling | Mammalian ortholog? | 43, 63, 98, 121 |
| GABA | GABA | Larvae | Olfaction | Brain neurons | Lymph gland | GABA-B-R | Preservation of hematopoietic progenitor cells | Role in mammals? Other roles of systemic GABA? |
135 |
| PGRPs | PGRPs | Adults | Aging-associated FB inflammation | FB | Gut | PGRP-LC | Altered gut inflammation and increased intestinal stem cell proliferation | Are PGRPs acting directly or indirectly on the gut? | 135 |
| GBPs | EGF? | Larvae | Dietary sugar, lipids, and proteins | FB | IPCs | ? | Increase insulin secretion | Direct action on IPCs? Neural circuitry and receptor? Mechanism of BBB crossing? Similar mammalian mechanisms? Relationship to Upd2 and CCHa2 |
72 |
| CCHa2 | ? | Larvae, adult (feeding) | Dietary sugar and proteins | FB?, gut? | IPCs, brain | CCHa2R | Increase insulin secretion, induce feeding | Produced from FB or gut? Mechanism of BBB crossing? Presence in hemolymph? Do lipids induce CCHa2? Similar mammalian mechanisms? Relationship to Upd2 and GBPs? |
122, 129 |
Abbreviations: AdipoQ, adiponectin; AKH, adipokinetic hormone; AKHR, AKH receptor; babo, baboon; BBB, blood-brain barrier; CC, Drosophila corpora cardiaca; Daw, dawdle; Dilp, Drosophila insulin-like peptide; dome, domeless; EGF, epidermal growth factor; Egr, eiger; FB, fat body; GABA, γ-aminobutyric acid; GBPs, growth-blocking peptides; GDF-11, growth-differentiation factor 11; Grnd, grindelwald; Hh, hedgehog; IGF, insulin-like growth factor; IGFBP, IGF binding protein; InR, insulin receptor; IPCs, insulin-producing cells; ISC, intestinal stem cell; JH, juvenile hormone; Lst, limostatin; Myo, myoglianin; NMU, neuromedin U; PGRPs, peptidoglycan recognition proteins; PTTH, prothoracicotropic hormone; SDR, secreted decoy of InR; Shh, sonic hedgehog; TGF-β, transforming growth factor beta; TACE, TNF-α converting enzyme; TNF-α, tumor necrosis factor alpha; TOR, target of rapamycin; Wit, wishful-thinking.
Figure 5.
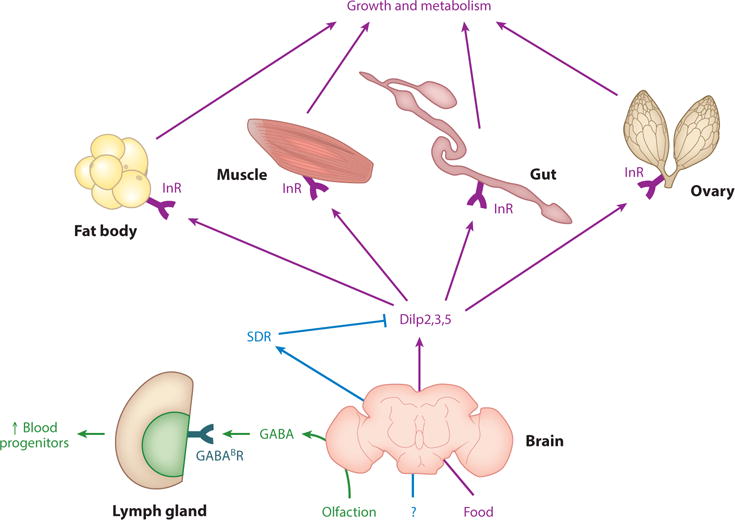
Drosophila central nervous system–derived systemic factors include the Dilps (Drosophila insulin-like peptides), SDR [secreted decoy of insulin receptor (InR)], and GABA (γ-aminobutyric acid). SDR binds to and negatively regulates Dilps. Dilps are secreted from insulin-producing cells (IPCs) and act through InR found in most tissues to regulate growth and metabolism. Note that not all organs on which Dilps act are shown. GABA is produced by the brain to preserve hematopoietic progenitor cells. Data from References 15, 62, 75, 102, 104, 127, 135, 145, and 152.
Figure 9.
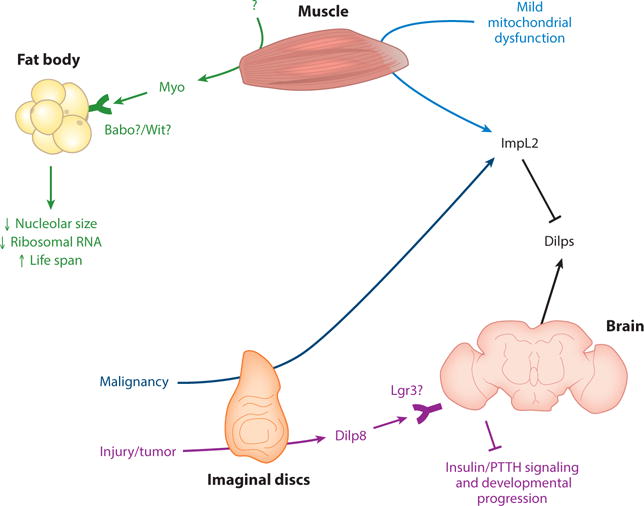
Muscle-derived Myo (myoglianin) may act through Babo or Wit receptors to decrease fat body nucleolar size and ribosomal RNA and increase life span. ImpL2 is secreted from muscle upon mild mitochondrial dysfunction. Injured imaginal discs secrete Dilp8 (Drosophila insulin-like peptide 8), which acts on Lgr3 (leucine rich repeat–containing G-protein–coupled receptor 3)-positive neurons to inhibit insulin and prothoracicotropic hormone (PTTH) signaling and developmental progression. Also, ImpL2 is secreted by malignant scrib and scrib RasV12 tumor-like larval imaginal discs transplanted to adults. Data from References 26, 27, 32, 41, 48, 49, 108, and 147.
Drosophila Brain-Derived Systemic Factors
Drosophila brain-derived systemic factors include insulin-like peptides, secreted decoy of InR, and γ-aminobutyric acid (Figure 5).
Drosophila insulin-like peptides 2, 3, and 5
Drosophila insulin-like peptides (Dilps) and mammalian insulin and insulin-like growth factors (IGFs) are homologous (15, 102, 152). In mammals, insulin is produced by β-cells, whereas in Drosophila, Dilps 2, 3, and 5 are secreted from median neurosecretory cells [insulin-producing cells (IPCs)] of the brain (15, 62, 102, 127, 152). Insulin and Dilps act through the highly homologous insulin receptor (InR) on all organs to increase growth, nutrient storage, and anabolism (15, 62, 102, 127, 152). In adults, Dilps also regulate germline stem cells (GSCs) (75) and aging (145). It will be important to discover the functional differences between Drosophila Dilps (102).
Secreted decoy of InR
Secreted decoy of InR (SDR) is an extracellular Dilp binding protein that is orthologous to InR. In Drosophila larva, SDR is secreted from glial cells on the surface of the brain. SDR binds to and inhibits Dilp function, negatively regulates growth, and is necessary for starvation resistance (104). It is not clear what regulates SDR secretion (104). In mammals, soluble decoy versions of transmembrane receptors have been identified (91); soluble InR can be generated by transcript termination before the transmembrane domain (149).
γ-Aminobutyric acid
Larval olfaction induces secretion of the neurotransmitter γ-aminobutyric acid (GABA) into the hemolymph (insect functional equivalent of blood) (4, 109) from brain neurons (135). GABA targets the GABAB metabotropic receptor on the hematopoietic lymph gland, induces intracellular calcium signaling, and preserves hematopoietic progenitor cells, suggesting that environmental sensing can be translated into a change in physiology of an organism. It raises the questions of whether similar mechanisms operate in mammals and whether systemic brain-derived GABA has additional roles (135).
Drosophila Fat Body–Derived Systemic Factors
Drosophila FB-derived systemic factors include unpaired-2, eiger, growth-blocking peptides, CCHamide-2, dawdle, ImpL2, peptidoglycan recognition proteins, and Dilp6 (Figure 6).
Figure 6.
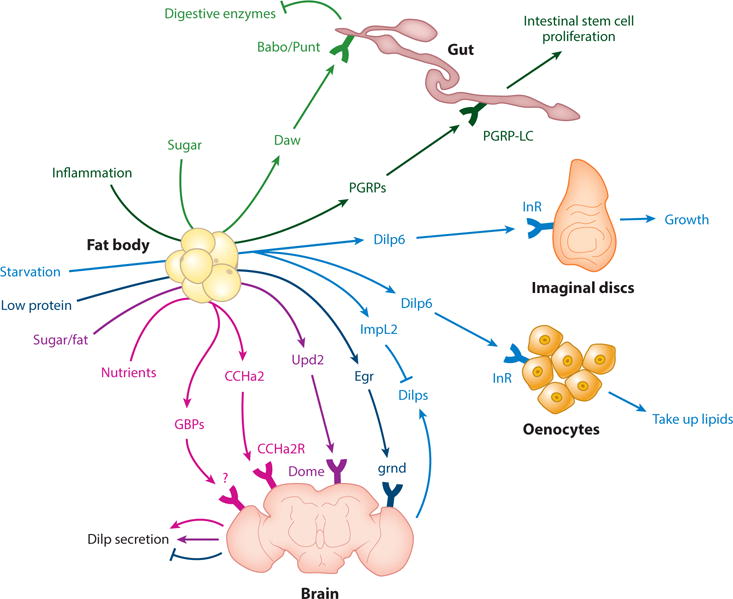
Drosophila fat body (FB)-derived systemic factors include the transforming growth factor (TGF)-related Daw (dawdle), PGRPs (peptidoglycan recognition proteins), Dilp6 (Drosophila insulin-like peptide 6), ImpL2, GBPs (growth-blocking peptides), CCHa2 (CCHamide2), Egr (Eiger), and leptin-like Upd2 (unpaired-2). Daw acts through the receptor Babo/Punt to regulate metabolism, gut digestive enzymes, pH equilibrium, and Dilp (Drosophila insulin-like peptide) secretion (not shown as it may be indirect). It is not clear whether Daw is a systemic factor, as it is found in multiple organs. PGRPs are secreted by age-inflamed FBs and act directly or indirectly to regulate gut inflammation and stem cell proliferation. Upd2 regulates Dilp secretion through the receptor dome (domeless). Egr negatively regulates Dilp secretion through the receptor Grnd (Grindelwald). GBP1/2 induces Dilp secretion through unknown mechanisms. CCHa2 induces Dilp secretion through the CCHa2 receptor. CCHa2 may also be produced by the gut (not shown). Dilp6 acts through insulin receptor (InR) on oenocytes and imaginal discs to regulate lipid uptake and growth, respectively. Dilp6 may also regulate IPC Dilp secretion (not shown). Also, ImpL2 is secreted from larval FBs upon starvation to inhibit extracellular Dilp. Data from References 1, 6, 7, 21–23, 50, 58, 72, 105, 118, 122, 129, and 139.
Unpaired-2
High fat and sugar diets induce secretion of unpaired-2 (Upd2) from FBs. Upd2 is a JAK/STAT cytokine and sensed by its receptor domeless (dome) on neurons adjacent to the IPCs to positively regulate Dilp secretion (Figure 6). Dilp secretion in turn promotes growth in larvae and nutrient storage in larvae and adults. Interestingly, Upd2 is the structural and functional ortholog of mammalian leptin (118). A number of questions remain. First, what is the mechanism of Upd2 secretion from the FB in response to diet, given that Upd2 has no signal peptide (118)? Second, is Upd2 a systemically secreted factor: Is it present in the hemolymph (blood)? How does Upd2 cross the blood-brain barrier (BBB)? Also, in mammals, leptin may be sensed by adipose tissue neurons (100); does Drosophila have neurons innervating the FB, and can it sense Upd2? Neuronal mechanisms of Upd2 sensing remain to be clarified (118). Finally, FB amino acids regulate Dilp secretion (28) independent of Upd2 (118), suggesting that additional FB-derived signals exist.
Eiger
Eiger (Egr) is a tumor necrosis factor (TNF)-family cytokine expressed as a transmembrane protein. In larvae, low protein diet induces Egr secretion from FBs into the hemolymph through inhibition of TOR (target of rapamycin) and induction of TNFα-converting enzyme (TACE), which cleaves Egr from the membrane (1). Egr binds to IPCs through the Grindelwald (Grnd) receptor, which acts through the Jun N-terminal kinase (JNK) pathway to inhibit Dilp2/5 mRNA expression and larval growth (1). In addition, under high sugar diet, Grnd signaling in FBs induces insulin resistance. In adult flies, Egr induces TAG storage under starvation; however, it remains to be seen whether this occurs through Dilp secretion. Intriguingly, treatment of mammalian islets with TNFα reduces insulin mRNA expression (1). Issues for future study include mechanisms of Egr crossing of BBB, Egr-interacting proteins in the hemolymph, and mechanisms of TACE induction downstream of TOR (1). In addition, mammalian TNFα forms a similar protein structure to adiponectin (134), which raises the question of whether fly FB-derived Egr is an adiponectin ortholog. This question can be addressed using rescue of egr mutants by expressing human adiponectin, as done previously for Upd2-leptin (118).
Growth-blocking peptides
Growth-blocking peptides (GBPs) are epidermal growth factor (EGF)-like cytokines and immune regulators without known receptors. Dietary lipids, proteins, and sugars induce larval FB GBP1/2, which act on IPCs to induce Dilp secretion and growth (72, 146). Future studies may determine whether GBPs act directly on the brain, their neural circuitry and receptors, or the mechanism of BBB crossing, and whether similar mechanisms exist in adult flies and mammals (72, 146).
CCHamide-2
CCHamide-2 (CCHa2) is a neuropeptide acting on G-protein–coupled CCHa2 receptor (122). Proteins and sugars induce larval FB CCHa2, which acts on IPCs to induce Dilp secretion and growth (129). In adults and larvae, CCHa2 regulates feeding (122). However, it is unclear whether CCHa2 is produced by the gut, brain, or FB (122). Future studies may determine the mechanism of BBB crossing, whether CCHa2 is present in the hemolymph, whether lipids induce CCHa2, and whether similar mechanisms exist in adult flies and mammals (129). Also, what is the relationship between Upd2, GBPs, and CCHa2, given their induction by similar nutrients (72, 118, 129)?
Dawdle
In adults, the TGF-β/activin-like ligand dawdle (Daw) is induced primarily in FBs by dietary sugar. Directly or indirectly, Daw acts through Baboon (Babo)C/Punt receptors on intestinal enterocytes to phosphorylate Smad-2, which then represses the transcription of digestive enzymes. Thus, adult FB-derived Daw acts as a negative feedback to repress digestion (23). Also, Daw from adult muscle can negatively regulate muscle protein homeostasis and directly or indirectly positively regulate Dilp secretion (6). Finally, larval Daw from several organs positively regulates insulin secretion and affects systemic metabolism, including triacylglycerol, glucose, and glycogen storage, mitochondrial genes, and hemolymph pH (50). Together, these studies suggest that Daw is an important regulator of metabolism (6, 23, 50). Future studies may address whether Daw acts as a local or a systemic factor, as it is found in many organs (50). Studies of Daw in the hemolymph may address this question. Mammalian activin signaling also regulates mitochondrial activity and organ growth (82), suggesting a number of further studies.
ImpL2
ImpL2 is an immunoglobulin-superfamily member similar to mammalian IGFBP7 (58). It binds to Dilps extracellularly and inhibits insulin signaling. Starvation induces expression of Drosophila larval FB ImpL2, thereby increasing survival (58).
Peptidoglycan recognition proteins
Peptidoglycan recognition proteins (PGRPs) are immune factors that act through PGRP-LC receptor and the immune deficiency (IMD) pathway (22). In adults, old age-associated FB inflammation causes secretion of PGRPs, which act on the gut to alter inflammation and increase intestinal stem cell proliferation. It is not clear whether PGRPs act directly or indirectly on the gut (e.g., by acting on immune cells) (22). PGRPs are conserved (38), raising the question of whether similar mechanisms operate in mammals.
Dilp6
In larvae, the IGF-like factor Dilp6 is induced in the FB upon cessation of feeding—before/during pupariation or starvation. The induction is dependent on the FOXO transcription factor and the metamorphosis steroid hormone ecdysone (153). Dilp6 promotes growth of imaginal discs that give rise to adult organs. As this happens in the absence of other Dilps and food intake, Dilp6 acts as a switch between energy storage and growth of organs (105, 139). In adults, FB-derived Dilp6 acts through the InR to regulate turnover of lipids from FBs to oenocytes and oenocyte lipid uptake. This is important for starvation resistance (21). In addition, Dilp6 may directly or indirectly regulate brain Dilp secretion (7). Dilp6 has similarities to mammalian IGFs (139).
Drosophila Gut-Derived Systemic Factors
Drosophila gut-derived systemic factors include hedgehog and ImpL2 (Figure 7).
Figure 7.
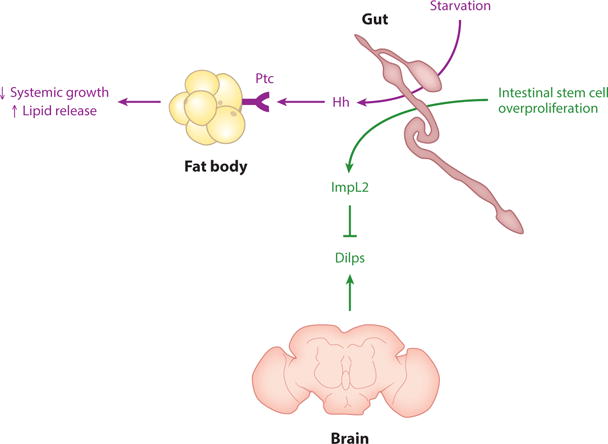
The Drosophila gut-derived factors are the insulin growth factor binding protein (IGFBP)-like factor ImpL2 and Hh (hedgehog). ImpL2 is secreted from the gut upon intestinal stem cell overproliferation and binds to and inhibits Dilps (Drosophila insulin-like peptides). Hh binds to Ptc (patched) on fat bodies and the prothoracic gland (not shown) to regulate growth and lipid mobilization. CCHa2 (CCHamide2) may also be produced by the gut (not shown). Data from References 74, 122, 125.
Hedgehog
Hedgehog (Hh) was previously known as a paracrine developmental regulator. In Drosophila larvae, starvation induces gut Hh, which is secreted into the hemolymph bound to lipoproteins. Hh targets the FB through the Patched (Ptc) receptor to negatively regulate systemic growth and positively regulate lipid release due to starvation, thus affecting larval sensitivity to starvation. Moreover, Hh integrates growth and metamorphosis by acting on the prothoracic gland to reduce ecdysone production, thus negatively regulating pupariation (125). The gut has been proposed to be the major source of Hh acting on FBs because FBs have little to no Hh mRNA and reduction of Hh in the gut results in reduction of Hh protein in FBs (125). However, it is possible that neurons that innervate the FB could be a source of Hh, as neurons and enterocytes can both produce Hh (56) and knockdown of Hh in neurons results in at least a twofold reduction in hemolymph Hh (125). It would be interesting to address whether mammalian sonic hedgehog (Shh) and other Hh proteins have similar roles, as Shh is detected in the blood (110, 125).
ImpL2
In adults, Hippo pathway–induced intestinal stem cell (ISC) hyperproliferation causes production of Dilp-inhibitor ImpL2 from the gut. This results in decreased Dilp signaling; nutrient uptake from hemolymph; nutrient storage; and ovary, muscle, and FB atrophy. In the FB, energy stores are depleted, and hemolymph sugar levels rise (74). Interestingly, in the gut hyperplasia itself, insulin signaling and nutrient consumption increase through upregulation of insulin and glycolysis pathway components, including Dilp3 (74), which may bind poorly to ImpL2 (104). It would be interesting to determine whether related mechanisms occur during physiological processes involving cell proliferation and mammalian cancer cachexia (74).
Drosophila Corpora Cardiaca– and Corpora Allata–Derived Systemic Factors
The following three factors are produced by the corpora cardiaca (CC) and corpora allata (CA) endocrine gland complex, which is located close to the brain, esophagus, and proventriculus in adults, and brain in larvae (29, 43) (Figure 8).
Figure 8.
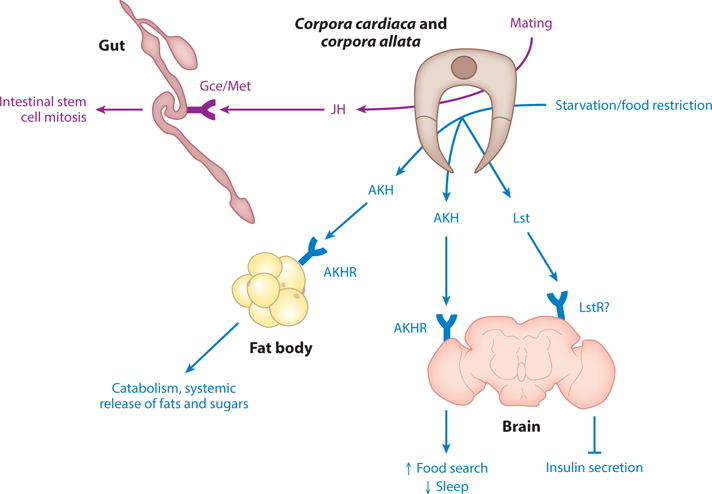
Drosophila corpora cardiaca (CC)- and corpora allata (CA)-derived factors are glucagon-like AKH (adipokinetic hormone), JH (juvenile hormone), and NMU (neuromedin U)-like Lst (limostatin). AKH is secreted by CC cells and acts through the AKHR (AKH receptor) found in many tissues to regulate growth and metabolism. Lst is secreted from CC cells and acts through its putative receptor LstR on insulin-producing cells to decrease Dilp (Drosophila insulin-like peptide) secretion. JH is secreted from CA cells and acts through the receptor Met found in several organs to regulate growth and metabolism. Note that CC/CA cells are found close to the esophagus, proventriculus, and brain in adults and brain in larvae. Although JH and AKH have other target organs, selected organs and functions discussed in the text are shown for simplicity. Data from References 3, 29, 43, 47, 51, 63, 66, 69, 78, 96, 98, 121, and 150.
Adipokinetic hormone
Adipokinetic hormone (AKH) is an endocrine peptide produced by CC cells that acts on the AKH receptor (AKHR) in multiple tissues in both larvae and adults. AKH regulates metabolism in an opposing way to Dilps by acting to promote catabolism and systemic release of fats and sugars from FBs (47, 51, 69, 78). In addition, AKH signaling regulates life span, food search behavior, sleep, and resistance to oxidative stress (47, 66, 78, 96, 150). Future studies may identify systemic factors that regulate AKH signaling. Interestingly, AKH and CC cells have been likened to the mammalian glucagon and α-cell systems, respectively (47, 69).
Limostatin
Limostatin (Lst) is a novel protein secreted by CC cells in response to food restriction in adult Drosophila. Lst acts as a decretin, as it decreases insulin secretion by acting through the G-protein–coupled CG9918/LstR receptor on IPCs (3). However, binding experiments using purified Lst and CG9918 proteins will need to be performed to demonstrate that CG9918 is the bona fide LstR. Also, whether Lst is present in fly hemolymph is not known. Moreover, future studies may address whether Lst acts on CC cells themselves and on the FB. Interestingly, mammalian neuromedin U (NMU) and NMU receptors are orthologous to fly Lst signaling (3). However, the mechanisms of NMU action remain to be investigated in detail.
Juvenile hormone
Juvenile hormone (JH) is a sesquiterpenoid molecule secreted by CA cells that regulates larval growth and development and adult reproduction through its Gce/Met receptor (43, 63). Recently, additional functions of JH emerged, including regulation of insulin signaling in larvae and intestinal stem cell proliferation in adults (98, 121).
Drosophila Muscle-Derived Systemic Factors
Drosophila muscle-derived systemic factors include myoglianin and ImpL2 (Figure 9).
Myoglianin
In addition to Daw (described above) (50), myoglianin (Myo), a related TGF-family factor, performs a distinct role in regulating physiology. Adult muscle-derived Myo decreases FB nucleolar size and ribosomal RNA, and increases life span (32). The detection of Myo in fly hemolymph would strengthen the conclusion that Myo acts systemically. Although the receptor in this context is unclear, previous work showed that Myo signals through wishful-thinking (Wit) and Babo coreceptors (77). Moreover, the physiological inducer of Myo secretion from muscle is uncharacterized. Also, what is the relationship of Myo to other TGF-β family members also involved in physiology, including Daw as described above (50)?
ImpL2
Moderate mitochondrial perturbation in Drosophila adult muscle results in secretion of ImpL2, which inhibits whole-body insulin signaling and increases life span (108). Overall, Drosophila systemic insulin signaling is regulated by extracellular binding factors from the periphery, including SDR (104) and ImpL2 (74, 108). What is the relationship between these, and does secretion of ImpL2 and SDR from different organs have diverse effects on physiology? Interestingly, SDR and ImpL2 bind to different Dilps in vitro: Dilp3 binds well to SDR but poorly to ImpL2 (104).
Drosophila Imaginal Disc–Derived Systemic Factors
Drosophila imaginal disc–derived systemic factors include Dilp8, ImpL2, and possibly Hh (Figure 9).
Dilp8
Dilp8 is a relaxin/insulin/IGF-like peptide. In larvae, changes in imaginal disc growth caused by injury or overproliferation induce secretion of Dilp8 from imaginal discs. Dilp8 delays entry to pupariation and growth of other imaginal discs, thereby acting as a signal for developmental consistency (27, 48). Dilp8 may directly or indirectly bind to leucine rich repeat–containing G-protein–coupled receptor 3 (Lgr3) in four neurons in the brain pars intercerebralis. These neurons delay growth and pupariation by inhibiting prothoracicotripic hormone (PTTH; 153) neurons and thus JH production, as well as Dilp3 and 5 secretion from IPCs (26, 49, 147). Issues for future studies include detection of endogenous Dilp8 in the hemolymph, Dilp8 functions in adult animals, and Dilp8 crossing of the BBB as well as whether Lgr3 is the bona fide Dilp8 receptor (49, 147). Of note, it is not clear whether Lgr3 is the bona fide Dilp8 receptor (49); in vitro binding assays using purified Lgr3 and Dilp8 proteins need to be done. Dilp8 is related to mammalian relaxin, insulin, and IGF, whereas Lgr3 is a relaxin-type receptor. This raises the possibility that a similar mechanism occurs in mammals (26, 27, 48, 49, 147).
ImpL2
Tumor-like malignant scrib and scrib RasV12 larval imaginal discs transplanted to adults secrete ImpL2, which inhibits systemic Dilps (41). This results in ovary, muscle, and FB atrophy in a phenotype similar to that resulting from ImpL2 secretion from overproliferating ISCs described above (41, 74).
Hedgehog
Larval imaginal discs have been suggested to secrete Hh into the fly hemolymph (92). It remains to be investigated whether imaginal disc–derived Hh has a physiological function on distal organs.
ROBUST READOUTS FOR FUNDAMENTAL ORGAN FUNCTIONS ARE ESSENTIAL FOR SYSTEMATIC DISCOVERY OF NOVEL FACTORS
Systematic genetic screens aimed at discovering interorgan communication factors have not been done in any organism. Therefore, we expect that many molecules involved in organ communication under physiological and pathological conditions remain to be identified. Indeed, a number of connections between organs remain uncharacterized. These include muscle to GSCs, gut to GSCs, FBs to GSCs, digestive tract to muscle, brain to adipose tissue (non-neural), and others. For instance, in humans, adult-onset muscle dystrophies (111) and poor nutrition (45) are associated with poor fertility. Moreover, Drosophila FBs regulate GSCs in response to nutrients through unknown signals (5). Importantly, approximately half of fly hemolymph proteins and 30–40% of fly-secreted proteins have unknown or poorly characterized functions, and most have no known functions in interorgan communication (I.A. Droujinine, Y. Hu, J.M. Asara, N. Perrimon, unpublished results). We expect that a fraction of these proteins have a signaling role in organ communication.
The factor and its source and destination can be determined from a perturbation or at homeostasis by bioinformatics, biochemistry, RNA sequencing, organ transplants, parabiosis (25), or genetic screens. In an organ-sensing screen, secreted factors are mutated in one organ, and an organ function is measured in another (Figure 10) (37). Drosophila organ-specific perturbations can be achieved using the Gal4/UAS system driving the expression of a secreted factor cDNA, RNAi, or Cas9/CRISPR (86, 103, 114). Cas9 proteins with dead nuclease activity (dCas9) fused to either transcriptional activators (dCas9-a) or repressors (dCas9-i) can be used for gene activation and repression in cells expressing the sgRNAs (86). For a successful organ-sensing screen (37), a critical parameter is a robust distal organ function readout.
Figure 10.
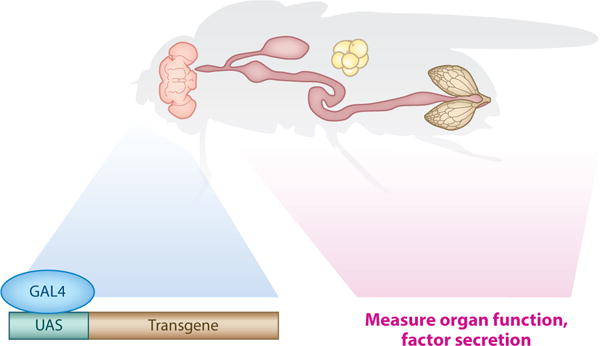
Design of an organ-sensing screen in Drosophila. The Gal4-UAS system drives the tissue-specific expression of a transgene (RNA interference), cDNA, or Cas9, and the function of a distal organ is measured. Alternatively, secretion of a key systemic factor can be measured to address questions about the integration of pathways in interorgan communication.
There are a number of basic organ functions that have been measured in the organ communication literature (Table 3 and references therein). For instance, fatty acid (FA) and sugar metabolism in FBs, Dilps retention in IPCs, and systemic growth have been popular readouts, given their simplicity, widespread reagent availability, and physiological relevance (e.g., see 118). However, a significant number of organ functions have been largely overlooked in Drosophila (Table 3). These include heat generation (30) (see Evolutionary Considerations below) and detoxification by FBs; behavioral control by the central nervous system (CNS); cell division, growth, differentiation, chromosomal stability, and cell migration associated with tumors and wounds; and waste production by the digestive system. In addition, a number of organs have not been extensively studied, including the reproductive system, oenocytes, Malpighian tubules, trachea, and the heart. Thus, it is important to develop assays for these key tissues, as they may reveal novel biological pathways and/or be more sensitive readouts than are the current ones. In conclusion, systematic screens that measure robust organ function readouts in response to secreted factor perturbation identify novel interorgan communication pathways.
Table 3.
Selected Drosophila organ functions in interorgan communication
| Drosophila organ | Mammalian functional equivalent | Possible functions in interorgan communication | What has been measured in Drosophila? | Selected references |
|---|---|---|---|---|
| Insulin-producing cells | Pancreatic β-cells | Insulin storage and secretion; nutrient sensing; secreted factor sensing; electrical activity; synapses | Insulin storage and secretion; nutrient sensing; secreted factor sensing; synapses | 3, 15, 26, 27, 33, 48, 49, 62, 72, 75, 102, 118, 127, 129, 145, 147, 152 |
| Corpora cardiaca and corpora allata | Pancreatic α-cells and other specialized endocrine glands? | AKH/glucagon and other factor storage and secretion; nutrient sensing; secreted factor sensing | AKH/glucagon and Lst storage and secretion | 3, 43, 47, 51, 63, 66, 69, 78, 96, 98,121,150 |
| Muscle | Muscle | Contraction; glycogen storage; protein homeostasis; glucose, and FA turnover; heat generation; mitochondria; interaction with immune system; secreted factor production; secreted factor sensing | Contraction; protein homeostasis; glucose and FA turnover; mitochondria; secreted factor production (e.g., Myo, ImpL2) | 4, 30, 32, 33, 66, 81, 108, 109, 156 |
| Fat body | Adipose tissue and liver | Nutrient sensing; glycogen, glucose and FA synthesis, storage and release; heat generation; detoxification; mitochondrial biogenesis and function; protein homeostasis; interaction with immune system; secreted factor production; secreted factor sensing | Nutrient sensing; glucose and FA synthesis, storage and release; mitochondria; protein homeostasis; interaction with the immune system; secreted factor production (Upd2, Daw, PGRPs, Dilp6, ImpL2, Egr, GBPs, CCHa2); secreted factor sensing (Myo, Dilps, AKH) | 4, 6, 7, 17, 21–23, 30, 32, 33, 35, 47, 50, 51, 58, 66, 69, 72, 78, 81, 105, 109, 118, 129, 139 |
| Oenocyte | Liver | Nutrient sensing; sugar and FA homeostasis; detoxification; cuticular hydrocarbon production; secreted factor production; secreted factor sensing | Nutrient sensing; sugar and FA homeostasis; secreted factor sensing (Dilp6) | 10, 21, 54, 81, 109, 118 |
| Digestive system | Digestive system | Digestion; nutrient uptake; nutrient sensing; glucose, FA, and protein metabolism; detoxification/waste excretion; regeneration and stem cell proliferation; host-microbiome interactions; interaction with immune system; secreted factor production; secreted factor sensing | Digestion; nutrient uptake; nutrient sensing; glucose and FA metabolism; stem cell proliferation; interaction with the immune system; secreted factor production (ImpL2, Hh); secreted factor sensing (Daw, PGRPs) | 4, 17, 23, 35, 74, 81, 109, 125, 135, 141 |
| Reproductive system | Reproductive system | Cell division, growth, and migration; germline stem cell proliferation and differentiation; meiosis; epigenetic inheritance; glucose, FA, and protein metabolism; mitochondria; secreted factor production; secreted factor sensing | Germline stem cell proliferation; secreted factor sensing (insulin) | 5, 75, 155 |
| Central nervous system | Central nervous system | Outside environment sensing; interorganism communication; nutrient homeostasis; behavioral control; electrical activity; synapses; neural regulation of organs and immunity; central regulation of glycogen, glucose, FA, and protein homeostasis, mitochondria; secreted factor (including classical hormone) production; secreted factor sensing | Outside environment sensing; nutrient homeostasis; electrical activity; synapses; central regulation of glucose and FA homeostasis; secreted factor production (insulin, SDR, GABA); secreted factor sensing (Upd2, Dilp8, GBPs, Egr, CCHa2) | 15, 17, 62, 72, 73, 75, 102, 104, 118, 127, 129, 135, 145, 152 |
| Malpighian tubule | Kidney | Water balance; waste removal; glucose, FA, and protein metabolism; regeneration; interaction with immune system; secreted factor production; secreted factor sensing | Has not been extensively investigated in interorgan communication | 9, 17, 137 |
| Heart | Heart | Contraction; blood propulsion; glucose, FA, and protein metabolism; mitochondria; secreted factor production; secreted factor sensing | Contraction, lipids (unknown secreted factors) | 11, 35 |
| Trachea | Air tract, lungs, trachea, blood vessels for oxygen delivery | Exchange of oxygen, carbon dioxide, and other gases and volatiles between the environment and the body; buoyancy; interaction with immune system; secreted factor production; secreted factor sensing | Has not been extensively investigated in interorgan communication | 4, 17, 109 |
| Tumor and wounds | Tumor and wounds | Cell division, growth, differentiation, and migration; glucose, FA, and protein metabolism; nutrient sensing; mitochondria; interaction with immune system; chromosomal stability; secreted factor production; secreted factor sensing | Glucose and FA metabolism; secreted factor production (ImpL2, Dilp8) | 26, 27, 41, 48, 49, 74, 147 |
Abbreviations: AKH, adipokinetic hormone; Daw, dawdle; Dilp, Drosophila insulin-like peptide; EGF, epidermal growth factor; Egr, eiger; FA, fatty acid; GBPs, growth-blocking peptides; Hh, hedgehog; Lst, limostatin; Myo, myoglianin; PGRPs, peptidoglycan recognition proteins; SDR, secreted decoy of InR.
SYSTEMS BIOLOGY OF INTERORGAN COMMUNICATION: COORDINATED REGULATION OF SYSTEMIC FACTORS AND PHYSIOLOGY
In the above sections, we have reviewed the Drosophila interorgan communication factors, their physiological inducers, and their outputs. After elucidating the pathways involved in interorgan communication, the challenge is to determine how these pathways integrate with each other in coordinating body function, integration meaning two or more pathways interacting to regulate systemic physiology. For example, dietary restriction pathways interact with FA turnover in lifespan regulation through unclear systemic factors (66). Also, high-fat-diet–induced heart toxicity is rescued by inhibition of TOR and promotion of lipid breakdown in FBs through unknown secreted factors (11). These systems biology interorgan communication questions are best addressed in Drosophila, given its powerful genetic tools.
Most, if not all, integration studies so far have been centered on the regulation of Dilp (insulin) signaling by secreted factors (Figure 11). Dietary nutrients induce FB secretion of Upd2, GBPs, and CCHa2, which positively regulate IPC Dilp secretion to promote nutrient storage and growth (72, 118, 129). The opposing physiological condition of starvation induces Egr secretion from FBs and Lst secretion from corpora cardiaca to inhibit Dilp secretion from IPCs, thus reducing nutrient storage and promoting breakdown (1, 3). In addition, developing organ (imaginal disc) growth perturbation in the form of an injury or tumor causes secretion of Dilp8 to negatively regulate IPC Dilp secretion and slowing down the growth of the organism (26, 27, 48, 49, 147). Also, imaginal disc malignancy, ISC overproliferation, mild muscle mitochondrial dysfunction, and FB starvation induce secretion of ImpL2, which inhibits Dilps extracellularly (41, 58, 74, 108). Induction of ImpL2 in these contexts makes sense because tumors are quickly dividing and growing tissues that require increased nutrient release from storage organs like FBs and decreased nutrient uptake to other organs (41, 74). Reduced insulin signaling promotes mitophagy of defective mitochondria (108), and extracellular inhibition of Dilps provides another level of regulation of insulin signaling during starvation (58). Together, these studies illustrate how various secreted systemic factors integrate information about overall organism status from multiple organs and translate this information to a key interorgan communication factor (in this situation, insulin).
Figure 11.
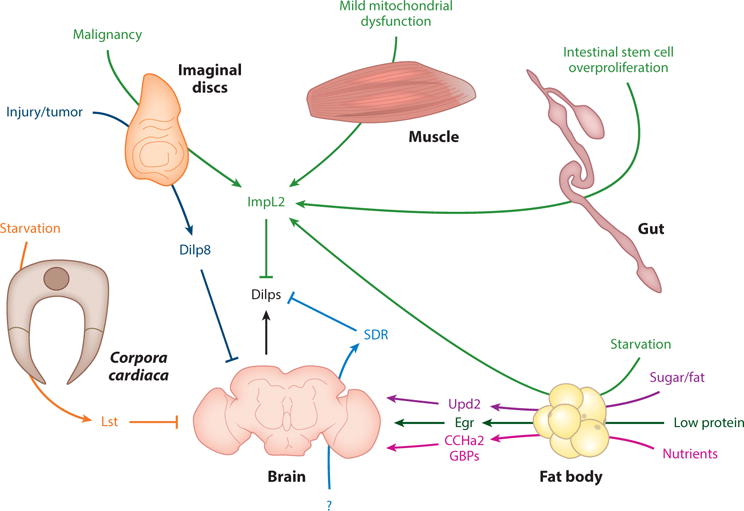
Various interorgan communication factors are integrated to regulate insulin signaling (1, 3, 26, 27, 41, 48, 49, 58, 72, 74, 108, 118, 129, 147). Abbreviations: CCHa2, CCHamide2; Dilp, Drosophila insulin-like peptide; Egr, Eiger; GBP, growth-blocking peptide; Lst, limostatin; SDR, secreted decoy of insulin receptor; Upd2, unpaired-2.
EVOLUTIONARY CONSIDERATIONS
Similarities and Differences Between Flies and Humans
Drosophila is a model system for interorgan communication between organs conserved to humans. Fly FBs and oenocytes (adipose tissue and liver in humans), brain, muscle, digestive system, gonads, and heart have human equivalents (Figure 3) (4, 35, 81, 109). A number of interorgan communication factors identified in mammals are conserved to Drosophila, including leptin (fly Upd2) (118) and GDF-11 (fly myoglianin) (32) (Table 2). However, flies have no bone, blood vessels, adaptive immunity, or potent central thermoregulation (4, 17, 35, 81, 109). Interestingly, Drosophila larvae keep their body temperature 0.5°C above that of the medium, suggesting limited thermoregulatory capacity (30).
Interorgan Communication Enabled and Evolved as a Result of Specialization of Organs
The origin of multicellularity has been extensively looked at, whereas evolution and maintenance of multiorgan organisms have been largely overlooked (20, 52, 71). Multicellular organisms are stabilized by the large organism size, efficiency of cell specialization, and codependency of specialized/differentiated cells on each other (52, 83, 99). Stable organisms may lead to increasingly differentiated organs (83). As organs became increasingly differentiated, interorgan communication may have evolved for organs to regulate and coordinate each other’s functions (37). Interorgan communication ensures that differentiated cell/organ functions are carried out faithfully and that cells remain differentiated, specialized, and dependent on the rest of the organism. For example, IPCs are dependent on other organs for nutrients, and other organs are dependent on insulin (102). In turn, interorgan communication leads to increasingly differentiated organs, which stabilize organisms (Figure 12). For instance, in the absence of Drosophila larval Dilp8, developmental robustness and synchrony are affected (26, 27, 48, 49, 147). Thus, interorgan communication coevolved with multicellularity and is necessary for the evolution of organs.
Figure 12.

Interorgan communication enabled and evolved as a result of specialization of organs (20, 37, 52, 71, 83, 99). Large organism size, overall efficiency of organ specialization, and codependency of specialized/differentiated organs stabilize organisms. This leads to increasingly differentiated organs, which results in interorgan communication. Interorgan communication (e.g., through insulin) enables regulation and coordination of organ functions and ensures organ dependency on the rest of the organism. In turn, interorgan communication leads to increasingly differentiated organs, which stabilize organisms.
Potential Properties of Evolved Interorgan Communication Networks
To faithfully execute each organ’s function, the action of local signaling networks needs to be limited. For instance, to prevent tumorigenesis, proliferation must be limited to the stem cell niche (131). As hypothesized from network theory (16, 99), in organisms there is a compromise between the number of cell types and connectivity: If there are too many different directly communicating cells, the system becomes unstable. Organs allow the number of cell types that directly interact with each other to be limited (through hierarchy). For example, regulated by other neurons, only IPCs in the Drosophila brain produce insulin (118). Spatiotemporal segregation of cells and reduction in number and distance of action of competitive and cooperative interactions may increase organism stability (52, 99). In addition, different organs’ functions or signaling networks do not interfere with each other. Thus, long-distance communication may connect organs through a limited number of links.
Reorganization of local secreted factor networks (71, 85, 123) may have led to long-distance communication. Developmental pathways, including JAK/STAT (Upd2) (118) and TGFβ (Myo, Daw) (23, 32, 50), have physiological functions in interorgan communication. This pleiotropy of pathways may stabilize complex organisms and allow fewer links or system components (99). For example, Dictyostelium discoideum dimA has dual functions in responding to secreted DIF-1 and cell fitness (44). Long-distance signals act on key nodes between organs, affecting organ-specific networks. These nodes can be niche cells, such as neurons that are adjacent to and regulate Drosophila IPCs in response to Upd2 (118). Alternatively, because developmental ligands are potent regulators of cell fates, such as proliferation, a systemically released binding factor can regulate niche signaling extracellularly (e.g., ImpL2) (37, 58). Hence, interorgan signaling coordinates and limits the action of organ-specific networks. In addition, interorgan communication is a distinguishing feature and necessity of complex organisms.
PERSPECTIVES
After elucidating the pathways involved in interorgan communication, the challenge will be to determine how these pathways integrate with each other in coordinating body function. Given the powerful genetic tools available in Drosophila, these questions in systems biology of interorgan communication are best addressed in the fly. In the studies focused on the regulation of insulin signaling by various systemic factors, readouts such as body size, lipid and sugar metabolism, Dilp retention in IPCs, and Dilp secretion have been used (3, 74, 108, 118). From these studies, it is apparent that robust, reliable readouts are essential for understanding the integration of pathways regulating systemic physiology.
An informative and robust readout of pathway integration is the induction or repression of known key secreted physiological regulators, such as AKH, Myo, ImpL2, Upd2, and others (Table 2; Figure 10). Studies may help to elucidate how a change in the physiological state of one organ causes a change in production of a secreted factor. That is, does the secretion of a physiological regulator depend on an unknown systemic signal from another organ? However, many of the described interorgan communication factors have not been detected in the fly hemolymph at endogenous levels. Therefore, it is essential to develop reliable assays for such factors before major screens are undertaken. Moreover, does one systemic factor act through another systemic factor to regulate physiology? For this question, mutants can be made in two or more tissues using Gal4, Q, and LexA systems (14, 76, 115). In conclusion, after identifying the basic components of interorgan communication, studies will be required to investigate how these parts are integrated into the broader interorgan communication network (ICN) in physiology and disease.
Interorgan communication allows and evolved as a result of specialization of organs. Studies in vertebrates and Drosophila have identified a number of organ communication factors. Future studies will focus on identifying novel conserved organ communication factors through systematic genetic screens with robust organ function readouts. In addition, it is important to identify how known organ communication pathways are integrated. Together, we anticipate that studies within the next decade will establish a framework ICN that will have implications for evolution, function, and dysfunction of multiorgan organisms.
SUMMARY POINTS.
A number of mammalian interorgan communication factors have been identified.
A number of conserved Drosophila interorgan communication factors have been found using organ-specific genetic perturbations. These include Dilp1–8, ImpL2, SDR, Upd2, Egr, Lst, AKH, Myo, Daw, Hh, CCHa2, GBP1/2, JH, and GABA.
Interorgan communication evolved as a result of, and enabled, specialization of organs.
FUTURE ISSUES.
A number of connections between organs remain uncharacterized.
Future studies will aim to identify conserved interorgan communication factors using organ-sensing genetic screens.
Organ-sensing screens require development of robust organ function readouts.
After identifying the key interorgan communication factors, studies will aim to determine how these pathways are integrated with each other into an ICN.
Acknowledgments
I.A.D. acknowledges support from an NSERC Postgraduate Fellowship, the Brigham and Women’s Hospital Osher Center for Integrative Medicine Pre-doctoral Fellowship, and the Harvard Medical School Cell Biology Department Innovative Grant Program. We thank our students from the Harvard Medical School Interorgan Communication Nanocourse for discussions. We thank Luye Mu for drawing organs and help with figure preparation, and Akhila Rajan, Ben Ewen-Campen, and Nirmalya Chatterjee for comments on the review. N.P. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- TGF
transforming growth factor
- FBs
fat bodies
- Dilps
Drosophila insulin-like peptides
- IGFs
insulin-like growth factors
- IPCs
insulin-producing cells
- GSCs
germline stem cells
- Hemolymph
insect equivalent of vertebrate blood; bathes organs and provides a medium for interorgan communication
- JAK/STAT
janus kinase/signal transducer and activator of transcription
- BBB
blood-brain barrier
- TNF
tumor necrosis factor
- JNK
Jun N-terminal kinase
- EGF
epidermal growth factor
- IGFBP
IGF binding protein
- Ecdysone
insect hormone produced by the prothoracic gland that induces molting and pupariation
- ISC
intestinal stem cell
- AKH
adipokinetic hormone
- Prothoracicotropic hormone (PTTH)
neuropeptide that induces ecdysone release
- Organ-sensing screen
experimental approach for identifying systemic factors through systematic genetic perturbation in one tissue and scoring the effect on another tissue
- FA
fatty acid
- CNS
central nervous system
- ICN
interorgan communication network
Footnotes
NOTE ADDED IN PROOF
During the proof stage of this review, a recent report by Pierre Leopold and colleagues (30a) added another component of the interorgan communication network regulating insulin secretion. Interestingly, a subunit of the mitochondrial ATPase Stunted (Sun) is secreted into Drosophila hemolymph in response to nutrient uptake and binds to its GPCR Methuselah (Mth) on IPCs to induce Dilp secretion (30a).
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Agrawal N, Delanoue R, Mauri A, Basco D, Pasco M, et al. The Drosophila TNF Eiger is an adipokine that acts on insulin-producing cells to mediate nutrient response. Cell Metab. 2016;23:675–84. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–52. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 3.Alfa RW, Park S, Skelly K-R, Poffenberger G, Jain N, et al. Suppression of insulin production and secretion by a decretin hormone. Cell Metab. 2015;21:323–33. doi: 10.1016/j.cmet.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen D, Colombani J, Leopold P. Coordination of organ growth: principles and outstanding questions from the world of insects. Trends Cell Biol. 2013;23:336–44. doi: 10.1016/j.tcb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong AR, Laws KM, Drummond-Barbosa D. Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila. Development. 2014;141:4479–88. doi: 10.1242/dev.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai H, Kang P, Hernandez AM, Tatar M. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLOS Genet. 2013;9:e1003941. doi: 10.1371/journal.pgen.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai H, Kang P, Tatar M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell. 2012;11:978–85. doi: 10.1111/acel.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–98. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 9.Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–74. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- 10.Billeter J-C, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–91. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 11.Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, et al. High-fat-diet–induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–44. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, et al. A PGC1-α–dependent myokine that drives brown-fat–like development of white fat and thermogenesis. Nature. 2012;481:463–68. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. doi: 10.1038/ncomms1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 15.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–21. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 16.Buchman TG. The community of the self. Nature. 2002;420:246–51. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 17.Buchon N, Silverman N, Cherry S. Immunity in Drosophila melanogaster: from microbial recognition to whole-organism physiology. Nat Rev Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–37. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17:768–78. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll SB. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001;409:1102–9. doi: 10.1038/35059227. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee D, Katewa SD, Qi Y, Jackson SA, Kapahi P, Jasper H. Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. PNAS. 2014;111:17959–64. doi: 10.1073/pnas.1409241111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Zheng X, Zheng Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell. 2014;159:829–43. doi: 10.1016/j.cell.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chng WB, Bou Sleiman MS, Schüpfer F, Lemaitre B. Transforming growth factor β/activin signaling functions as a sugar-sensing feedback loop to regulate digestive enzyme expression. Cell Rep. 2014;9:336–48. doi: 10.1016/j.celrep.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 24.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–33. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 25.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–99. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 26.Colombani J, Andersen DS, Boulan L, Boone E, Romero N, et al. Drosophila Lgr3 couples organ growth with maturation and ensures developmental stability. Curr Biol. 2015;25:2723–29. doi: 10.1016/j.cub.2015.09.020. Demonstrates that Lgr3 positive neurons are the site of Dilp8 action. [DOI] [PubMed] [Google Scholar]
- 27.Colombani J, Andersen DS, Léopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–85. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 28.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–49. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 29.Dai J-D, Gilbert LI. Metamorphosis of the corpus allatum and degeneration of the prothoracic glands during the larval-pupal-adult transformation of Drosophila melanogaster: a cytophysiological analysis of the ring gland. Dev Biol. 1991;144:309–26. doi: 10.1016/0012-1606(91)90424-2. [DOI] [PubMed] [Google Scholar]
- 30.Da-Ré C, De Pittà C, Zordan MA, Teza G, Nestola F, et al. UCP4C mediates uncoupled respiration in larvae of Drosophila melanogaster. EMBO Rep. 2014;15:586–91. doi: 10.1002/embr.201337972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Delanoue R, Meschi E, Agrawal N, Mauri A, Tsatskis Y, et al. Drosophila insulin release is triggered by adipose Stunted ligand to brain Methuselah receptor. Science. 2016;253:1553–56. doi: 10.1126/science.aaf8430. [DOI] [PubMed] [Google Scholar]
- 31.de Laval B, Pawlikowska P, Petit-Cocault L, Bilhou-Nabera C, Aubin-Houzelstein G, et al. Thrombopoietin-increased DNA-PK-dependent DNA repair limits hematopoietic stem and progenitor cell mutagenesis in response to DNA damage. Cell Stem Cell. 2013;12:37–48. doi: 10.1016/j.stem.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep. 2014;7:1481–94. doi: 10.1016/j.celrep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–25. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013;12:943–49. doi: 10.1111/acel.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diop SB, Bodmer R. Gaining insights into diabetic cardiomyopathy from Drosophila. Trends Endocrinol Metab. 2015;26:618–27. doi: 10.1016/j.tem.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donath MY, Burcelin R. GLP-1 effects on islets: hormonal, neuronal, or paracrine? Diabetes Care. 2013;36:S145–48. doi: 10.2337/dcS13-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Droujinine IA, Perrimon N. Defining the interorgan communication network: systemic coordination of organismal cellular processes under homeostasis and localized stress. Front Cell Infect Microbiol. 2013;3:82. doi: 10.3389/fcimb.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–74. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, et al. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteom. 2011;10:M110.006353. doi: 10.1074/mcp.M110.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueroa-Clarevega A, Bilder D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell. 2015;33:47–55. doi: 10.1016/j.devcel.2015.03.001. Shows the role of Dilp-binding protein ImpL2 in systemic physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2015;78:223–41. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 43.Flatt T, Tu M-P, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 44.Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CR. Pleiotropy as a mechanism to stabilize cooperation. Nature. 2004;431:693–96. doi: 10.1038/nature02894. Demonstrates the role of multifunctionality of signaling pathways in the evolution to multicellularity. [DOI] [PubMed] [Google Scholar]
- 45.Frisch RE. Malnutrition and fertility. Science. 1982;215:1272–73. doi: 10.1126/science.7199206. [DOI] [PubMed] [Google Scholar]
- 46.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Gáliková M, Diesner M, Klepsatel P, Hehlert P, Xu Y, et al. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics. 2015;201:665–83. doi: 10.1534/genetics.115.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336:579–82. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- 49.Garelli A, Heredia F, Casimiro AP, Macedo A, Nunes C, et al. Dilp8 requires the neuronal relaxin receptor Lgr3 to couple growth to developmental timing. Nat Commun. 2015;6:8732. doi: 10.1038/ncomms9732. Demonstrates that Lgr3 positive neurons are the site of Dilp8 action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh AC, O’Connor MB. Systemic activin signaling independently regulates sugar homeostasis, cellular metabolism, and pH balance in Drosophila melanogaster. PNAS. 2014;111:5729–34. doi: 10.1073/pnas.1319116111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grönke S, Müller G, Hirsch J, Fellert S, Andreou A et al. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLOS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grosberg RK, Strathmann RR. The evolution of multicellularity: a minor major transition? Annu Rev Ecol Evol Syst. 2007;38:621–54. [Google Scholar]
- 53.Gusarova V, Alexa CA, Na E, Stevis PE, Xin Y, et al. ANGPTL8/betatrophin does not control pancreatic beta cell expansion. Cell. 2014;159:691–96. doi: 10.1016/j.cell.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–80. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- 55.Hall JE. Historical perspective of the renin-angiotensin system. Mol Biotechnol. 2003;24:27–39. doi: 10.1385/MB:24:1:27. [DOI] [PubMed] [Google Scholar]
- 56.Han H, Pan C, Liu C, Lv X, Yang X, et al. Gut-neuron interaction via Hh signaling regulates intestinal progenitor cell differentiation in Drosophila. Cell Discov. 2015;1:15006. doi: 10.1038/celldisc.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartwig S, Raschke S, Knebel B, Scheler M, Irmler M, et al. Secretome profiling of primary human skeletal muscle cells. Biochim Biophys Acta. 2014;1844:1011–17. doi: 10.1016/j.bbapap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Honegger B, Galic M, Köhler K, Wittwer F, Brogiolo W, et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. Shows the role of Dilp-binding protein ImpL2 in systemic physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong HS, Lee J, Lee E, Kwon YS, Lee E, et al. A new role of substance P as an injury-inducible messenger for mobilization of CD29+ stromal-like cells. Nat Med. 2009;15:425–35. doi: 10.1038/nm.1909. [DOI] [PubMed] [Google Scholar]
- 60.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 61.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily 1. Endocr Rev. 1999;20:761–87. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 62.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol. 2002;12:1293–300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 63.Jindra M, Uhlirova M, Charles J-P, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLOS Genet. 2015;11:e1005394. doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jørgensen SB, Honeyman J, Oakhill JS, Fazakerley D, Stöckli J, et al. Oligomeric resistin impairs insulin and AICAR-stimulated glucose uptake in mouse skeletal muscle by inhibiting GLUT4 translocation. Am J Physiol Endocrinol Metab. 2009;297:E57–66. doi: 10.1152/ajpendo.90744.2008. [DOI] [PubMed] [Google Scholar]
- 65.Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of interorgan communication. Cell. 2016;164:1248–56. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katewa SD, Demontis F, Kolipinski M, Hubbard A, Gill MS, et al. Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab. 2012;16:97–103. doi: 10.1016/j.cmet.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–34. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339:746–54. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 69.Kim SK, Rulifson EJ. Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature. 2004;431:316–20. doi: 10.1038/nature02897. [DOI] [PubMed] [Google Scholar]
- 70.Kir S, White JP, Kleiner S, Kazak L, Cohen P, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knoll AH. The multiple origins of complex multicellularity. Annu Rev Earth Planet Sci. 2011;39:217–39. [Google Scholar]
- 72.Koyama T, Mirth CK. Growth-blocking peptides as nutrition-sensitive signals for insulin secretion and body size regulation. PLOS Biol. 2016;14:e1002392. doi: 10.1371/journal.pbio.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–46. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 74.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell. 2015;33:36–46. doi: 10.1016/j.devcel.2015.02.012. Shows the role of Dilp-binding protein ImpL2 in systemic physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–73. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 76.Lai S-L, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–9. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 77.Lee-Hoeflich ST, Zhao X, Mehra A, Attisano L. The Drosophila type II receptor, wishful thinking, binds BMP and myoglianin to activate multiple TGFβfamily signaling pathways. FEBS Lett. 2005;579:4615–21. doi: 10.1016/j.febslet.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 78.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–23. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehr S, Hartwig S, Lamers D, Famulla S, Müller S, et al. Identification and validation of novel adipokines released from primary human adipocytes. Mol Cell Proteom. 2012;11:M111.010504. doi: 10.1074/mcp.M111.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–88. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- 82.Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, et al. Activin signaling: effects on body composition and mitochondrial energy metabolism. Endocrinology. 2009;150:3521–29. doi: 10.1210/en.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Libby E, Ratcliff WC. Ratcheting the evolution of multicellularity. Science. 2014;346:426–27. doi: 10.1126/science.1262053. Illustrates early evolution to multicellularity and/or multicellular communities. [DOI] [PubMed] [Google Scholar]
- 84.Liddle RA. Cholecystokinin cells. Annu Rev Physiol. 1997;59:221–42. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- 85.Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell. 2010;142:661–67. doi: 10.1016/j.cell.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin S, Ewen-Campen B, Ni X, Housden BE, Perrimon N. In vivo transcriptional activation using CRISPR-Cas9 in Drosophila. Genetics. 2015;201:433–42. doi: 10.1534/genetics.115.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu T, Qian W-J, Gritsenko MA, Xiao W, Moldawer LL, et al. High dynamic range characterization of the trauma patient plasma proteome. Mol Cell Proteom. 2006;5:1899–913. doi: 10.1074/mcp.M600068-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo C-M, Obici S, Dong HH, Haas M, Lou D, et al. Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes. 2011;60:2000–7. doi: 10.2337/db10-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–39. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–36. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- 92.Matusek T, Wendler F, Polès S, Pizette S, D’Angelo G, et al. The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature. 2014;516:99–103. doi: 10.1038/nature13847. [DOI] [PubMed] [Google Scholar]
- 93.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20:54–60. doi: 10.1016/j.cmet.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McPherron AC. Metabolic functions of myostatin and GDF11. Immunol Endocr Metab Agents Med Chem. 2010;10:217. doi: 10.2174/187152210793663810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metaxakis A, Tain LS, Grönke S, Hendrich O, Hinze Y, et al. Lowered insulin signalling ameliorates age-related sleep fragmentation in Drosophila. PLOS Biol. 2014;12:e1001824. doi: 10.1371/journal.pbio.1001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meyer C. Final answer: Ghrelin can suppress insulin secretion in humans, but is it clinically relevant? Diabetes. 2010;59:2726–28. doi: 10.2337/db10-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mirth CK, Tang HY, Makohon-Moore SC, Salhadar S, Gokhale RH, et al. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. PNAS. 2014;111:7018–23. doi: 10.1073/pnas.1313058111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitri S, Foster KR. The genotypic view of social interactions in microbial communities. Annu Rev Genet. 2013;47:247–73. doi: 10.1146/annurev-genet-111212-133307. Illustrates early evolution to multicellularity and/or multicellular communities. [DOI] [PubMed] [Google Scholar]
- 100.Murphy KT, Schwartz GJ, Nguyen NLT, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–47. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLOS ONE. 2006;1:e31. doi: 10.1371/journal.pone.0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nässel DR, Broeck JV. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci. 2016;73:271–90. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ni J-Q, Markstein M, Binari R, Pfeiffer B, Liu L-P, et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okamoto N, Nakamori R, Murai T, Yamauchi Y, Masuda A, Nishimura T. A secreted decoy of InR antagonizes insulin/IGF signaling to restrict body growth in Drosophila. Genes Dev. 2013;27:87–97. doi: 10.1101/gad.204479.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, et al. A fat body–derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell. 2009;17:885–91. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oury F, Sumara G, Sumara O, Ferron M, Chang H, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owusu-Ansah E, Perrimon N. Cellular stress signaling between organs in metazoa. Annu Rev Cell Dev Biol. 2015;31:497–522. doi: 10.1146/annurev-cellbio-100814-125523. [DOI] [PubMed] [Google Scholar]
- 108.Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155:699–712. doi: 10.1016/j.cell.2013.09.021. Shows the role of Dilp-binding protein ImpL2 in systemic physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Padmanabha D, Baker KD. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol Metab. 2014;25:518–27. doi: 10.1016/j.tem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Palm W, Swierczynska MM, Kumari V, Ehrhart-Bornstein M, Bornstein SR, Eaton S. Secretion and signaling activities of lipoprotein-associated hedgehog and non-sterol-modified hedgehog in flies and mammals. PLOS Biol. 2013;11:e1001505. doi: 10.1371/journal.pbio.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Passeri E, Bugiardini E, Sansone VA, Pizzocaro A, Fulceri C, et al. Gonadal failure is associated with visceral adiposity in myotonic dystrophies. Eur J Clin Investig. 2015;45:702–10. doi: 10.1111/eci.12459. [DOI] [PubMed] [Google Scholar]
- 112.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 113.Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur A, et al. Circulating growth differentiation factor 11/8 levels decline with age. Circ Res. 2016;118:29–37. doi: 10.1161/CIRCRESAHA.115.307521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Port F, Chen H-M, Lee T, Bullock SL. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. PNAS. 2014;111:E2967–76. doi: 10.1073/pnas.1405500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–48. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, et al. Bone marrow–derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol. 2015;78:85–107. doi: 10.1146/annurev-physiol-021115-105305. [DOI] [PubMed] [Google Scholar]
- 118.Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151:123–37. doi: 10.1016/j.cell.2012.08.019. Illustrates the physiologic role for leptin structural ortholog Upd2 and the neural circuit it acts through. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–91. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rehfeld J, Larsson L-I, Goltermann N, Schwartz T, Holst J, et al. Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature. 1980;284:33–38. doi: 10.1038/284033a0. [DOI] [PubMed] [Google Scholar]
- 121.Reiff T, Jacobson J, Cognigni P, Antonello Z, Ballesta E, et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife. 2015;4:e06930. doi: 10.7554/eLife.06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, et al. CCHamide-2 is an orexigenic brain-gut peptide in Drosophila. PLOS ONE. 2015;10:e0133017. doi: 10.1371/journal.pone.0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richter DJ, King N. The genomic and cellular foundations of animal origins. Annu Rev Genet. 2013;47:509–37. doi: 10.1146/annurev-genet-111212-133456. [DOI] [PubMed] [Google Scholar]
- 124.Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19:96–108. doi: 10.1016/j.cmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodenfels J, Lavrynenko O, Ayciriex S, Sampaio JL, Shevchenko A, Eaton S. Production of systemically circulating Hedgehog by the intestine couples nutrition to growth and development. Genes Dev. 2014;28:2636–51. doi: 10.1101/gad.249763.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, et al. Asprosin, a fastin-induced glucogenic protein hormone. Cell. 2016;165:566–79. doi: 10.1016/j.cell.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–20. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- 128.Sabio G, Das M, Mora A, Zhang Z, Jun JY, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–43. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sano H, Nakamura A, Texada MJ, Truman JW, Ishimoto H, et al. The nutrient-responsive hormone CCHamide-2 controls growth by regulating insulin-like peptides in the brain of Drosophila melanogaster. PLOS Genet. 2015;11:e1005209. doi: 10.1371/journal.pgen.1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Savage DB, Sewter CP, Klenk ES, Segal DG, Vidal-Puig A, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator–activated receptor-γaction in humans. Diabetes. 2001;50:2199–202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 131.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–79. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 132.Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–15. doi: 10.1016/j.cmet.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–80. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol. 1998;8:335–40. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 135.Shim J, Mukherjee T, Mondal BC, Liu T, Young GC, et al. Olfactory control of blood progenitor maintenance. Cell. 2013;155:1141–53. doi: 10.1016/j.cell.2013.10.032. An example of how sensory signals translate to physiological control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Silverthorn DU, Ober WC, Garrison CW, Silverthorn AC, Johnson BR. Human Physiology: An Integrated Approach. San Francisco, CA: Pearson/Benjamin Cummings; 2009. [Google Scholar]
- 137.Singh SR, Liu W, Hou SX. The adult Drosophila Malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sinha M, Jang YC, Oh J, Khong D, Wu EY, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–52. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell. 2009;17:874–84. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, et al. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 2014;19:1050–57. doi: 10.1016/j.cmet.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song W, Veenstra JA, Perrimon N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9:40–47. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, et al. Tumor necrosis factor α–induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–74. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 143.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 144.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 145.Tatar M, Kopelman A, Epstein D, Tu M-P, Yin C-M, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–10. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 146.Tsuzuki S, Ochiai M, Matsumoto H, Kurata S, Ohnishi A, Hayakawa Y. Drosophila growth-blocking peptide-like factor mediates acute immune reactions during infectious and non-infectious stress. Sci Rep. 2012;2:210. doi: 10.1038/srep00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vallejo DM, Juarez-Carreño S, Bolivar J, Morante J, Dominguez M. A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science. 2015;350:aac6767. doi: 10.1126/science.aac6767. Demonstrates that Lgr3 positive neurons are the site of Dilp8 action. [DOI] [PubMed] [Google Scholar]
- 148.Voet D, Voet JG. Biochemistry. Hoboken, NJ: John Wiley Sons; 2004. [DOI] [PubMed] [Google Scholar]
- 149.Vorlová S, Rocco G, LeFave CV, Jodelka FM, Hess K, et al. Induction of antagonistic soluble decoy receptor tyrosine kinases by intronic polyA activation. Mol Cell. 2011;43:927–39. doi: 10.1016/j.molcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Waterson MJ, Chung BY, Harvanek ZM, Ostojic I, Alcedo J, Pletcher SD. Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. PNAS. 2014;111:8137–42. doi: 10.1073/pnas.1315461111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu J, Spiegelman BM. Irisin ERKs the fat. Diabetes. 2014;63:381–83. doi: 10.2337/db13-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 153.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 2013;58:497. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23. doi: 10.1016/j.beem.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 155.Yan D, Neumüller RA, Buckner M, Ayers K, Li H, et al. A regulatory network of Drosophila germline stem cell self-renewal. Dev Cell. 2014;28:459–73. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang H, Kronhamn J, Ekström JO, Korkut GG, Hultmark D. JAK/STAT signaling in Drosophila muscles controls the cellular immune response against parasitoid infection. EMBO Rep. 2015;16:1664–72. doi: 10.15252/embr.201540277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yi P, Park J-S, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–58. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]


