Abstract
Aim
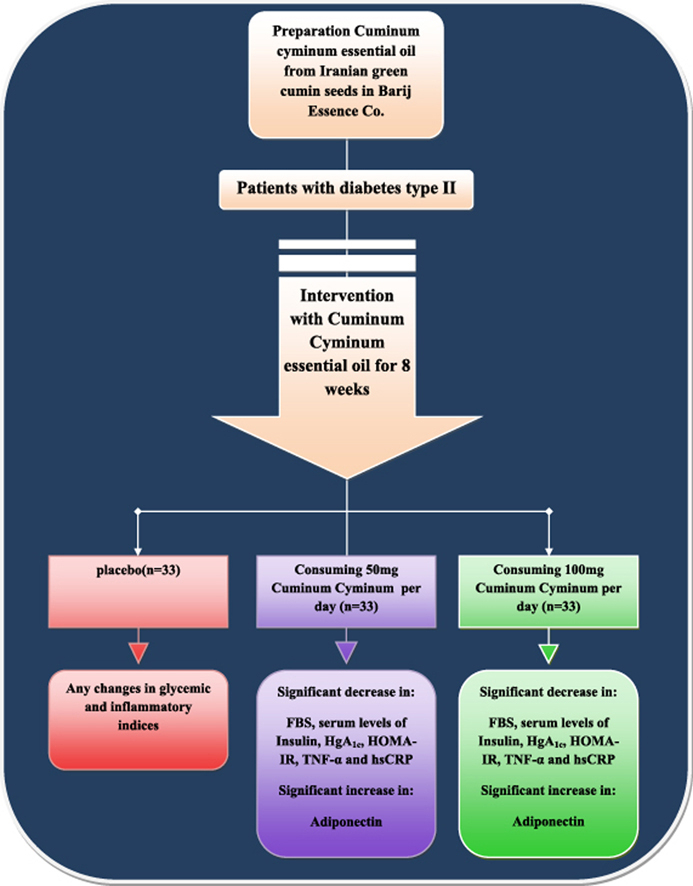
The purpose of this study was to evaluate the effect of 50 and 100 mg doses of green cumin essential oil on glycemic and inflammatory indices in patients with diabetes type II.
Method
In this randomized double-blind placebo-controlled clinical trial, subjects were divided into three groups after selecting them randomly according to the inclusion criteria: 1 – Cuminum cyminum capsule (100 mg/day,n = 33), 2 – C. cyminum capsule (50 mg/day, n = 33), and 3 – placebo (n = 33). Before and after 8 weeks of intervention, a blood sample was taken.
Findings
The findings demonstrated that the mean of the FBS, glycosylated hemoglobin (HgA1c) and the serum levels of insulin were significantly decreased and insulin sensitivity (HOMA-IR) was significantly increased in the groups receiving the 100 mg supplement (P < 0.001) and 50 mg supplement, but these indices were increased in the placebo group. The mean serum levels of TNF-α and hsCRP were significantly decreased, and serum levels of adiponectin was significantly increased at the end of the study in the groups receiving 100 mg (P < 0.001) and 50 mg (P = 0.008) supplement as well as in the group who received the placebo. There were no significant change in HOMA-B and QUICKI as insulin resistance parameters.
Discussion and conclusion
Administering C. cyminum supplement in patients with diabetes type II may decrease the serum levels of insulin, FBS, and glycosylated hemoglobin and also the inflammatory indices of TNF-α and hsCRP and increased the serum levels of adiponectin. In addition it may control the complications of diabetes type II in these patients.
Keywords: Cuminum cyminum, Diabetes type II, Glycemic indices, Inflammatory indices, Green cumin
Graphical abstract
1. Introduction
Diabetes is a metabolic disorder which is known by hyperglycemia due to impairment of insulin secretion, insulin function or both. Diabetes type II is a kind of diabetes which affects 90–95% of diabetic people, the treatment of which is independent of insulin. The signs and symptoms of this disease are the elevated level of blood glucose, decreased peripheral absorption of glucose due to impairment of insulin secretion and peripheral resistance to insulin.1
During recent years, the prevalence of diabetes type II has increased.2 Diabetes type II is one of the risk factors for developing cardiovascular diseases including congestive heart failure and coronary vascular diseases, chronic neural diseases such as diabetic neuropathy, renal diseases such as diabetic nephropathy and the end stage of renal disease, optic diseases including cataracts and non-healing wounds such as foot wounds. Any of the diseases mentioned are due to hyperglycemia. Therefore, diabetes type II has severe effects on the neural system, the renal system and the eyes, and eventually it results in blindness, renal dysfunction and/or amputation of the affected body organ.3, 4, 5
This disease imposes a huge expense on society such that all countries throughout the world spend the 5%–13% of the costs allocated to disease treatment for diabetes.
The first step in treatment of diabetes is to control the level of blood glucose6, 7 through some form of therapy regime, including physical activity, use of hypoglycemic medications and insulin therapy. In one study in England, it was seen that patients need to start insulin therapy after 10 years of hypoglycemic drug usage.6, 8, 9 Insulin therapy has some side effects, including lipo-hypertrophy and lipo-atrophy.10, 11
Herbal treatment is one way of managing this disease.12, 13 Until now, many plants have been tested and used in the prevention and treatment of diabetes. Cuminum cyminum or green cumin is a plant of the Apiaceae family. The native regions for this plant are India, Iran, the Mediterranean and Egypt.14 This plant has antioxidant properties and it has been used in traditional medicine as a stimulant, carminative, and coagulant.15 Studies on laboratory animals have shown that essential oil of C. cyminum has anti-diabetic properties.16 The effective components of this plant are Cumin aldehyde, γ-Terpinine, α-Sabinin, α-Flandren and α-Kadinin,17 of which Cuminaldehyde or 4-Isopropil Benzaldhyde is the most effective. This component is the enzyme inhibitor for α-glycosidase and aldose reductase in the carbohydrate metabolism pathway. It is possible that the anti-diabetic property is due to the existence of these enzyme inhibitors.18
Studies have shown that the blood levels of tumor necrosis factor-α (TNF-α) and high sensitivity C-reactive protein (hsCRP) are increased and the blood levels of adiponectin decreased in patients with diabetes type II.19, 20 Also, a study on rats with hypertension has shown that the supplementary treatment with C. cyminum resulted in decrease in inflammatory factors such as TNF-α.21, 22
Until now, studies for evaluating the effect of C. cyminum on diabetes type II have been performed almost exclusively on laboratory animals; however, one study has been done on a human sample with diabetes type II with 10 subjects in each group and 20 subjects total.23 The result of this study indicated that green cumin has positive and significantly meaningful effect on glucose of serum and lipid profile. But in another study performed on overweight subjects, no meaningful effect of C. cyminum on plasma glucose in a fasting state and lipid profile was detected.24 As most studies have been performed on laboratory animals,16, 25, 26, 27, 28, 29, 30 and due to lack of comparison on the rate of effectiveness of the different doses of this plant on patients with diabetes type II as well as controversial outcomes of the studies, it is essential to compare the effectiveness of the different doses of green cumin extract. Therefore, the present study was designed for the purpose of evaluating the effect of 50 and 100 mg doses of essential oil of C. cyminum on the glycemic indices and some inflammatory indices (TNF-α, adiponectin and hsCRP) in patients with diabetes type II.
2. Method and materials
2.1. Participants
This study was a randomized controlled-placebo double-blind clinical trial conducted from December 2015 to February 2016 in Urmia, Iran. Diabetic type II patients aged 20–60 were recruited for the current study. For estimating sample size, we used the standard formula suggested for clinical trials by considering type one error (α) of 0.05 and type two error(ß) of 0.20 (power = 80%). Therefore, we needed 33 patients in each group. The inclusion criteria were FBS > 126 mg/dL and FBS < 200 mg/dL, subjects under control for the glucose and lipid of the blood and lack of changes in the dosage of their medicines during the study, having the unified medication plan in doses and terms of the type of the medication, lack of using the insulin therapy, lack of using of tobacco and alcohol, lack of chronic or acute diseases, lack of pregnancy or lactation, lack of menopause, lack of hormone therapy, lack of following the diet for weight loss as well as lack of using the supplementary extract of C. cyminum. The exclusion criteria included sensitivity to the extract of C. cyminum, pregnancy during the study, variation in the dosage of the medicines during the study, developing to the abovementioned diseases during the study and unwillingness to cooperation by subject.
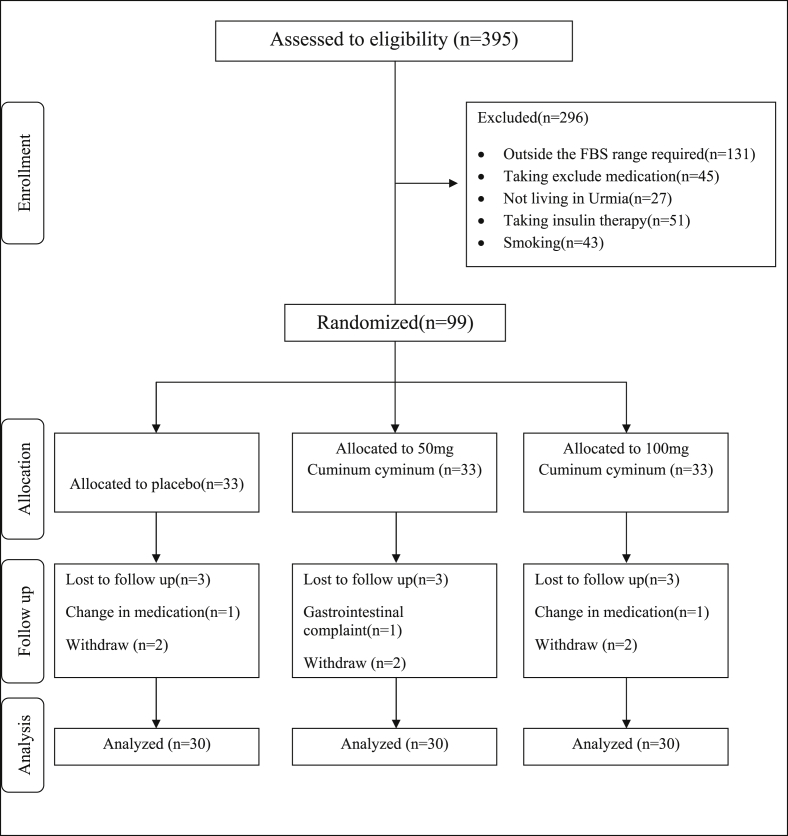
A total of 395 patients attended the endocrinology clinic affiliated with Urmia University of Medical Sciences; 99 patients met the inclusion criteria and enrolled in the study.
After selecting the samples, general information questionnaire and 24 h's dietary recall were completed. All participants provided written informed consent after receiving an explanation of the purposes of the study, inclusion criteria, exclusion criteria, disadvantages and advantages of the study which were approved by the ethics committee of the Urmia University of Medical Sciences. The study was recorded in the clinical trial record center of Iran under registration No. IRCT2015101113677N9.
2.2. Study design
At the study baseline and after stratification for gender, preintervention weight and age, subjects were randomly assigned to three groups of 33 subjects each as follows: The first group received the 100 mg supplement of essential oil of C. cyminum; the second group received the 50 mg supplement of essential oil of C. cyminum; and the third group received the placebo containing oral paraffin which was similar to the supplementary extract of green cumin in appearance, shape, and color. The supplementary essential oil of C. cyminum were prepared by Barij Essence Co. and the placebo was prepared by Zahravi Company. The cumin capsules were produces from Iranian green cumin seeds in Barij Essence, Kashan, Iran. For this purpose first, cumin essential oil was solved in polyethylene glycol (PEG) 4000 at 25 °C, then it was inserted into a capsule. C. cyminum. capsules was analysed in the laboratory of Barij Essence company by the gas chromatography spectrometry (GC) method. Following analysis of the C. cyminum, it was determined that the major components were cumin aldehyde, thymoquinone, p-cymene, γ-penllandrene, limonene, myrcene, terpinene, safranal, 2-ethoxy-3-isopro-pylpyrazine, sterol compounds and β-pinene. The diet of the participant was evaluated by using of 24 h recal dietary records and four physical activity measurements in weeks 0, 2, 4 and 6 (one weekend and 3 weekdays) to make sure that they maintained their usual diet and physical activity during intervention. Also, the consuming evaluation was analyzed by Nutritionist IV software (version 15).
2.3. Biochemical assessment
Fasting blood samples (10 ml) were drawn from the brachial artery of all participants after 12 h fasting for biochemistry tests. Blood samples were immediately centrifuged (Hettich D-78532, Tuttlingen, Germany) at 3500 rpm for 10 min to separate serum. Then the blood samples were stored at −70 °C before analysis at the Urmia University of Medical Sciences reference laboratory. Commercial kits were used to measure fasting blood glucose (Pars Azmoon, Tehran, Iran), serum levels of insulin (Monobind, California, United States), glycosilated hemoglobin (Paadco, Tehran, Iran), hsCRP (Diagnostic Biochem, Ontario, Canada), adiponectin (Mercodia, Uppsala, Sweden), and TNF-α (Koma Biotech, Seol, Korea). For assessment of insulin resistance, we used the homeostatic model of assessment for insulin resistance (HOMA-IR), homeostatic model assessment-β cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) indices, that they was calculated based on suggested formulas.31
Interventions were followed by phone contact. After 8 weeks, again 10 ml blood samples were drawn from the brachial artery of each participant after 12 h fasting for repetition of biochemical tests. Samples were conserved at a temperature of 70 °C until analysis. Fasting plasma glucose was measured by the method of glucose oxidase. The serum level of insulin and serum concentrations of TNF-α and hsCRP were measured by the ELISA method.
2.4. Statistical methods
Data were analyzed by SPSS software, version 22. In this study, less than 0.05 was considered as the meaningful level. All quantitative variables were reported as mean ± SD and qualitative variables were reported as a number (percent). The K–S test was used for determining the normality of the data. The T-test paired test was used for comparing the mean of pre-intervention and post-intervention data with normal distribution. Also, The Wilcoxon test was used for data with abnormal distribution. The ANOVA method was used for comparing the mean of the three groups. In addition, MANCOVA and regression were used for adjustment of the confounding factors such as age, pre-intervention variants and diet. The method of analyses of variance with repetitive measurements and followup tests were used for evaluating the meaningfulness of diet determined by 4 measurements during the intervention period The confidence level for all variables was considered 95%. The statistically meaningful level was P < 0.05.
3. Findings
Among 99 participants, one sample due to variation in the dose of the medicine; one sample due to the sensitivity of the gastrointestinal tract; and 6 samples due to lack of cooperation were excluded from the study and 90 samples were analyzed (Fig. 1).
Fig. 1.
Summary of patient flow diagram.
The mean age of the all participant was 47.33 ± 6.36. (Table 1).
Table 1.
General characteristics of study participants.
| Variable | Complementary group 100 mg/day (Mean ± SD) |
Complementary group 50 mg/day (Mean ± SD) |
Placebo group (Mean ± SD) |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | Number | Percent | ||
| Sex | Male | 12 | 40.0 | 9 | 30.0 | 7 | 23.3 | 28 | 31.1 |
| Female | 18 | 60.0 | 21 | 70.0 | 23 | 76.7 | 62 | 68.9 | |
| Age (year) (Mean ± SD) |
46.93 ± 5.98 | 47.56 ± 5.76 | 47.50 ± 7.41 | 47.33 ± 6.36 | |||||
The mean of daily dietary consumption in 4 steps of the measurement showed no significant variation during the study (Table 2).
Table 2.
Dietary intakes of study participants throughout the study.
| Variable | Complementary group 100 mg/day (Mean ± SD) |
Complementary group 50 mg/day (Mean ± SD) |
Placebo group (Mean ± SD) | P-valuea |
|---|---|---|---|---|
| Energy (Kcal/day) | 2030.7 ± 261.2 | 2028.6 ± 315.3 | 1969.0 ± 214.4 | 0.18 |
| Carbohydrate (g/day) | 265.4 ± 40.7 | 263.9 ± 56.2 | 255.1 ± 49.3 | 0.11 |
| Protein (g/day) | 58.9 ± 10.5 | 59.0 ± 11.0 | 61.7 ± 14.1 | 0.62 |
| Fat (g/day) | 75.6 ± 15.4 | 75.6 ± 13.4 | 71.3 ± 13.4 | 0.74 |
| SFA (g/day) | 21.9 ± 5.0 | 20.8 ± 6.2 | 21.1 ± 4.8 | 0.96 |
| PUFA (g/day) | 25.4 ± 5.2 | 24.9 ± 6.1 | 26.1 ± 6.9 | 0.16 |
| Cholesterol (mg/day) | 202.4 ± 115.9 | 207.1 ± 101.4 | 201.1 ± 101.6 | 0.74 |
| MUFA (mg/day) | 20.1 ± 1.3 | 22.7 ± 3.6 | 20.9 ± 5.5 | 0.86 |
| Fiber (gm/day) | 19.2 ± 3.7 | 17.9 ± 5.7 | 16.7 ± 1.9 | 0.25 |
| Magnesium (μg/day) | 271.5 ± 85.7 | 283.7 ± 75.3 | 296.1 ± 68.7 | 0.34 |
| Selenium (μg/day) | 95.8 ± 22.1 | 93.2 ± 32.9 | 97.1 ± 18.4 | 0.91 |
Data are means ± standard deviations. SFA = Saturated fatty acid; PUFA = polyunsaturated fatty acid; MUFA = monounsaturated fatty acid.
Obtained from ANOVA test.
Findings demonstrated that mean and SD of the glycemic indices of the FBS, glycosylated hemoglobin (HbA1c) and the serum level of insulin were significantly decreased in the groups receiving 100 mg and 50 mg supplementary essential oil of green cumin (P < 0.001), but it was increased in the group who received the placebo.
Regarding inflammatory indices, the results in both groups receiving the supplementary essential oil of green cumin and the placebo demonstrated that the mean of the TNF-α and hsCRP indices were decreased meaningfully at the beginning and end of the study in the two groups who consumed C. cyminum (P < 0.001 and P = 0.008), but the SD slightly increased in the all groups except the group who received 50 mg supplementary essential oil of C. cyminum. The SD of hsCRP remained stable in this group. In addition, the mean serum levels of adiponectin were increased significantly at the beginning and end of the study. Data related to comparing the mean of considered variables in the three groups is shown in Table 3 and Fig. 1.
Table 3.
Effect of cumin intake on glycemic indices and some of inflammatory factor.
| Variable | Complementary group 100 mg/day (Mean ± SD) |
Complementary group 50 mg/day (Mean ± SD) |
Placebo group (Mean ± SD) |
P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Changes | Before | After | Changes | Before | After | Changes | ||
| FBS (mg/dl) | 170.33 ± 29.19 | 114.43 ± 21.86 | −55.9 ± 7.9 | 169.86 ± 28.03 | 163.63 ± 23.01 | −6.23 ± 9.4 | 159.56 ± 31.19 | 165.26 ± 32.42 | −5.7 ± 14.9 | 0.002 |
| HgA1c (%) | 7.09 ± 0.79 | 5.31 ± 0.70 | −1.78 ± 0.21 | 7.27 ± 1.18 | 7.27 ± 1.15 | 0.00 ± 0.56 | 6.72 ± 1.32 | 6.73 ± 1.35 | −0.35 ± 2.6 | 0.024 |
| Serum insulin (μIU/ml) | 15.17 ± 1.78 | 10.45 ± 1.05 | −4.72 ± 0.78 | 14.65 ± 2.00 | 13.88 ± 1.61 | −0.77 ± 0.96 | 12.61 ± 2.52 | 13.14 ± 2.38 | 0.53 ± 1.5 | 0.015 |
| HOMA-IR | 0.003 ± 0.001 | 0.0029 ± 0.0005 | 0.003 ± 0.001 | 0.006 ± 0.001 | 0.005 ± 0.001 | 0.0005 ± 0.00072 | 0.005 ± 0.001 | 0.005 ± 0.001 | −0.0003 ± 0.0004 | 0.008 |
| HOMA-B | 29.5 ± 7.1 | 30.6 ± 7.4 | −1.08 ± 6.9 | 28.2 ± 5.8 | 27.5 ± 5.2 | 0.6 ± 5.4 | 26.0 ± 8.4 | 26.2 ± 8. | −0.21 ± 3.7 | 0.103 |
| QUICKI | 3.57 ± 0.08 | 3.03 ± 0.09 | 0.03 ± 0.08 | 3.08 ± 0.07 | 3.08 ± 0.06 | −0.01 ± 0.05 | 3.1 ± 0.1 | 3.1 ± 0.1 | 0.005 ± 0.07 | 0.061 |
| hsCRP (pg/ml) | 4.77 ± 0.52 | 3.39 ± 0.58 | −1.38 ± 1.2 | 4.31 ± 0.54 | 4.15 ± 0.54 | −0.16 ± 0.89 | 4.46 ± 0.51 | 4.42 ± 0.71 | −0.04 ± 1.3 | 0.007 |
| TNF-α (ng/ml) | 12.86 ± 0.45 | 11.08 ± 0.52 | −1.78 ± 0.97 | 12.44 ± 0.63 | 12.30 ± 0.64 | −0.14 ± 1.9 | 12.08 ± 0.63 | 12.04 ± 0.68 | −0.04 ± 0.49 | 0.008 |
| Adiponectin (μg/l) | 102.08 ± 59 | 159.19 ± 71 | 57.11 ± 29 | 106.21 ± 71 | 122.88 ± 41 | 16.67 ± 85 | 103.19 ± 85 | 105.01 ± 23 | 1.82 ± 13 | 0.010 |
All values are means ± SD.
Obtained from ANOVA test.
Also, the serum levels of these variables after intervention had statistically significant differences among the three groups as shown by using the ANCOVA test, regression, and by omitting the confounding effect of the insulin, FBS, HbA1c, hsCRP, adiponectin and TNF-α before intervention (P < 0.001) (Table 4).
Table 4.
Adjusted changes in metabolic variables in study participants.
| Variable | Complementary group 100 mg/day (Mean ± SD) |
Complementary group 50 mg/day (Mean ± SD) |
Placebo group (Mean ± SD) |
P-valuea |
|---|---|---|---|---|
| FBS | ||||
| Model 1b | −56.2 ± 3.01 | −5.9 ± 2.9 | 5.6 ± 3.1 | 0.00 |
| Model 2c | −56.2 ± 3.01 | −5.4 ± 2.9 | 5.2 ± 3.1 | 0.00 |
| Insulin | ||||
| Model 1 | −4.3 ± 0.2 | −0.56 ± 0.2 | −0.03 ± 0.2 | 0.01 |
| Model 2 | −4.3 ± 0.2 | −0.59 ± 0.2 | −0.01 ± 0.2 | 0.01 |
| HOMA-IR | ||||
| Model 1 | 0.003 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.00 |
| Model 2 | −0.003 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.00 |
| HOMA-B | ||||
| Model 1 | 2.03 ± 0.96 | −0.2 ± 0.93 | −1.01 ± 1.005 | 0.10 |
| Model 2 | 2. 04 ± 0.95 | −0.4 ± 0.9 | −0.9 ± 1.008 | 0.10 |
| QUICKI | ||||
| Model 1 | −0.05 ± 0.01 | −0.004 ± 0.01 | 0.01 ± 0.01 | 0.06 |
| Model 2 | −0.06 ± 0.06 | −0.003 ± 0.01 | 0.01 ± 0.01 | 0.05 |
| HgA1c | ||||
| Model 1 | −1.88 ± 0.42 | 0.00 ± 0.46 | −0.45 ± 2.6 | 0.02 |
| Model 2 | −1.81 ± 0.21 | 0.00 ± 0.46 | −0.45 ± 2.6 | 0.02 |
| TNF-α | ||||
| Model 1 | −1.40 ± 1.2 | −0.17 ± 1.8 | −0.05 ± 0.61 | 0.00 |
| Model 2 | −1.61 ± 2.4 | −0.7 ± 2.3 | 0.08 ± 0.3 | 0.00 |
| Adiponectin | ||||
| Model 1 | 59.18 ± 31 | 17.63 ± 76 | 2.63 ± 9 | 0.00 |
| Model 2 | 58.17 ± 49 | 16.41 ± 25 | 2.12 ± 8 | 0.00 |
| hsCRP | ||||
| Model 1 | −1.49 ± 1.2 | −0.17 ± 0.99 | −0.03 ± 1.1 | 0.01 |
| Model 2 | −1.48 ± 1.1 | −0.17 ± 0.89 | −0.03 ± 1.3 | 0.01 |
All values are means ± standard errors. FBS = Fasting blood sugar; HOMA-IR = homeostasis model of assessment-insulin resistance; HOMAB = homeostatic model assessment-Beta cell function; QUICKI = quantitative insulin sensitivity check index; TNF-α = Tumor necrosis factor; hsCRP = high sensitivity C reactive protein.
Obtained from ANCOVA.
Adjusted for baseline values.
Further adjusted for Model 1 + age.
4. Discussion
In the present study, a daily supplement 50 and 100 mg essential oil of C. cyminum during 8 weeks produced significantly greater changes in serum level of insulin, FBS, HbA1c, hsCRP and TNF-α than with the placebo.
Until now, most studies have been done on laboratory animals; the effect of green cumin supplement has been demonstrated on healthy and diabetic rats. In a study performed by Jagtap, the effect of supplement of three different doses of green cumin per body weight was indicated in diabetic and normal rats. The results demonstrated that the serum level of insulin significantly increased after treatment by green cumin.27 However, in this study green cumin and glibenclamide were used for treatment of the two different groups, but the results of the comparison of these two factors did not have provided in rats awkward.
In the study by Dhandapani, the supplement of green cumin resulted in a significant decrease of the level of glucose. Also, the level of HgA1c decreased after receiving the supplement of green cumin. In this study, it was found that the reductive effect of the supplement of green cumin was more than treatment with glibenclamide.25 In the both recent studies, the methods used for preparing the green cumin supplement and measuring the dose of the supplement are unclear.
In an animal trial by Patil, the results were similar to the above studies. In the diabetic rats, the serum level of insulin became normal and the level of FBS decreased after receiving the green cumin. Also, it was observed that the increased serum levels of insulin depended on the used dose of the green cumin.16 The result of the animal trial on diabetic rats which was performed by Willatgamuwa was similar to the above-mentioned studies, but it did not change the above indices in healthy rats.28 The studies of Ardakani et al. on rats demonstrated an effect similar to the other studies. Yet, in these studies some weaknesses such the as short time of the study and unclear rate of blood glucose and the lack of limitation in the level of the blood glucose in selected rats has resulted in inconclusive results in the studied rats.29, 30
Although the findings of the studies on laboratory animal trials for the effect of green cumin on glycemic indices are significantly meaningful; however, the rate of the effect of the different doses of green cumin remains controversial.
In one study on humans which was been performed by Asemi et al., the evaluation of the effect of Orlistat supplemented by green cumin on overweight people showed any significant changes in FBS32 that is different from the results of the other studies on laboratory animal trials and also from the present study. In addition, the cause of the inconsistency between these studies to our study is the unclear result of the rate of blood sugar in the studied subjects and also there is not any limitation for the rate of blood sugar. The discrepancy of the samples in the terms of blood sugar is the main confounding factor in this study and it compromises the effect of the extract of green cumin on the glycemic indices according to its result.
In a study on humans which was performed by Andallu et al., the results confirmed the effect of green cumin on glycemic indices in patients with diabetes type II23; however, in this study some factors such as small sample population, and unclear methods for either sampling or using the supplement of the green cumin were restrictive factors and they may have produced error in the results.
In the other study which has been done by Badr et al, it was shown that during the pregnancy and breastfeeding in the diabetic rats, the anti-diabetic food supplementary has been associated with thymoquinone(one active element of cumin). In addition this material protected their offspring against development of diabetic compound and it significantly decreased the serum levels of insulin and increased the sensitivity to insulin.33
Since Cuminaldehyde, or 4-isopropylbenzaldehyde is the most effective material in green cumin, the other potential mechanism for improving the glycemic indices is the inhibitory effect of two enzymes of α-glycosidase and Adlose reductase in the metabolic pathway of the carbohydrates digestion and it may have anti-diabetic effect. It should be stated that this dose-dependent effect is more effective on improved glycemic indices at the higher doses of C. cyminum.18
Among several studies on green cumin, there is not any study which has evaluated the effect of green cumin on inflammatory factors. Nevertheless, in the present study the green cumin significantly reduced the serum levels of TNF-α, adiponectin and hsCRP in both groups of receiving 50 and 100 mg doses of extract of C. cyminum compare to placebo. As previously stated, decreased sensitivity to insulin resulted in increased serum level of TNF-α and HsCRP and decreased level of adiponectin.34, 35 Therefore, according to the performed studies and the present study the complementary to extract of green cumin causes improvement of the serum level of insulin. So, it is possible that improvement of the condition of insulin by the unclear method will be resulted in decreased level of the inflammatory factors.
In the most of the studies, the supplement of green cumin was compared to the other hypoglycemic drugs or it was compared to the placebo. However, the aim of this study was the evaluation the effective rate of supplement on the examined indices. On the other hand, more studies have been performed on laboratory animals and only one study using green cumin has been done on a human sample with diabetes type II. In several studies, only the effect of the green cumin on glycemic factors was evaluated; however, the inflammatory factors in these patients have been less studied. Also, with regard to both studies which have been performed on the human sample, with their controversial results, it appeared that further investigations were required for evaluating the effect of the different doses of the supplement of green cumin on glycemic and inflammatory factors in patients with diabetes type II. Also, the time of the supplementary in this study was 8 weeks. Nevertheless, the effect of the supplement on the aforementioned factors may be more clearly indicated in the long term, or a better effect may be observed by the lower doses of herbal medicine.
Conflict of interest
Authors don't have any conflict of interests.
Acknowledgement
This study was done in the Health and Treatment Center of the Urmia University of Medical Science with the cooperation of the board members and clinic clients. The authors gratefully acknowledge their contribution.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Association AD Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteghamati A., Meysamie A., Khalilzadeh O. Third national surveillance of risk factors of non-communicable diseases (SuRFNCD-2007) in Iran: methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9(1):1. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hongyan Z., Jian Z., Lei Z., Jianhui M., Yongning S., Yuwu Z. Characteristics of blood glucose excursions in type 2 diabetes mellitus patients with three different traditional chinese medicine syndromes. J Tradit Chin Med. 2015;35(5):537–545. doi: 10.1016/s0254-6272(15)30136-9. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E. Diabetes and heart disease. Diabetes Care. 2003;26(10):2947–2958. doi: 10.2337/diacare.26.10.2947. [DOI] [PubMed] [Google Scholar]
- 5.Caballero A.E. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11(11):1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 6.Farshchi A., Esteghamati A., Sari A.A. The cost of diabetes chronic complications among Iranian people with type 2 diabetes mellitus. J Diabetes & Metab Disord. 2014;13(1):42. doi: 10.1186/2251-6581-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdollahi M., Farshchi A., Nikfar S., Seyedifar M. Effect of chromium on glucose and lipid profiles in patients with type 2 diabetes; a meta-analysis review of randomized trials. J Pharm Pharm Sci. 2013;16(1):99–114. doi: 10.18433/j3g022. [DOI] [PubMed] [Google Scholar]
- 8.Stratton I.M., Adler A.I., Neil H.A.W. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association AD Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury T.A., Escudier V. Poor glycaemic control caused by insulin induced lipohypertrophy. Br Med J. 2003;327(7411):383. doi: 10.1136/bmj.327.7411.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matteucci E., Giampietro O. Oxidative stress in families of type 1 diabetic patients. Diabetes Care. 2000;23(8):1182–1186. doi: 10.2337/diacare.23.8.1182. [DOI] [PubMed] [Google Scholar]
- 12.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 13.Kadish A.H., Litle R.L., Sternberg J.C. A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem. 1968;14(2):116–131. [Google Scholar]
- 14.Group UPDS Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 15.Thippeswamy N., Naidu K.A. Antioxidant potency of cumin varieties—cumin, black cumin and bitter cumin—on antioxidant systems. Eur Food Res Technol. 2005;220(5–6):472–476. [Google Scholar]
- 16.Patil S.B., Takalikar S.S., Joglekar M.M., Haldavnekar V.S., Arvindekar A.U. Insulinotropic and β-cell protective action of cuminaldehyde, cuminol and an inhibitor isolated from Cuminum cyminum in streptozotocin-induced diabetic rats. Br J Nutr. 2013;110(8):1434–1443. doi: 10.1017/S0007114513000627. [DOI] [PubMed] [Google Scholar]
- 17.Parthasarathy V.A., Chempakam B., Zachariah T.J. 2008. Chemistry of Spices: CABI. [Google Scholar]
- 18.Lee H.-S. Cuminaldehyde: aldose reductase and α-glucosidase inhibitor derived from Cuminum cyminum L. seeds. J Agric Food Chem. 2005;53(7):2446–2450. doi: 10.1021/jf048451g. [DOI] [PubMed] [Google Scholar]
- 19.Mugabo Y., Li L., Renier G. The connection between C-reactive protein (CRP) and diabetic vasculopathy. Focus on preclinical findings. Curr Diabetes Rev. 2010;6(1):27–34. doi: 10.2174/157339910790442628. [DOI] [PubMed] [Google Scholar]
- 20.Mirza S., Hossain M., Mathews C. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine. 2012;57(1):136–142. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalaivani P., Saranya R.B., Ramakrishnan G. Cuminum cyminum, a dietary spice, attenuates hypertension via endothelial nitric oxide synthase and NO pathway in renovascular hypertensive rats. Clin Exp Hypertens. 2013;35(7):534–542. doi: 10.3109/10641963.2013.764887. [DOI] [PubMed] [Google Scholar]
- 22.Yuanyuan W., Minghua J., Lina Z. Effect of a combination of calorie-restriction therapy and Lingguizhugan decoction on levels of fasting blood lipid and inflammatory cytokines in a high-fat diet induced hyperlipidemia rat model. J Tradit Chin Med. 2015;35(2):218–221. doi: 10.1016/s0254-6272(15)30031-5. [DOI] [PubMed] [Google Scholar]
- 23.Andallu B., Ramya V. Antihyperglycemic, cholesterol-lowering and HDL-raising effects of cumin (Cuminum cyminum) seeds in type-2 diabetes. J Nat Remedies. 2007;7(1):142–149. [Google Scholar]
- 24.Taghizadeh M., Memarzadeh M.R., Asemi Z., Esmaillzadeh A. Effect of the Cumin cyminum L. intake on weight loss, metabolic profiles and biomarkers of oxidative stress in overweight subjects: a randomized double-blind placebo-controlled clinical trial. Ann Nutr Metab. 2015;66(2–3):117–124. doi: 10.1159/000373896. [DOI] [PubMed] [Google Scholar]
- 25.Dhandapani S., Subramanian V.R., Rajagopal S., Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res. 2002;46(3):251–255. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]
- 26.Shahnaz H., Hifza A., Bushra K., Khan J. Lipid studies of Cuminum cyminum fixed oil. Pak J Bot. 2004;36(2):395–402. [Google Scholar]
- 27.Jagtap A., Patil P. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48(8):2030–2036. doi: 10.1016/j.fct.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 28.Willatgamuwa S., Platel K., Saraswathi G., Srinivasan K. Antidiabetic influence of dietary cumin seeds (Cuminum cyminum) in streptozotocin induced diabetic rats. Nutr Res. 1998;18(1):131–142. [Google Scholar]
- 29.Mohiti-Ardekani J., Akbarian Z., Piri-Ardekani M.R., Mohiti-Ardekani A. Comparison of the effects of cuminum cyminuml and sibutramine on weight, serum leptin, glucose and lipids in rat. Iran J Diabetes Obes. 2012;4(2):74–78. [Google Scholar]
- 30.Mohiti Ardekani J., Akbarian Z., Nazarian A. Effects of Cumin (Cuminum cyminum L) oil on serum glucose and lipid levels of rats. SSU J. 2011;19(3):388–397. [Google Scholar]
- 31.Pisprasert V., Ingram K.H., Lopez-Davila M.F., Munoz A.J., Garvey W.T. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845–853. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asemi Z., Zare Z., Shakeri H., Sabihi S.-S., Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9. doi: 10.1159/000349922. [DOI] [PubMed] [Google Scholar]
- 33.Badr G., Mahmoud M.H., Farhat K., Waly H., Al-Abdin O.Z., Rabah D.M. Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids Health Dis. 2013;12(1):1. doi: 10.1186/1476-511X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst S.E. The role of TNF-α in insulin resistance. Endocrine. 2004;23(2–3):177–182. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- 35.Yaturu S., Rains J., Jain S.K. Relationship of elevated osteoprotegerin with insulin resistance, CRP, and TNF-α levels in men with type 2 diabetes. Cytokine. 2008;44(1):168–171. doi: 10.1016/j.cyto.2008.07.471. [DOI] [PubMed] [Google Scholar]