Abstract
The biomarkers are needed to be defined for standardization purposes so that safe and effective herbal formulations can be catered to the society. There is an urgent need for statistical support of herbal drugs because most of the herbal products are still used in the non-standardized form. This study is based on the development of a simple and sensitive RP-HPTLC method for concurrent estimation of two biomarkers ent-phyllanthidine and rutin in the methanol extract of aerial parts of Flueggea virosa. The developed method was found to be simple, economic and sensitive. Separation and quantification were performed with acetonitrile: water (4:6 V/V) used as the mobile phase on glass-backed RP-HPTLC plate. Detection of absorption maxima and quantification was done at 310 nm of UV region. The developed chromatographic system was found to give a sharp band for ent-phyllanthidine and rutin at Rf 0.73 ± 0.01 and 0.68 ± 0.01, respectively. The linearity ranges for ent-phyllanthidine, and rutin were found to be 200–1600 ngband−1 and 100–1400 ngband−1, respectively, with correlation coefficients (r2 values) of 0.998 and 0.997, respectively. The percentage of ent-phyllanthidine and rutin was found to be 9.121 ± 0.02% and 1.018 ± 0.04% (w/w), respectively. The resolution of bands and separation of constituents in FVME exhibited the perfect optimization of the developed method. The validation statistics supports the proposed method for standardizing crude drugs as well as formulations of a natural product containing ent-phyllanthidine and rutin.
Keywords: Flueggea virosa, Rutin, ent-Phyllanthidine, HPTLC, Quantification, Biomarkers
1. Introduction
The plant Flueggea virosa (Euphorbiaceae) also known as Chinese water berry, grow wild in tropical Africa, tropical Asia, Japan, Middle East, Australia and other parts of the world. Various organs of F. virosa are used to treat ailments such as arrhythmia, hepatitis, diabetes, HIV-related infections, fever, malaria, epilepsy. Several other biological effects of F. virosa include antiplasmodial, trypanocidal and antioxidant (Siddiqui et al., 2015). A wide range of chemical constituents include alkaloids, triterpenoids, resins, steroids, cardiac glycosides, bergenin, menisdaurin and anthraquinones have been reported in F. virosa (Siddiqui et al., 2015). Flavonoids, saponins (Magaji et al., 2008), 11-O-acetyl bergenin, virosecurinine, ent-phyllanthidine, kaempferol, quercetin, gallic acid, daucosterol and β-sitosterol (Wang et al., 2008) are some other phytoconstituents reported in F. virosa.
(+)-Phyllanthidine or ent-phyllanthidine (Fig. 1) is a Securinega alkaloid (securinane-type) reported in few plants of family Euphorbiaceae (Phyllanthaceae) such as Securinega, Phyllanthus, Margaritaria, Breynia, Flueggea. ent-Phyllanthidine was first isolated from Breynia coronata though its enantiomer (−)-Phyllanthidine was isolated twenty years earlier to this from the plant Phyllanthus discoides. ent-Phyllanthidine possesses significant therapeutic potential against many ailments such as leishmaniasis and hepatitis B virus (Moraes et al., 2015).
Figure 1.
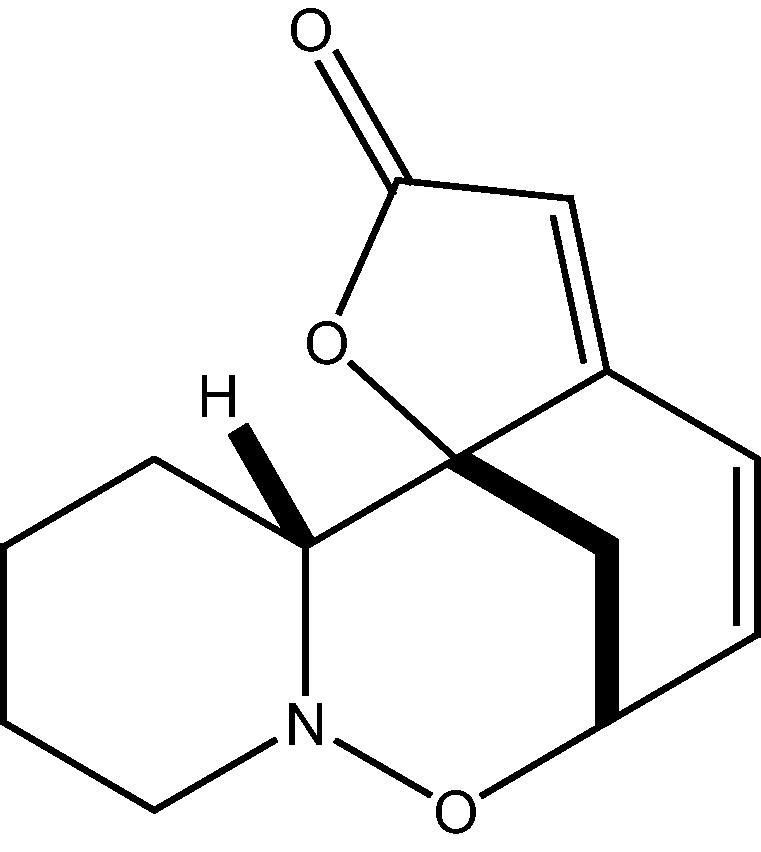
Chemical structure of ent-phyllanthidine.
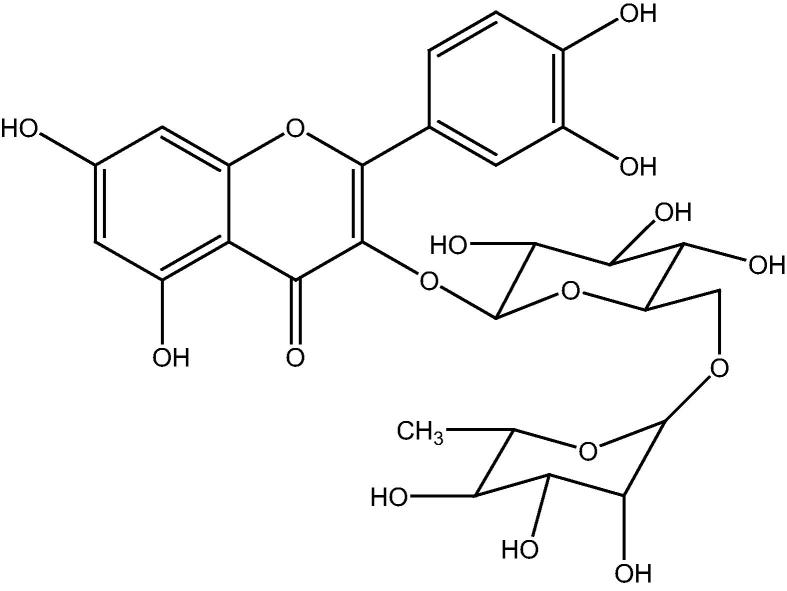
Rutin (Fig. 2) is an important flavonoid and almost an integral part of our daily diet. It is a vital constituent of many food items, vegetables, and beverages. Rutin possesses hepatoprotective, gastroprotective, anti-inflammatory and anti-diabetic effects (Hosseinzadeh and Nassiri-Asl, 2014). Some other, biological activities of rutin include antitumor, anti-asthmatic, antioxidant and antimicrobial. Due to the tremendous therapeutic potential rutin is considered as one of the essential flavonoids in pharmaceutical industry (Lee, 2013).
Figure 2.
Chemical structure of rutin.
During last few decades, herbs and herbal products have largely been adapted as complementary and alternative medicines. Natural products have already been proved to be a potential source for the treatment of many diseases but usually lack scientific verification and data to support. Due to deficiency of scientific data the World Health Organization (WHO) has given the emphasis on scientific evaluation of the effectiveness of plant-based drugs (Lee, 2013). Several methods reported the quantification of rutin, and these include HPTLC, RP-HPTLC (Al Ajmi et al., 2015), HPLC-ED (Dilberovic et al., 2010), LC-MS/MS (Ćirić et al., 2012), and electroanalytical methods (Mirel et al., 2008) in various samples of plant extracts, marketed formulations, and biological fluids, but no reporting was found regarding the estimation of ent-phyllanthidine along with rutin by chromatographic methods. Keeping in view a wide spectrum of biological activities and increasing demand for natural products this study was performed for concurrent analysis of rutin and ent-phyllanthidine in the methanol extract of F. virosa. This study was the first reporting of simultaneous quantification of above mentioned two biomarkers by a validated HPTLC method. The validation of developed method was done according to International Conference on Harmonization (ICH) guideline (ICH, Geneva, 2006).
2. Materials and methods
2.1. Materials
The plant material (F. virosa) was collected during spring season from the southern part of Saudi Arabia and identified by Dr. Muhammad Yousaf, Department of Pharmacognosy, College of Pharmacy, King Saud University (KSU), Riyadh, Saudi Arabia (SA). A specimen voucher (Number 15745a) was preserved in the departmental herbarium, Department of Pharmacognosy, College of Pharmacy, KSU, Riyadh, SA.
2.2. Apparatus, reagents and reference standards
Standards of rutin and ent-phyllanthidine were obtained from Sigma-Aldrich. Reagents and solvents of analytical grade were purchased from Fluka Chemicals (Busch, Switzerland) and Acros Organics (Hamilton, NJ), respectively. Pre-coated reverse phase HPTLC glass plates of silica gel 60 RP-18F254 were purchased (E. Merck, Germany). The standard and the extracts were applied to RP-HPTLC plates band wise with the help of CAMAG automatic TLC sampler-4 and developed in automatic development chamber-II. Scanning and documentation of developed RP-HPLTLC plate were done by CATS 4 and CAMAG TLC Reprostar 3, respectively.
2.3. Extraction conditions and preparation of sample
The extraction of F. virosa was done according to the method proposed for the estimation of rutin by Al Ajmi et al. (2015) in which the aerial parts of selected plant were dried in shade and coarsely powdered. 50 g of the powdered plant material was packed in a muslin fabric and extracted by Soxhletation using methanol (95%) as extractive solvent. The obtained extract (FVME) was concentrated by using rotary evaporator and finally dried. The percent yield of extract was found to be 9.14% w/w. The selection of an extractive solvent (methanol) was based on the solubility of marker compounds.
2.4. Preparation of reference standards
Methanol was used to prepare a stock solution of reference standards for ent-phyllanthidine and rutin (1 mg mL−1). To furnish the concentration of 100 μg mL−1, one milliliter of the stock solutions was again diluted to 9 mL each of methanol. For calibration, standard solution (2–16 μL) of ent-phyllanthidine and (1–14 μL) of rutin was applied on RP-HPTLC plate to keep the concentration in the range of 200–1600 ngband−1 and 100–1400 ngband−1, respectively.
2.5. HPTLC instrumentation and chromatographic conditions
The quantitative analysis of ent-phyllanthidine and rutin was carried out on 20 × 10 cm RP-HPTLC plate. Automatic HPTLC Sampler 4 (ATS4) fitted with Hamilton Gastight Syringe (1700 Series) of volume 25 μL was used to apply the samples as well as standard on RP-HPTLC plate. The samples were applied to the plate at the rate of 160 nL/s. The plate was developed in previously saturated (saturation time 20 min at 25 °C with vapors of mobile phase) automatic developing chamber (ADC2) in linear ascending mode with acetonitrile: water (4:6 V/V) used as mobile phase. After development, the plate was dried at room temperature and derivatized with freshly prepared Dragendorff reagent for detection of ent-phyllanthidine, scanned, and quantified at 310 nm wavelength in absorbance mode with CATS 4 operated by WinCATS software (Version 1.2.0).
2.6. Preparation of calibration graph
A series of spots of different volumes were applied to prepare the calibration graph of ent-phyllanthidine and rutin. Different volumes of spots have provided a different concentrations of biomarkers per spot for making calibration graph. Area and height versus quantity per spot were taken into consideration for preparing the calibration graph.
2.7. Method development
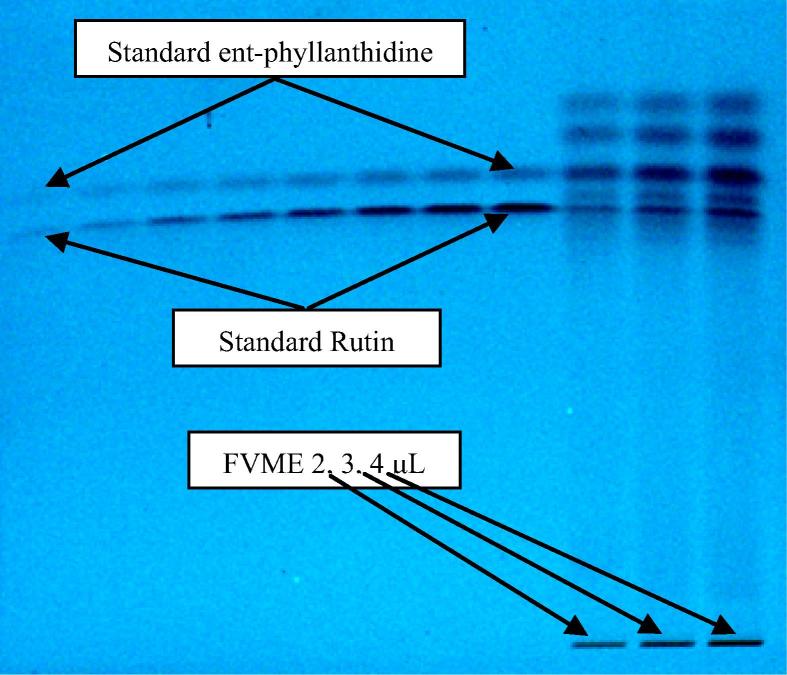
After trying several combinations of mobile phase and its optimization, the chromatogram was developed. The selected mobile phase [ACN: H2O (4:6 V/V)] provides the best resolution. The same mobile phase (Fig. 3) has been employed for the separation of methanol extracts of the sample (FVME) applied as three bands of 2, 3 and 4 μL volume. The optimized saturation time was observed as 20 min. The analysis was done densitometrically at absorption maxima of wavelength 310 nm in absorbance mode.
Figure 3.
Picture of developed RP-HPTLC plate at 254 nm wavelength.
2.8. Method validation
As per ICH guidelines the parameters such as linearity range, precision, accuracy, robustness, recovery, limit of detection (LOD) and limit of quantification (LOQ) have been considered for the validation of the proposed method. Replicate analyses (n = 6) of samples at three quality control (QC) levels have been performed to evaluate accuracy and precision. These three QC levels were low, medium and high with a concentration of 200, 400 and 600 ngband−1, respectively for ent-phyllanthidine and 150, 300 and 600 ngband−1, respectively for rutin.
The intraday assay was repeated for three different days to determine the inter-day precision and accuracy. Percentage of the coefficient of variation (% CV) of measured concentration for each calibration level was used to express precision while percentage recovery was used to express accuracy. The robustness of the proposed method was evaluated in triplicate at 300 ngband−1 by introducing some deliberate changes to the volume of mobile phase, the composition of mobile phase and duration of saturation. The results were evaluated in terms of relative standard deviation (RSD %) and standard error of peak areas. The mobile phases prepared from acetonitrile: water (4:6, V/V) in different proportions (4.5:5.5, V/V; 3.5:6.5, V/V; 4.25:5.75, V/V) were used for chromatography. The duration of saturation and mobile phase volume investigated was 20 ± 10 min (10, 20 and 30 min) and 20 ± 2 mL (18, 20 and 22 mL), respectively.
The calculation of LOD and LOQ was made on the basis of standard deviation (SD) of the response and the slope (s) of the calibration graph using formulae [LOD = 3.3 (SD/S) and LOQ = 10 (SD/S)]. The study of recovery was made by applying the method to drug samples to which known quantity of the marker corresponding to 50%, 100% and 150% of the ent-phyllanthidine and rutin had been added. The analysis of each level was made in triplicate. The utility of this method was exploited to check the recovery of ent-phyllanthidine and rutin at different levels in the extracts.
3. Results and discussion
3.1. Method development and validation
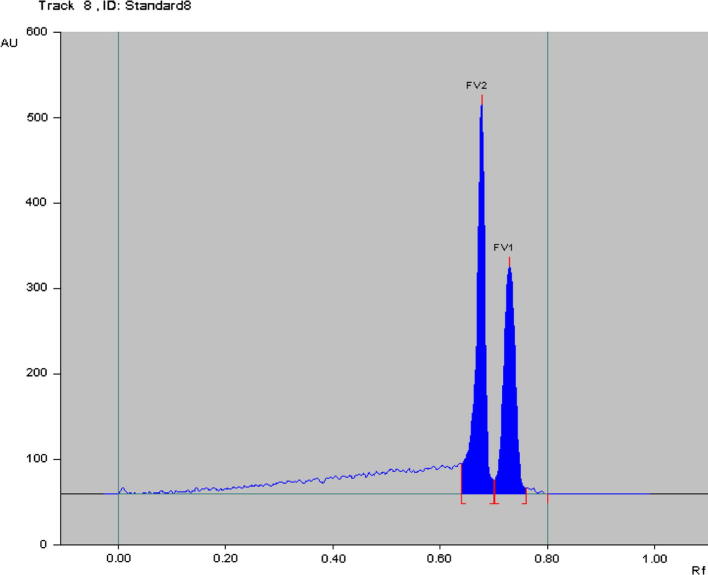
The selected mobile phase [ACN: H2O (4:6 V/V)] gives compact, symmetrical, sharp and high-resolution bands of ent-phyllanthidine (FV1) and rutin (FV2) at Rf 0.73 ± 0.01 and 0.68 ± 0.01, respectively (Fig. 3). The suggested method was found to be specific with excellent baseline resolution (Fig. 4). The outcome of this experiment can be recommended as a maiden method for simultaneous quantification of ent-phyllanthidine and rutin in F. virosa as well as in herbs and herbal formulations containing ent-phyllanthidine and rutin together by a cost effective, simple and sensitive RP-HPTLC method.
Figure 4.
Chromatogram of standards FV1 (ent-phyllanthidine; 1600 ng/spot) and FV2 (Rutin; 1400 ng/spot) at 310 nm wavelength; mobile phase: ACN: H2O (4:6 V/V) in RP-HPTLC.
3.2. RP-HPTLC study of FVME
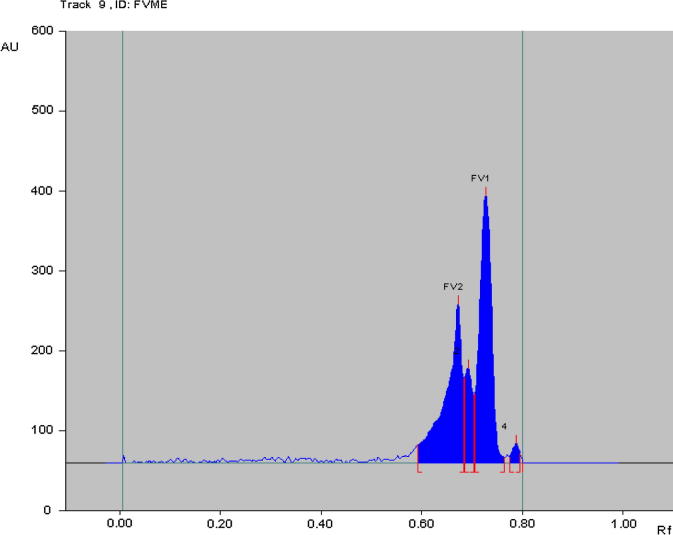
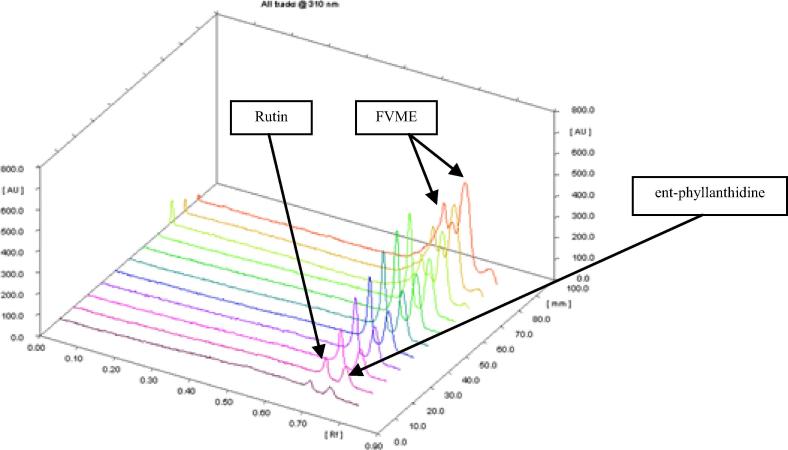
The proposed method was utilized for the quantitation of ent-phyllanthidine and rutin in FVME (Fig. 5). Three volumes 2, 3 and 4 μL of FVME were applied on RP-HPTLC plate for quantitative analysis. The bands of ent-phyllanthidine and rutin in sample extract (FVME) were confirmed by the 3D display of all tracks of standards as well as a sample (Fig. 6). The concentration of ent-phyllanthidine and rutin in the FVME was found to be 9.121 ± 0.02 and 1.018 ± 0.04% (w/w), respectively. This is the maiden method developed for concurrent estimation of biomarkers ent-phyllanthidine and rutin in F. virosa collected from Saudi Arabia.
Figure 5.
Chromatogram of FVME (2 μL) [ent-phyllanthidine (FV1), Rf = 0.73 and Rutin (FV2), Rf = 0.68] at 310 nm wavelength; mobile phase: ACN: H2O (4:6 V/V) in RP-HPTLC.
Figure 6.
3D-display of all tracks @310 nm developed on RP-HPTLC plate; mobile phase: ACN: H2O (4:6 V/V).
3.3. Method validation
Linear regression and correlation coefficient were used for validation of linearity for the biomarkers ent-phyllanthidine and rutin. The calibration graph was made with eight concentrations of ent-phyllanthidine and rutin and showed the good linear relationship in the range of 200–1600 ngband−1 and 100–1400 ngband−1, respectively. The linear regression equations and r2 values for the standard reference ent-phyllanthidine and rutin were observed as Y = 2.722x + 209.141 & 0.998 and Y = 3.567x + 316.119 & 0.997, respectively, which revealed a significant linearity response for developed method (Table 1). The mean value with ± SD of the slope and intercept was 2.722 ± 0.003 and 209.141 ± 0.011 for ent-phyllanthidine and 3.567 ± 0.002 and 316.119 ± 0.013 for rutin. No substantial difference was detected on the slopes of standard plots (P > 0.05). Table 2 presents intra- and interday precision and accuracy of the assay for ent-phyllanthidine and rutin at triplicate QC levels (200, 400 and 600 ngband−1 and 150, 300 and 600 ngband−1), respectively. Both intra- and interday precisions were determined regarding percent of coefficient of variation (% CV). Intra- and interday precisions (n = 6) for ent-phyllanthidine and rutin were found to be in the range of 1.31–1.84% and 1.37–1.85%, and 1.24–1.61% and 1.45–1.80%, respectively, which demonstrated the good precision of proposed method. However, the intra- and interday accuracies of ent-phyllanthidine and rutin were observed as 98.5–99.5% and 98.3–98.7% and 98.1–99.6% and 97.4–99.4%, respectively. These results indicated the accuracy of the proposed method. In Table 3, the SD and % RSD were calculated at 300 ngband−1 concentration level of ent-phyllanthidine and rutin. The robustness of the proposed method was depicted by the low values of SD and % RSD obtained after putting into small deliberate changes in the optimized method. LOD/LOQ for ent-phyllanthidine and rutin were observed as 48/145 ngband−1 and 39/117 ngband−1, respectively (Table 1). The observations for robustness indicated that the proposed method exhibited an excellent sensitivity for the quantification of above compounds. After spiking good recoveries were observed at three QC levels of ent-phyllanthidine and rutin. As shown in Table 4, after sample treatment and application excellent recoveries for ent-phyllanthidine (98.4–99.6%) and rutin (99.3–99.7%) were witnessed.
Table 1.
Rf, linear regression data for the calibration curve and sensitivity parameter for ent-phyllanthidine (FV1) and rutin (FV2).
| Parameter | ent-Phyllanthidine | Rutin |
|---|---|---|
| Rf | 0.73 ± 0.01 | 0.68 ± 0.01 |
| Linearity range (ngband−1) | 200–1600 | 100–1400 |
| Regression equation | Y = 2.722x + 209.141 | Y = 3.567x + 316.119 |
| Correlation coefficient (r2) | 0.998 | 0.997 |
| Slope ± SD | 2.722 + 0.003 | 3.567 ± 0.002 |
| Intercept ± SD | 209.141 + 0.011 | 316.119 ± 0.013 |
| Standard error of slope | 0.003 | 0.002 |
| Standard error of intercept | 0.011 | 0.013 |
| LOD | 48 ngband−1 | 39 ngband−1 |
| LOQ | 145 ngband−1 | 117 ngband−1 |
Table 2.
Precision and accuracy of ent-phyllanthidine (FV1) and rutin (FV2).
| Marker compound | Nominal concentration (a) | Concentration obtained ± SD (b) | Precision (c) (CV, %) | Accuracy (d) |
|---|---|---|---|---|
| Intraday batch | ||||
| FV1 | 200 | 197.0 ± 3.62 | 1.84 | 98.5 |
| 400 | 395.8 ± 5.70 | 1.44 | 98.9 | |
| 600 | 596.9 ± 7.83 | 1.31 | 99.5 | |
| FV2 | 150 | 147.2 ± 2.37 | 1.61 | 98.1 |
| 300 | 296.5 ± 4.11 | 1.39 | 98.8 | |
| 600 | 597.8 ± 7.43 | 1.24 | 99.6 | |
| Interday batch | ||||
| FV1 | 200 | 196.6 ± 3.65 | 1.85 | 98.3 |
| 400 | 394.8 ± 6.53 | 1.65 | 98.7 | |
| 600 | 591.3 ± 8.15 | 1.37 | 98.5 | |
| FV2 | 150 | 146.1 ± 2.12 | 1.45 | 97.4 |
| 300 | 294.3 ± 5.32 | 1.80 | 98.1 | |
| 600 | 596.1 ± 10.47 | 1.75 | 99.4 | |
a: Concentration in ngband−1; b: Mean from six determinations (n = 6), c: Precision as coefficient of variation (CV, %) = [(standard deviation)/(concentration found)] × 100, d: Accuracy (%) = [(concentration found)/(nominal concentration)] × 100.
Table 3.
Robustness of the method.
| Optimization condition |
ent-Phyllanthidine |
Rutin |
||
|---|---|---|---|---|
| SD | % RSD | SD | % RSD | |
| Mobile phase from acetonitrile: water (4:6 V/V) | ||||
| (4.5:5.5, V/V; 3.5:6.5, V/V; 4.25:5.75, V/V) | 6.46 | 0.027 | 6.43 | 0.021 |
| Mobile phase volume (18, 20 and 22 mL) | 5.61 | 0.019 | 5.06 | 0.018 |
| Duration of saturation (10, 20 and 30 min) | 3.31 | 0.012 | 4.18 | 0.015 |
Table 4.
Recovery studies of ent-phyllanthidine (FV1) and rutin (FV2).
| Marker compound | Concentration added to analyte (%) | Theoretical quantity (ng) | Added quantity (ng) | Detected quantity (ng) ± SD | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| ent-Phyllanthidine | (FV1) | 300 | ||||
| 50 | 450 | 443.1 ± 8.12 | 98.4 | 1.83 | ||
| 100 | 600 | 593.8 ± 6.19 | 98.9 | 1.04 | ||
| 150 | 750 | 746.7 ± 7.86 | 99.6 | 1.05 | ||
| Rutin (FV2) | 300 | |||||
| 50 | 450 | 446.9 ± 6.31 | 99.3 | 1.41 | ||
| 100 | 600 | 597.1 ± 6.21 | 99.5 | 1.04 | ||
| 150 | 750 | 748.11 ± 8.78 | 99.7 | 1.17 | ||
According to the available literature, ent-phyllanthidine seems to be a very promising compound for the treatment of liver diseases and leishmaniasis. Along with the development of a method for quantification, the findings of this experiment also revealed that Flueggea virosa is a rich source of ent-phyllanthidine (9.121 ± 0.02%w/w). The presence of ent-phyllanthidine in such a substantial percentage justifies the use of F. virosa as folkloric medicine for the treatment of hepatitis (Ezeonwumelu et al., 2013). Natural products especially functional food appear to be good alternatives for making the life easy and healthy. Some examples of fruits and vegetables are proved to be a rich source of a bioflavonoid rutin. Bioflavonoids possess a broad spectrum of biological activities. Rutin is a very common component of many fruits and vegetables. A wide spectrum of physiological and pharmacological activities of rutin in mammals either in vivo or in vitro has been scientifically proved (Lee, 2013). It is reported that more than 100 registered medicinal preparations contain rutin in their formulations (Lee, 2013). Due to awesome scavenging potential rutin is considered as potent anti-oxidant (Duthie and Dobson, 1999, Nagasawa et al., 2002, Abraham et al., 2008). Being excellent anti-oxidant rutin acts as anti-inflammatory (Guardia et al., 2001), anti-tumor (Ramanathan et al., 1993), anti-microbial and anti-asthmatic agent (Lee, 2013).
In many of the cases, the results of succeeding clinical trials of formulations do not show coherence with the previous results which may be due to the nonuniform process of extraction, the usage of different species, climatic variations and the differences at the site of harvest, which may directly affect the quantity of active constituents in the extract (Siddiqui et al., 2014). The proposed HPTLC method may provide a reliable tool for monitoring and production of standardized extracts, effective herbal formulations as well as accurate grading of crude drugs based on the levels of concentration of ent-phyllanthidine and rutin.
4. Conclusion
The experimental findings suggested a simple and sensitive method for the simultaneous detection and quantification of ent-phyllanthidine and rutin in F. virosa. It is revealed from this study that F. virosa is an excellent source of ent-phyllanthidine which supports its traditional use in liver diseases and can also be recommended to treat leishmaniasis.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the research through the research group project number RGP-073.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nasir A. Siddiqui, Email: nasiratksu@gmail.com.
Ramzi A. Mothana, Email: r_mothana@yahoo.com.
Adnan J. Al-Rehaily, Email: ajalreha@ksu.edu.sa.
Perwez Alam, Email: alamperwez007@gmail.com.
Muhammad Yousaf, Email: yousaf_phd@yahoo.com.
Sarfaraz Ahmed, Email: ahmsarfaraz@gmail.com.
Abdulrahman Alatar, Email: aalatar@ksu.edu.sa.
References
- Abraham L.C.N., Masakuni T., Isao H., Hajime T. Antioxidant flavonoid glycosides from the leaves of Ficus pumila L. Food Chem. 2008;109:415–420. doi: 10.1016/j.foodchem.2007.12.069. [DOI] [PubMed] [Google Scholar]
- Al Ajmi M.F., Alam P., Siddiqui N.A., Basudan O.A., Hussain A. Quantitative analysis of biomarker rutin in different species of genus Ficus by validated NP and RP-HPTLC methods. Pak. J. Pharm. Sci. 2015;28(6 Suppl.):2213–2220. [PubMed] [Google Scholar]
- Ćirić A., Prosen H., Jelikić-Stankov M., Đurđević P. Evaluation of matrix effect in determination of some bioflavonoids in food samples by LC-MS/MS method. Talanta. 2012;15(99):780–790. doi: 10.1016/j.talanta.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Dilberovic B., Salihovic M., Krvavac J., Toromanovic J., Tahirovic I., Sofic E. Quantification of rutin in some plants of family Lamiaceae using high performance liquid chromatography with electrochemical detection. Planta Med. 2010;76(12):289–291. [Google Scholar]
- Duthie S.J., Dobson V.L. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur. J Nutr. 1999;38:28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- Ezeonwumelu J.O.C., Matuki E.K., Ajayi A.M., Okoruwa A.G., Tanayen J.K., Adiukwu C.P. Phytochemical screening, acute toxicity and analgesic properties of aqueous extract of Flueggea virosa’s root in rats. Ibnosina J. Med. Biomed. Sci. 2013;5(1):15–21. [Google Scholar]
- Guardia T., Rotelli A.E., Juarez A.O., Pelzer L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–687. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Invest. 2014;37(9):783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization, 2006. ICH guideline Q2B on validation of analytical procedures: Text and Methodology, Geneva.
- Lee S.C. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013;150:805–817. doi: 10.1016/j.jep.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Magaji M.G., Anuka J.A., Abdu-Aguye I., Yaro A.H., Hussaini I.M. Behavioural effects of the methanolic root bark extract of Securinega virosa in rodents. Afr. J. Tradit. Complement. Altern. Med. 2008;5:147–153. doi: 10.4314/ajtcam.v5i2.31266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirel S., Oprean R., Mirel V., Sandulescu R. Voltametric determination of rutin in pharmaceutical dosage forms. Farmacia LVI. 2008;2:196–203. [Google Scholar]
- Moraes L.S., Donza M.R.H., Rodrigues A.P.D., Silva B.J.M., Brasil D.S.B., Zoghbi M.G.B., Andrade E.H.A., Guilhon G.M.S.P., Silva E.O. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae) Molecules. 2015;20:22157–22169. doi: 10.3390/molecules201219829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Tabata N., Ito Y., Nishizawa N. Suppression of early and advanced glycation by dietary water-soluble rutin derivative in diabetic rats. Int. Congr. Ser. 2002;1245:403–405. [Google Scholar]
- Ramanathan R., Das W.P., Tan C.H. Inhibitory effects of 2-hydroxy chalcone and other flavonoids on human cancer cell-proliferation. Int. J Oncol. 1993;3:115–119. doi: 10.3892/ijo.3.1.115. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.A., Alam P., Al-Rehaily A.J., Al-Oqail M.A., Parvez M.K. Simultaneous quantification of biomarkers Bergenin and Menisdaurin in the methanol extract of aerial parts of Flueggea virosa by validated HPTLC densitometric method. J. Chromatogr. Sci. 2015;53:824–829. doi: 10.1093/chromsci/bmu231. [DOI] [PubMed] [Google Scholar]
- Siddiqui N.A., Basudan O.A., Alajmi M.F., Al-Rehaily A.J., Alqasoumi S.I., Abdel-Kader M.S., Alam P., Donia A.R.M., Alam P. Estimation of germanicol by validated HPTLC densitometric method in leaves of different species of genus Ficus. Asian J. Chem. 2014;26(22):7638–7642. [Google Scholar]
- Wang G.C., Liang J.P., Wang Y., Qian L.I., Wen-Cai Y.E. Chemical constituents from Flueggea virosa. Chin. J. Nat. Med. 2008;6(4):251–253. [Google Scholar]