Abstract
Herbal medicines have been played an important role in the human civilization since very ancient time as a food, cloth, medicine and other aspects. Some of the important drugs in the modern medicine were derived from the natural sources such as aspirin, digitalis, quinine, vincristine, vinblastine etc. Hispidulin (4′, 5, 7-trihydroxy-6-methoxyflavone) is a flavones derivative found in plant such as Grindelia argentina, Arrabidaea chica, Saussurea involucrate, Crossostephium chinense, Artemisia and Salvia species. Hispidulin have antioxidant, antifungal, anti-inflammatory, antimutagenic, and antineoplastic properties. So far, various analytical methods have been investigated and developed for detection of hispidulin in the plant materials. Productions of hispidulin through different tissue culture techniques have been also investigated. Present review summarized medicinal uses, pharmacological activities and analytical aspects of hispidulin. From the above mentioned aspects, we can conclude that, this review will be helpful to the researcher in the field of natural product for the development of novel molecule for the treatment of different disorders.
Keywords: Analytical aspects, Herbal medicine, Hispidulin, Medicinal aspects, Pharmacological activities
Graphical abstract
1. Introduction
Herbs play an important role in the human civilization as they have been used for different purpose in different field such as medicine, nutraceuticals, perfumery, beverages, fragrances, cosmetics and dyeing industry. From the ancients times herbs were mainly used for the treatments of various disorders until the synthetic drugs developed in the world. More than 40% of prescription drugs in the world were mainly derived from herbal source. Herbs, vegetables and fruits contain numerous phytochemicals such as phenolic compounds, nitrogen compounds, carotenoids, ascorbic acid etc.1 Different color, flavor and smell of plants were mainly due to the presence of different phytoconstituent present in the plants. They play an important role in the plant's defense mechanism against various diseases.2 For the search of better therapeutic goal, plants are still considered as one of the important sources of materials. More than 50% of the prescribed drugs in the Europe and USA are derived from natural sources such as plants or their derivatives.3 Many of these plants products and their crude extracts were used in different types of traditional medicine. Medicinal plants play a key role in health care as more than 80% of the world's populations relying on the traditional medicine for their primary health care. In spite of tremendous development in the field of allopathy, medicinal plants and their derived products are still used in the modern medicine throughout the world. In India more than 7300 plant species are used in traditional health care systems for the treatment of different disorders. The most important chemical constituents of plants are alkaloids, tannin, flavonoid and phenolic compounds etc. In recent years, treatment of infectious disease using antimicrobial drugs has developed multiple drug resistance.4 Medicinal herbs have been used as remedy for the treatment of pain throughout history including some of most important analgesic prototypes i.e. salicylic acid and morphine was originally derived from plant sources. Natural products are believed to be an important source of new chemical substances for the developments of Nobel medicine for the treatment of various disorders.5 Plants play a dominant role in the maintenance of human health since ancient times till today. According to World Health Organization (WHO), medicinal plants would be the best source to get a variety of drugs. Plants develop different bioactive molecules, making them a rich source of different types of medicinal compound. About 80% of individuals from developed countries use traditional medicine for their primary healthcare needs, which contain different compound derived from medicinal plants.6 For the determination of identity, purity and strength of the drug phytochemical standards are generally used in the herbal field. These parameters are also used to evaluate its genuine nature compared to the adulterated drugs. Phytochemical evaluation also plays an important role in the possible steps of adulteration.7
2. An overview of hispidulin
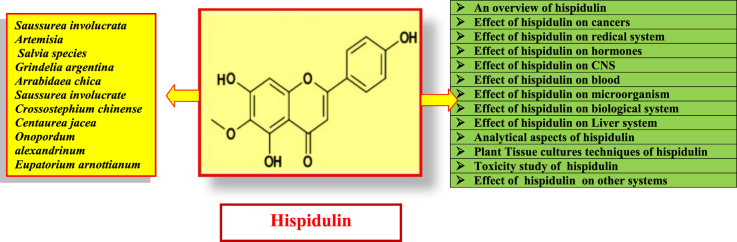
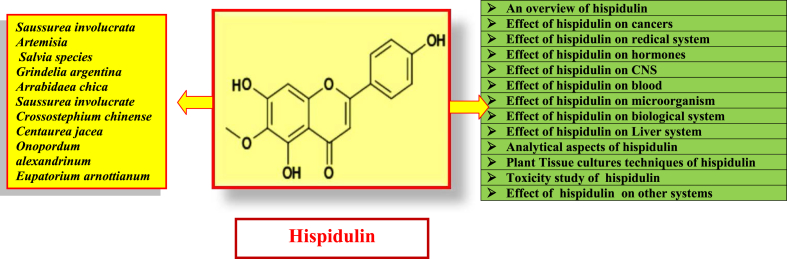
Hispidulin (4′, 5, 7-trihydroxy-6-methoxyflavone) is a naturally occurring flavone found in different plant materials such as Saussurea involucrata Kar. et Kir., a rare traditional Chinese medicinal herb, several Artemisia and Salvia species. Several in vitro studies have demonstrated its potent antioxidative, antifungal, anti-inflammatory, antimutagenic, and antineoplastic properties.8, 9 Recently, hispidulin is identified as a potent ligand of the central human benzodiazepine (BZD) receptor in vitro. It also acts as a partial positive allosteric modulator at γ-aminobutyric acid (GABA) receptors, penetrates the blood-brain barrier and possesses anticonvulsant activity in the central nervous system.8, 9 Hispidulin (Fig. 1) is the active compound which is also proven to be antimycobacterial, antiasthma, antimicrobial, antiproliferative, and insect larvicidal. This natural flavone is reported to be 100-fold more potent than theophylline in its property of inhibiting platelet aggregation.10
Fig. 1.
Chemical structure and overview of hispidulin.
3. Pharmacological activities
3.1. Effect of hispidulin on cancers
Treatments of cancer have focused the main attention and interest of researchers due to their great impact on the human population's and health. A considerable ratio of deaths (2–3%) recorded worldwide annually due to different types of cancer.3 Effect of hispidulin on its anti-tumor effect of Temozolomide (TMZ) in glioblastoma was studied and revealed that hispidulin enhanced the anti-tumor activity of TMZ in glioblastoma because of its inhibiting effect on cell proliferation and cell apoptosis induction.11 Effect of the hispidulin, with sunitinib on renal cell carcinoma (RCC) cell proliferation in vitro and on in vivo tumor growth was studied. Hispidulin dose-dependently inhibited proliferation and induced apoptosis in both of the tested RCC cell lines. Inhibiting pStat3 signaling was found to be one of the main mechanisms for its antitumor activity. The result revealed that the combination treatment will be better therapeutic option for patients with RCC.12 Gastric cancer is one of the most common malignant cancers due to poor prognoses and high mortality rates worldwide. Hispidulin inhibits the growth of gastric cancer cells through induced G1/S phase arrest and apoptosis in time- and concentration-dependent manners.13 In another study, antiproliferative effects of hispidulin isolated from Inula viscosa (L.) were tested and were found to be active at the tested concentration.3 Hispidulin significantly inhibited human pancreatic tumor growth in xenograft mice when treated at a dosage of 20 mg/kg daily. Further hispidulin also inhibited vascular endothelial growth factor (VEGF)-induced cell migration, invasion, and capillary-like structure formation in a dose-dependent manner.14 Hispidulin potentiated the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human ovarian cancer cells and converted TRAIL-resistant cells to TRAIL-sensitive cells. Moreover hispidulin also downregulated the expression of Mcl-1, Bcl-2 and Bcl-xL.15 Glioblastoma multiforme (GBM) is the most common and lethal type of primary brain tumor. Treatment of hispidulin resulted in dose-dependent inhibition of GBM cellular proliferation. Moreover, hispidulin-activated AMPK decreases the activity and expression of lipogenic enzymes, such as fatty acid synthase and acetyl-CoA carboxylase.8 Effect of hispidulin on the cytotoxicity of the sesquiterpene lactone helenalin was studied in the human lung carcinoma cell line GLC4 using the microculture tetrazolium (MTT) assay. Hispidulin showed their modulating effect on helenalin-induced cytotoxicity in the significant range.16 Mutagenicity and antimutagenicity of hispidulin were performed using the liquid preincubation method of the Salmonella test. At the highest dose tested, compounds showed no mutagenicity and no cytotoxicity toward Salmonella typhimurium strains TA98 and TA100 either in the presence or absence of S9 mix.17 Hispidulin were evaluated for their inhibitory activity against LPS/IFN-γ-induced NO production in RAW 264.7 macrophages and for their cytotoxic activities against the human leukemic cell line CCRF-CEM and MRC-5 lung fibroblasts. Hispidulin markedly reduced LPS/IFN-γ-induced NO production in the tested cell lines.18 In another study, hispidulin induces cell death in a dose and time-dependent manner in HepG2 cells whereas no toxic reaction was observed in normal human liver cells. Observed effect of hispidulin induces apoptosis in HepG2 cells suggested that the pro-apoptotic effect of Hispidulin was mediated through mitochondrial dysfunction and inhibition of P13k/Akt signaling pathway.19
3.2. Effect of hispidulin on radical system
Effects of hispidulin on the oxidative metabolism of isolated rat liver mitochondria were investigated. Hispidulin inhibited enzymatic activities between complexes I and III of the respiratory chain. The results indicate that hispidulin as an uncoupler of oxidative phosphorylation, is able to release iron from ferritin.20 Hispidulin, were evaluated for free radical scavenging activity and tyrosinase inhibitory effect in cell-free systems for its antioxidant potentials was also evaluated. Hispidulin showed strong antioxidant potential at the tested concentration.21 Hispidulin isolated from Indian medicinal plants was tested for their activity as inhibitors of microsomal lipid peroxidation and scavengers of oxygen free radicals in vitro as well as in a model of xenobiotic toxicity in mouse. Hispidulin inhibited lipid peroxidation in vitro and further treatment of mice with hispidulin after bromobenzene intoxication decreased serum glutamate-pyruvate transaminase activity.22
3.3. Effect of hispidulin on hormones
Hispidulin have been proven to have estrogen-like and antiosteoporotic activity and can be potentially used for the treatment of osteoporosis.23 Effect of hispidulin on ovariectomy (OVX)-induced bone loss in mice was investigated. Female mice subjected to OVX were treated with Hispidulin for 8 weeks. Hispidulin treatment effectively prevented OVX-induced body weight loss and attenuated OVX-induced bone loss.24 In another study hispidulin significantly inhibited osteoclast activity in RAW 264.7 cell as well as stimulated the ALP activity of MC3T3E1 cells. Hispidulin was also found to inhibit RANKL-induced activation of Jun N-terminal kinase (JNK) and p38, in addition to NF-κB in vitro experiment.25
3.4. Effect of hispidulin on central nervous system
Hispidulin has been reported to have an antiepileptic profile. Hispidulin inhibited the release of glutamate evoked by the K⁺ channel blocker 4-aminopyridine (4-AP). Hispidulin inhibits glutamate release from cortical synaptosomes in rats through the suppression of presynaptic voltage-dependent Ca2+ entry and ERK/synapsin I signaling pathway.26 Hispidulin from Cirsium rivulare was studied for anxiolytic and pro-cognitive properties and results suggest that the flavonoids from C. rivulare possess anxiolytic and pro-cognitive effects.27 In another study, hispidulin was able to penetrate the blood-brain barrier and found to possess antiepileptic activity. Effect of hispidulin administration on bupivacaine-induced neurotoxicity was also studied. Treatment with hispidulin significantly attenuated bupivacaine-induced cell injury. In addition, hispidulin treatment also increased the levels of phospho-AMPK and phospho-GSK3β and attenuated bupivacaine-induced loss in mitochondrial membrane potential.28
3.5. Effect of hispidulin on blood
Hispidulin and theophylline inhibited platelet aggregation triggered by adenosine-5′-monophosphate, arachidonic acid, paf-acether and collagen. Hispidulin was 100-fold more potent than theophylline. A threshold concentration of PGE1 did not modify the anti-aggregatory effect of hispidulin but potentiated the effect of theophylline. These data suggest that hispidulin could inhibit platelet aggregation by elevating cAMP levels by a mechanism different from that of theophylline or PGE1.29 In small concentrations hispidulin caused concentration-dependent contraction of isolated guinea-pig ileum and only mild relaxation of guinea-pig tracheal rings. Larger concentrations caused concentration-dependent relaxation of the ileum and the trachea. All the effects on the ileum and the trachea are reversible upon removal of the compound. These observations suggest that hispidulin may interfere with Ca2+ binding to the Ca2+-receptor protein(s) in the smooth muscle cell and with the agonist-induced Ca2+-release from intracellular stores.30
3.6. Effect of hispidulin on microorganism
In vitro trypanocidal and leishmanicidal activities of the flavonoids hispidulin, from Ambrosia tenuifolia, are investigated and found to be active. The IC(50) values for hispidulin on Trypanosoma cruzi epimastigotes were 46.7 μM. On trypomastigotes, the IC(50) values were 62.3 μM for hispidulin. Hispidulin was found to be more active on promastigotes of Leishmania Mexicana.31 Hispidulin was extracted from the ethanolic extract of the aerial parts of Baccharis uncinella C. DC. and was found to have inhibitory effect against trypomastigotes of T. cruzi.32
3.7. Effect of hispidulin on biological system
Hispidulin as well as their metabolites are present in the Saussurea involucrate. It inhibits nonoxidative advanced glycation end products, which is one of the mediators involved in physiological inflammation.33 To elucidate the metabolism of hispidulin in the large intestine, its biotransformation by the pig caecal microflora was studied. Despite of the flavonoid subclass, the presence of a hydroxy group at the 4′-position is responsible for its fast breakdown. However an additional hydroxy group at the B-ring did not affect the degradation level.34
3.8. Effect of hispidulin on liver system
The effects of hispidulin on bromobenzene-induced hepatotoxicity in mice were investigated. Hispidulin at 50–150 mg/kg i.p. dose level were compared to the reference compound N-acetyl-l-cysteine for inhibition of liver injury and lipid peroxidation. Hispidulin at the highest dose was able to counteract reduced glutathione depletion induced by bromobenzene in starved mice. This hepatoprotective effect of hispidulin could be due to its antioxidant potentials.35
3.9. Effect of hispidulin on inflammation
Hispidulin, nepetin and jaceosidin were isolated from Eupatorium arnottianum Griseb and their anti-inflammatory activities were investigated in the TPA mouse ear edema and were found to be active. Nepetin and jaceosidin reduced the TPA mouse ear edema and also inhibited the NF kappaB induction.36 Topical anti-inflammatory activity of bioactivity-guided fractionation of methanolic extract of the leaves of Santolina insularis and all the isolated compounds including hispidulin were investigated in croton oil-induced dermatitis in mouse ear. Among all the active constituents and the crude fraction the most active compound was found to be luteolin and prevented ear edema more effectively compared to the standard drug indomethacin.37
4. Analytical aspects of hispidulin
High performance liquid chromatography (HPLC) with photodiode array (PDA) and mass spectrometry (MS) detection tool were used for the identification and quantification of flavonoids and phenolic acid derivatives in Clerodendrum petasites S. Moore. Hispidulin was found to be one of the main active constituents present in the C. petasites extract with other compounds.38 Nine compounds were successfully separated from Salvia plebeia R.Br. using two-step high-speed counter-current chromatography with three elution modes. Elution–extrusion counter-current chromatography was applied in the first step, while classical counter-current chromatography and recycling counter-current chromatography were used in the second step. The separation yielded nine compounds including hispidulin in the Salvia plebeian extract.39 High-speed countercurrent chromatography (HSCCC) was successfully applied for the isolation and purification of flavonoids from Herba salviae Plbeiae using stepwise and dual-mode elution with a pair of two-phase solvent systems. Hispidulin along with other phytoconstituents were separated from the H. salviae sample in a one-step separation, with more than 95% purities as determined by high-performance liquid chromatography.40 A bioassay-guided phytochemical analysis of the ethanolic extract of Grindelia argentina Deble and Oliveira-Deble lead to the isolation of hispidulin. Their structures were determined by extensive 1D- and 2D-NMR experiments along with mass spectrometry and chemical evidence.18
The phenolic composition of Arrabidaea chica leaves was studied through liquid chromatography coupled with diode array detection (LC-DAD) and liquid chromatography coupled with electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Hispidulin and some other phytochemicals were identified in the A. chica extract through this technique.41 Four chemical constituents were separated from ethyl acetate extracts and five chemical constituents were separated from n-butanol extracts of Centipeda minima using silica gel, reserved phase silica gel and Sephadex LH-20 gel column chromatography. Hispidulin was found to be one of the main active constituents in the C. minima through these techniques.42 High-speed countercurrent chromatography was used as main tools for the separation and purification of flavonoids from the extract of belamcanda. These peaks including hispidulin were detected by ESI-MS(n) and NMR spectroscopy method. Further the data were also compared with the standard reference compound.43 A bioactivity-guided isolation and purification process was used for the identification of the alpha-glucosidase inhibiting components from the Crossostephium chinense plant. Ethyl acetate and water layer fractions of C. chinense were subjected to column chromatography using various stationary phases. Hispidulin and some other components were separated from the plant extract.44
A sensitive high performance liquid chromatography electrospray ionization mass spectrometry method (LC-ESI-MS) was developed for the detection of different bioactive flavonoids including hispidulin in the S. involucrate.45 A bioassay-guided isolation was performed to isolate and identify the immunosuppressive components from Artemisia vestita. Nine known flavones including hispidulin were isolated and identified in the A. vestita.46 A simple and reliable method of high-performance liquid chromatography coupled with photodiode array detector (HPLC-DAD) was developed for the fingerprint analysis and quantitative analysis of Salvia plebeia R.Br. Seven bioactive compounds including hispidulin were identified in the Salvia plebeia R.Br.47 In another study six new diterpenoids and 12 known compounds including hispidulin, were isolated and identified in the aerial parts of Scoparia dulcis.48 Greek oregano (Origanum vulgare), marjoram (Origanum majorana), rosemary (Rosmarinus officinalis), and Mexican oregano (Lippia graveolens) are rich sources of bioactive compounds. Hispidulin and others phytochemicals were identified in greenhouse-grown Mexican oregano and rosemary through LC-ESI-MS method.49 Eighteen flavonoid including hispidulin, were identified in the C. chinense in Japan and Taiwan through UV, 1H and 13C NMR, LC-MS and HPLC method.50 Phytochemical studies of Artemisia herba alba commonly known as desert or white wormwood showed the presence of hispidulin.51 Foliar flavonoids of Nipponanthemum and hispidulin were isolated from Nipponanthemum nipponicum.52
Bioassay-guided fractionation of the chloroform extract of Centaurea jacea L. afforded the isolation of hispidulin. Further structures of the compounds were elucidated UV, MS and NMR spectroscopic method.53 LC-ESI-MS analysis was used for identification of phenolic compounds in the methanolic extracts of commercially available dried oregano, sage and thyme. In oregano, hispidulin and some other phytoconstituents were found to be present.54 A bioactivity-guided fractionation of the ethyl acetate fraction of the flowers of Onopordum alexandrinum L. (Asteraceae) yielded hispidulin. The isolated compounds were identified through UV, 1H NMR, 13C NMR, HMQC, HMBC, and COSY spectroscopic methods.55 Combined form of medium-pressure liquid chromatography and preparative high-pressure liquid chromatography, high-speed countercurrent chromatography was used for the separation and purification of isoflavonoids from the extract of belamcanda. Seven compounds including hispidulin were identified in the plant extract.56 Eleven compounds including hispidulin were isolated and identified from Iris dichotoma through various column chromatographic methods.57 Phytochemical analysis of Clerodendrum chinense (Osbeck) Mabberley cultivated in Egypt was performed. Hispidulin and other phytochemical were isolated from the leaves of C. chinense.58 Fractionation of hydroalcoholic extract of S. racemosa Pers resulted in the isolation of four main constituents including hispidulin.59 Phytochemical study of leaves of Leuzea carthamoides was performed and the result showed the presence of seven natural compounds including hispidulin.60 Cold stressed plants of both accessions showed different responses in both rosemary accessions accessions.61 Three anti-inflammatory compounds nepetin, jaceosidin and hispidulin have been isolated and identified from dichloromethane extract of Eupatorium arnottianum Griseb.38 Two elemanolide sesquiterpenes and two eudesmane-type sesquiterpene glycosides named hierapolitanins A-D, were isolated, with hispidulin from the aerial parts of Centaurea hierapolitana Boiss.62
Bioactivity-guided fractionation of the methanol extract of the leaves of Santolina insularis led to the isolation of hispidulin and other xanthone.39 Seven flavonoidal compounds including hispidulin were isolated from the flowers of C. rivulare (Jacq.) Further their structure were determined through chemical and UV, 1H NMR, 13C NMR spectroscopic methods.63 Hispidulin, pulchellin E and gaillardin were isolated from the aerial parts of Inula oculus-christi.64 Cytotoxicity-guided fractionation of the methanol soluble part of the dichloromethane extract of the leaves of Warionia saharae led to the isolation of the hispidulin and other phytochemicals.65 Hispidulin and other phytochemicals were isolated from the methanolic extracts of the aerial parts of Artemisia argyi. Further their structures were elucidated on the basis of spectral data.66 Benzodiazepine receptor binding assay-guided fractionation of the methanol extract of sage leaves (Salvia officinalis L.) revealed three flavones apigenin, hispidulin and cirsimaritin.67 Bioactivity-guided chemical investigation of the flavonoid fraction of the leaves of Lantana montevidensis Briq. has resulted in the isolation of hispidulin.68 A bioassay-guided fractionation of the ethanolic extract of C. petasites was performed through partitioning and centrifugal partition chromatography. Hispidulin was isolated and identified as the main active components.69
A reversed-phase high-performance liquid chromatography/diode-array detector method was used for the determination of phenolic compounds in sage. Six phenolic compounds including hispidulin were found to be present in the sage.70 Foliar flavonoids of 31 species of the Annonaceae native to Brazil were studied. More than 76 compounds, were isolated and identified including hispidulin from the samples.71 In another method, a reversed-phase HPLC method were developed and used for the determination of some flavonoids including hispidulin in vervain samples.72 Bioassay-directed fractionation of the flowers and leaves of Ratibida columnifera led to the isolation of 10 cytotoxic substances including hispidulin.73 Phytochemical analysis of the aerial parts of Artemisia giraldii var. giraldii lead to the isolation of hispidulin and some other components.74 The ethanolic extract of the aerial parts of Centaurea scoparia Sieb. afforded five polyoxygenated flavones including hispidulin.75 Hispidulin and some new labdane diterpenes have been isolated from the aerial parts of Baccharis gaudichaudiana.76 Activity-based fractionation of Eriodictyon californicum resulted in the isolation of 12 flavonoids including hispidulin.77 In another study, ratibinolide II, a new eudesmanolide, and known flavanone hispidulin were isolated from Ratibida latipalearis.78 The 6-methoxyflavones hispidulin and eupafolin have been identified from the aerial parts of Eupatorium cannabinum L.79 Methylated flavonoids hispidulin have been isolated and identified by spectroscopic methods from the flowers of Arnica chamissonis Less.80 Four flavanones and one flavone hispidulin have been isolated from Artemisia campestris L. ssp. glutinosa Gay.81 Five flavonoids including hispidulin have been isolated from Chenopodium botrys.82 Ten compounds including hispidulin were isolated from the methanolic extract of the aerial part of Phyla nodiflora.21 Bioassay-guided fractionation of ethanolic extract of aerial parts of B. uncinella C. DC. led to the isolation of hispidulin and pectolinaringenin.32 Chromatographic separation of the fractions of Centaurea sadleriana Janka led to the identification of the hispidulin and some other compounds.83 Flavonoid aglycones hispidulin, patuletin, and kaempferol were identified in the Eupatorium perfoliatum L extract.84 Chloroform fraction of Scutellaria barbata was subjected to bioassay-guided isolation of the active components through chromatographic techniques on silica gel and Sephadex LH-20. Hispidulin was isolated from chloroform fraction of S. barbata.85 An ethyl acetate extract of Artemisia herba-alba was partitioned by HPLC in 10 fractions and form the fraction hispidulin and cirsilineol were isolated.86 The phenolic composition of A. chica leaf was studied by liquid chromatography coupled with diode array detection (LC-DAD) and liquid chromatography coupled to electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Hispidulin, scutellarein, luteolin, and apigenin were identified in the A. chica leaf.41 Further some method used for the detection of phytochemical present in the plant materials were also presented in the Table 1.
Table 1.
Different method used for the detection of phytochemical including hispidulin in the plants.
| S. no | Plant material | Method used | Phytoconstituents | Reference |
|---|---|---|---|---|
| 1 | Clerodendrum petasites S. Moore | High performance liquid chromatography (HPLC) with photodiode array (PDA) and mass spectrometry (MS) detection. | Vanillic acid, verbascoside, 4-coumaric acid, ferulic acid, nepetin, luteolin, apigenin, naringenin, hispidulin, hesperetin and chrysin, | 38 |
| 2 | Saussurea involucrata | High-performance liquid chromatography electrospray ionization mass spectrometry method (LC-ESI-MS) | Apigenin, luteolin, hispidulin, luteolin-7-O-glucoside and rutin. | 45 |
| 3 | Salvia plebeia R.Br | High-performance liquid chromatography coupled with photodiode array detector (HPLC-DAD). | Caffeic acid, luteolin-7-glucoside, nepetin-7-glucoside, homoplantaginin, luteolin, nepetin and hispidulin. | 47 |
| 4 | Centaurea jacea L | Bioassay-guided fractionation, UV, MS and NMR spectroscopy. | Cirsiliol, apigenin, hispidulin, eupatorin, isokaempferide, axillarin, centaureidin, 6-methoxy kaempferol 3-methyl ether, trachelogenin, cnicin, 4′-acetylcnicin, aliphatic glucose diesters, and 1β-isobutanoyl-2-angeloyl-glucose. | 53 |
| 5 | Onopordum alexandrinum L. | UV, 1H NMR, 13C NMR, HMQC, HMBC, and COSY. | Acacetin-7-O-galacturonide, flavonoids; 6-methoxy-apigenin (hispidulin), acacetin, luteolin, kaempferol, eriodictyol, apigenin-7-O-glucoside, luteolin-7-O-glucoside, and kaempferol-3-O-rutinoside. | 55 |
| 6 | Belamcanda | Combined with medium-pressure liquid chromatography (MPLC) and preparative high-pressure liquid chromatography (Prep-HPLC), high-speed countercurrent chromatography (HSCCC). | Apocynin, mangiferin, 7-O-methylmangiferin, hispidulin, 3′-hydroxyltectoridin, iristectorin B and isoiridin. | 56 |
| 7 | Cirsium rivulare (Jacq.) | UV, 1H NMR, 13C NMR spectroscopy. | Tricin, apigenin, luteolin, hispidulin, acacetin 7-O-beta-d-rutinoside (linarin), apigenin 7-O-beta-D-glucuronide and apigenin 7-O-beta-D-glucoside. | 63 |
| 8 | Sage | Reversed-phase high-performance liquid chromatography/diode-array detector procedure. | Caffeic acid, luteolin 7-O-glucoside, rosmarinic acid, apigenin, hispidulin, and cirsimaritin. | 70 |
| 9 | Vervain | Reversed-phase HPLC. | Luteolin, nepetin, hispidulin, jaceosidin, cirsimaritin, cirsilineol, and eupatorin. | 72 |
5. Plant tissue cultures techniques data of hispidulin
Plant cell cultures techniques represent a potential renewable source of valuable medicinals, flavors, essences and colorants which cannot be produced by microbial cells or chemical synthesis. However, only a few cultures produce these compounds in a significant level. Different techniques using in vitro systems, have been extensively developed and studied with the aim of improving the production of these secondary metabolite.10 Cell cultures of Saussurea medusa produce valuable secondary metabolites in which jaceosidin and hispidulin were found to be the major bioactive compounds. Cultures were challenged with methyl jasmonate (MJ) and the highest jaceosidin and hispidulin concentrations were achieved with 5 μM MJ added to 9-d-old subcultures, being 2.2-fold and 4.2-fold, respectively.87 From the ethanolic extract of a hairy root culture of S. involucrata, syringin, rutin and hispidulin, were isolated and their chemical structures were confirmed by HPLC-ESI-MS. Quantitative study of hispidulin showed 3 times higher concentration in the culture compared to the wild plants.88 Three previously established cell lines (yellow, red and white) of S. medusa were investigated for the jaceosidin and hispidulin production. Maximum yields of the jaceosidin and hispidulin were found to be in the red cell line.89 Sage (S. officinalis L.) calli were established by culturing intermodal segments, excised from aseptic seedlings, on MS basal medium gelled with agar. Further the calli were also supplemented with dichlorophenoxyacetic acid (2,4-D) in presence of benzyladenine (BA) or zeatin (ZEA) or kinetin (KIN). Suspended cells were established by transferring one callus to 50 mL of liquid MS basal medium devoid of agar but having the hormonal supplementation. Further phytochemical analysis of the calli showed the presence of twelve phenolic compounds including hispidulin.90
6. Conclusion
Herbal remedies are used for the treatments of different disorders. Plants materials are tremendous source of natural drugs and some of the important drugs in the modern era were also derived from the herbal sources. Plant-derived products play an important role in the health care system in the developing countries and even in the developed country for the treatments of different disorders. Some of the important medicine such as aspirin, anti-malarial, anti-cancer and many more have derived from the herbal source. Herbal medicine can be used as a remedy against microorganism, inflammation, cardiovascular diseases, blood disorders, cerebral disorders, immune system, oxidative stress etc. A large number of the prescribed drugs including some of the most important medicine are derived from the plant sources.91, 92, 93, 94 A large number of the prescribed drugs in the world are derived from plants such as and in India, about 80% of the rural population uses medicinal herbs for the treatment of different types of disorders.95 As per the World Health Organization survey more than 21000 plants are used in the world as a medicine for the treatments of different ailments.96 Herbal medicines are gaining popularity both in developing and developed countries due to its fewer side effects. Plants contain different types of phytoconstituent in the form of either primary metabolite or secondary metabolite. So many drugs used in the modern medicine are mainly derived from the medicinal plants such as morphine, reserpine, vincristine, vinblastine, quinine etc. Now day's scientists are focusing on pure natural compound instead of the herbal extract for the development of better medicine and hispidulin as one of among. It has different pharmacological activities and has potential to treat different ailment from the human body. In the present review we have collected all the information of hispidulin in regards with its medicinal importance, pharmacological activities, extraction, isolation, and other analytical aspects. So the present review will be helpful to the scientist for the development of better medicine for the natural sources in the future.
Conflict of interest
None.
Acknowledgments
The authors want to acknowledge Banaras Hindu University, Varanasi for online article support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
- 2.Patel D.K., Patel K., Duraiswamy B., Dhanabal S.P. Phytochemical analysis and standardization of Strychnos nux-vomica extract through HPTLC techniques. Asian Pac J Trop Dis. 2012;2:S56–S60. [Google Scholar]
- 3.Talib W.H., Abu Zarga M.H., Mahasneh A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscose. Molecules. 2012;17:3291–3303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumari A., Sharma R.A. A review on Millingtonia hortensis Linn. Int J Pharm Sci Rev Res. 2013;19:85–92. [Google Scholar]
- 5.Mounnissamy V.M., Kavimani S., Balu V., Sankari G., Quine S.D. Anti-nociceptive activity of Cansjera rheedii J. Gmelin (Opiliaceae) Maejo Int J Sci Technol. 2009;3:306–312. [Google Scholar]
- 6.Singh M.K., Khare G., Iyer S.K., Sharwan G., Tripathi D.K. Clerodendrum serratum: a clinical approach. J Appl Pharm Sci. 2012;2:11–15. [Google Scholar]
- 7.Patel D.K., Patel K., Dhanabal S.P. Standardization of Berberis aristata extract through conventional and modern HPTLC techniques. Asian Pac J Trop Dis. 2012;2:S136–S140. [Google Scholar]
- 8.Lin Y.C., Hung C.M., Tsai J.C. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2010;58:9511–9517. doi: 10.1021/jf1019533. [DOI] [PubMed] [Google Scholar]
- 9.Kavvadias D., Sand P., Youdim K.A. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khamar D., Devkar R., Reshma K.K., Shreedhara C.S., Setty M.M., Hegde S. Enhanced hispidulin production in vitro from callus culture of millingtonia hortensis l.f. IJPBS. 2013;2:633–639. [Google Scholar]
- 11.Wang Y., Liu W., He X., Fei Z. Hispidulin enhances the anti-tumor effects of temozolomide in glioblastoma by activating AMPK. Cell Biochem Biophys. 2015 Mar;71(2):701–706. doi: 10.1007/s12013-014-0252-6. [DOI] [PubMed] [Google Scholar]
- 12.Gao H., Jiang Q., Han Y., Peng J., Wang C. Hispidulin potentiates the antitumor effect of sunitinib against human renal cell carcinoma in laboratory models. Cell Biochem Biophys. 2015 Mar;71(2):757–764. doi: 10.1007/s12013-014-0260-6. [DOI] [PubMed] [Google Scholar]
- 13.Yu C.Y., Su K.Y., Lee P.L. Potential therapeutic role of hispidulin in gastric cancer through induction of apoptosis via NAG-1 signaling. Evid Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/518301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L., Wu Y., Lin L. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–225. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang J.M., Hung C.M., Fu C.N. Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. J Agric Food Chem. 2010;58:10020–10026. doi: 10.1021/jf102304g. [DOI] [PubMed] [Google Scholar]
- 16.Woerdenbag H.J., Merfort I., Schmidt T.J. Decreased helenalin-induced cytotoxicity by flavonoids from Arnica as studied in a human lung carcinoma cell line. Phytomedicine. 1995;2:127–132. doi: 10.1016/S0944-7113(11)80057-7. [DOI] [PubMed] [Google Scholar]
- 17.Chulasiri M., Bunyapraphatsara N., Moongkarndi P. Mutagenicity and antimutagenicity of hispidulin and hortensin, the flavonoids from Millingtonia hortensis L. Environ Mol Mutagen. 1992;20:307–312. doi: 10.1002/em.2850200409. [DOI] [PubMed] [Google Scholar]
- 18.Alza N.P., Pferschy-Wenzig E.M., Ortmann S. Inhibition of NO production by Grindelia Argentina and isolation of three new cytotoxic saponins. Chem Biodivers. 2014;11:311–322. doi: 10.1002/cbdv.201300193. [DOI] [PubMed] [Google Scholar]
- 19.Gao H., Wang H., Peng J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem Biophys. 2014;69:27–34. doi: 10.1007/s12013-013-9762-x. [DOI] [PubMed] [Google Scholar]
- 20.Dabaghi-Barbosa P., Mariante Rocha A., Franco da Cruz Lima A. Hispidulin: antioxidant properties and effect on mitochondrial energy metabolism. Free Radic Res. 2005;39:1305–1315. doi: 10.1080/13561820500177659. [DOI] [PubMed] [Google Scholar]
- 21.Lin F.J., Yen F.L., Chen P.C. HPLC-fingerprints and antioxidant constituents of Phyla nodiflora. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/528653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanz M.J., Ferrandiz M.L., Cejudo M. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica. 1994;24:689–699. doi: 10.3109/00498259409043270. [DOI] [PubMed] [Google Scholar]
- 23.Yang L., Yu Z., Qu H., Li M. Comparative effects of hispidulin, genistein, and icariin with estrogen on bone tissue in ovariectomized rats. Cell Biochem Biophys. 2014;70:485–490. doi: 10.1007/s12013-014-9945-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhou R., Wang Z., Ma C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem Biophys. 2014;69:311–317. doi: 10.1007/s12013-013-9800-8. [DOI] [PubMed] [Google Scholar]
- 25.Nepal M., Choi H.J., Choi B.Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. Eur J Pharmacol. 2013;715:96–104. doi: 10.1016/j.ejphar.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Lin T.Y., Lu C.W., Wang C.C., Lu J.F., Wang S.J. Hispidulin inhibits the release of glutamate in rat cerebrocortical nerve terminals. Toxicol Appl Pharmacol. 2012;263:233–243. doi: 10.1016/j.taap.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Walesiuk A., Nazaruk J., Braszko J.J. Pro-cognitive effects of Cirsium rivulare extracts in rats. J Ethnopharmacol. 2010;129:261–266. doi: 10.1016/j.jep.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 28.Niu X., Chen J., Wang P., Zhou H., Li S., Zhang M. The effects of hispidulin on bupivacaine-induced neurotoxicity: role of AMPK signaling pathway. Cell Biochem Biophys. 2014;70:241–249. doi: 10.1007/s12013-014-9888-5. [DOI] [PubMed] [Google Scholar]
- 29.Bourdillat B., Delautier D., Labat C., Benveniste J., Potier P., Brink C. Hispidulin, a natural flavone, inhibits human platelet aggregation by increasing cAMP levels. Eur J Pharmacol. 1988;147:1–6. doi: 10.1016/0014-2999(88)90626-7. [DOI] [PubMed] [Google Scholar]
- 30.Abdalla S., Abu-Zarga M., Afifi F., Al-Khalil S., Sabri S. Effects of hispidulin, a flavone isolated from Inula viscosa, on isolated Guinea-pig smooth muscle. Gen Pharmacol. 1988;19:559–563. doi: 10.1016/0306-3623(88)90164-4. [DOI] [PubMed] [Google Scholar]
- 31.Sülsen V.P., Cazorla S.I., Frank F.M. Trypanocidal and leishmanicidal activities of flavonoids from Argentine medicinal plants. Am J Trop Med Hyg. 2007;77:654–659. [PubMed] [Google Scholar]
- 32.Grecco Sdos S., Félix M.J., Lago J.H. Anti-trypanosomal phenolic derivatives from Baccharis uncinella. Nat Prod Commun. 2014;9:171–173. [PubMed] [Google Scholar]
- 33.Su K.Y., Yu C.Y., Chen Y.P., Hua K.F., Chen Y.L. 3,4-Dihydroxytoluene, a metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-κB signaling. BMC Complement Altern Med. 2014;14:21. doi: 10.1186/1472-6882-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labib S., Hummel S., Richling E., Humpf H.U., Schreier P. Use of the pig caecum model to mimic the human intestinal metabolism of hispidulin and related compounds. Mol Nutr Food Res. 2006;50:78–86. doi: 10.1002/mnfr.200500144. [DOI] [PubMed] [Google Scholar]
- 35.Ferrándiz M.L., Bustos G., Payá M., Gunasegaran R., Alcaraz M.J. Hispidulin protection against hepatotoxicity induced by bromobenzene in mice. Life Sci. 1994;55:PL145–PL150. doi: 10.1016/0024-3205(94)00490-0. [DOI] [PubMed] [Google Scholar]
- 36.Clavin M., Gorzalczany S., Macho A. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J Ethnopharmacol. 2007;112:585–589. doi: 10.1016/j.jep.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Cottiglia F., Casu L., Bonsignore L. Topical anti-inflammatory activity of flavonoids and a new xanthone from Santolina insularis. Z Naturforsch C. 2005;60:63–66. doi: 10.1515/znc-2005-1-212. [DOI] [PubMed] [Google Scholar]
- 38.Thitilertdecha P., Guy R.H., Rowan M.G. Characterisation of polyphenolic compounds in Clerodendrum petasites S. Moore and their potential for topical delivery through the skin. J Ethnopharmacol. 2014;154:400–407. doi: 10.1016/j.jep.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 39.Ren D.B., Qin Y.H., Yun Y.H., Lu H.M., Chen X.Q., Liang Y.Z. Separation of nine compounds from Salvia plebeia R.Br.. using two-step high-speed counter-current chromatography with different elution modes. J Sep Sci. 2014;37:2118–2125. doi: 10.1002/jssc.201400293. [DOI] [PubMed] [Google Scholar]
- 40.Li J., Zhang X., Yu Q., Fu X., Wang W. One-step separation of four flavonoids from Herba salviae Plbeiae by HSCCC. J Chromatogr Sci. 2014;52:1288–1293. doi: 10.1093/chromsci/bmu007. [DOI] [PubMed] [Google Scholar]
- 41.Siraichi J.T., Felipe D.F., Brambilla L.Z. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in Southern Brazil. PLoS One. 2013;8:e72733. doi: 10.1371/journal.pone.0072733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao J., Li G. Chemical constituents of Centipeda minima. Zhongguo Zhong Yao Za Zhi. 2012;37:2301–2303. [PubMed] [Google Scholar]
- 43.Peng C., Liang Y., Wang X., Xie H., Zhang T., Ito Y. Preparative isolation and purification of flavonoids from the Chinese medicinal herb Belamcanda by high-speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2009;32:2451–2461. doi: 10.1080/10826070903188245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q., Yang X., Zou L., Fu D. Bioactivity guided isolation of alpha-glucosidase inhibitor from whole herbs of Crossostephium chinense. Zhongguo Zhong Yao Za Zhi. 2009;34:2206–2211. [PubMed] [Google Scholar]
- 45.Xu Y.J., Zhao D.X., Fu C.X. Determination of flavonoid compounds from Saussurea involucrata by liquid chromatography electrospray ionisation mass spectrometry. Nat Prod Res. 2009;23:1689–1698. doi: 10.1080/14786410802187742. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y., Gong F.Y., Wu X.X. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Jin X.F., Lu Y.H., Wei D.Z., Wang Z.T. Chemical fingerprint and quantitative analysis of Salvia plebeia R.Br. by high-performance liquid chromatography. J Pharm Biomed Anal. 2008;48:100–104. doi: 10.1016/j.jpba.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Liu Q., Yang Q.M., Hu H.J. Bioactive diterpenoids and flavonoids from the aerial parts of Scoparia dulcis. J Nat Prod. 2014;77:1594–1600. doi: 10.1021/np500150f. [DOI] [PubMed] [Google Scholar]
- 49.Bower A.M., Real Hernandez L.M., Berhow M.A., de Mejia E.G. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J Agric Food Chem. 2014;62:6147–6158. doi: 10.1021/jf500639f. [DOI] [PubMed] [Google Scholar]
- 50.Uehara A., Kitajima J., Kokubugata G., Iwashina T. Further characterization of foliar flavonoids in Crossostephium chinense and their geographic variation. Nat Prod Commun. 2014;9:163–164. [PubMed] [Google Scholar]
- 51.Moufid A., Eddouks M. Artemisia herba alba: a popular plant with potential medicinal properties. Pak J Biol Sci. 2012;15:1152–1159. doi: 10.3923/pjbs.2012.1152.1159. [DOI] [PubMed] [Google Scholar]
- 52.Uehara A., Iwashina T. Flavonoids from the Japanese monotypic genus, Nipponanthemum. Nat Prod Commun. 2012;7:1005–1006. [PubMed] [Google Scholar]
- 53.Forgo P., Zupkó I., Molnár J., Vasas A., Dombi G., Hohmann J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea jacea L. Fitoterapia. 2012;83:921–925. doi: 10.1016/j.fitote.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Nagy T.O., Solar S., Sontag G., Koenig J. Identification of phenolic components in dried spices and influence of irradiation. Food Chem. 2011;128:530–534. doi: 10.1016/j.foodchem.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Salama M.M., Ezzat S.M., Sleem A.A. A new hepatoprotective flavone glycoside from the flowers of Onopordum alexandrinum growing in Egypt. Z Naturforsch C. 2011;66:251–259. doi: 10.1515/znc-2011-5-608. [DOI] [PubMed] [Google Scholar]
- 56.Wang X., Liang Y., Peng C. Preparative isolation and purification of chemical constituents of belamcanda by MPLC, HSCCC and PREP-HPLC. J Liq Chromatogr Relat Technol. 2011;34:241–257. doi: 10.1080/10826076.2011.547058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L., Yang J., Peng Y., Xiao P. Chemical constituents of Iris dichotoma. Zhongguo Zhong Yao Za Zhi. 2010;35:3168–3171. [PubMed] [Google Scholar]
- 58.Wahba H.M., AbouZid S.F., Sleem A.A., Apers S., Pieters L., Shahat A.A. Chemical and biological investigation of some Clerodendrum species cultivated in Egypt. Pharm Biol. 2011;49:66–72. doi: 10.3109/13880209.2010.494674. [DOI] [PubMed] [Google Scholar]
- 59.Marques M.R., Stüker C., Kichik N. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia. 2010;81:552–556. doi: 10.1016/j.fitote.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Koleckar V., Opletal L., Brojerova E. Evaluation of natural antioxidants of Leuzea carthamoides as a result of a screening study of 88 plant extracts from the European Asteraceae and Cichoriaceae. J Enzyme Inhib Med Chem. 2008;23:218–224. doi: 10.1080/14756360701450806. [DOI] [PubMed] [Google Scholar]
- 61.Luis J.C., Martín R., Frías I., Valdés F. Enhanced carnosic acid levels in two rosemary accessions exposed to cold stress conditions. J Agric Food Chem. 2007;55:8062–8066. doi: 10.1021/jf0712393. [DOI] [PubMed] [Google Scholar]
- 62.Karamenderes C., Bedir E., Pawar R., Baykan S., Khan I.A. Elemanolide sesquiterpenes and eudesmane sesquiterpene glycosides from Centaurea hierapolitana. Phytochemistry. 2007;68:609–615. doi: 10.1016/j.phytochem.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Nazaruk J., Gudej J. Flavonoid compounds from the flowers of Cirsium rivulare (Jacq.) All. Acta Pol Pharm. 2003;60:87–89. [PubMed] [Google Scholar]
- 64.Vajs V., Nevesćanin M., Macura S., Juranić N., Menković N., Milosavljević S. Sesquiterpene lactones from the aerial parts of Inula oculus-christi. Fitoterapia. 2003;74:508–510. doi: 10.1016/s0367-326x(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 65.Hilmi F., Sticher O., Heilmann J. New cytotoxic sesquiterpene lactones from Warionia saharae. Planta Med. 2003;69:462–464. doi: 10.1055/s-2003-39703. [DOI] [PubMed] [Google Scholar]
- 66.Seo J.M., Kang H.M., Son K.H. Antitumor activity of flavones isolated from Artemisia argyi. Planta Med. 2003;69:218–222. doi: 10.1055/s-2003-38486. [DOI] [PubMed] [Google Scholar]
- 67.Kavvadias D., Monschein V., Sand P., Riederer P., Schreier P. Constituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptor. Planta Med. 2003;69:113–117. doi: 10.1055/s-2003-37712. [DOI] [PubMed] [Google Scholar]
- 68.Nagao T., Abe F., Kinjo J., Okabe H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol Pharm Bull. 2002;25:875–879. doi: 10.1248/bpb.25.875. [DOI] [PubMed] [Google Scholar]
- 69.Hazekamp A., Verpoorte R., Panthong A. Isolation of a bronchodilator flavonoid from the Thai medicinal plant Clerodendrum petasites. J Ethnopharmacol. 2001;78:45–49. doi: 10.1016/s0378-8741(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 70.Areias F., Valentão P., Andrade P.B., Ferreres F., Seabra R.M. Flavonoids and phenolic acids of sage: influence of some agricultural factors. J Agric Food Chem. 2000;48:6081–6084. doi: 10.1021/jf000440+. [DOI] [PubMed] [Google Scholar]
- 71.Santos D.Y., Salatino M.L. Foliar flavonoids of Annonaceae from Brazil: taxonomic significance. Phytochemistry. 2000;55:567–573. doi: 10.1016/s0031-9422(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 72.Valentão P., Andrade P.B., Areias F., Ferreres F., Seabra R.M. Analysis of vervain flavonoids by HPLC/Diode array detector method. Its application to quality control. J Agric Food Chem. 1999;47:4579–4582. doi: 10.1021/jf990444i. [DOI] [PubMed] [Google Scholar]
- 73.Cui B., Lee Y.H., Chai H. Cytotoxic sesquiterpenoids from Ratibida columnifera. J Nat Prod. 1999;62:1545–1550. doi: 10.1021/np990260y. [DOI] [PubMed] [Google Scholar]
- 74.Tan R.X., Lu H., Wolfender J.L. Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med. 1999;65:64–67. doi: 10.1055/s-1999-13965. [DOI] [PubMed] [Google Scholar]
- 75.Youssef D., Frahm A.W. Constituents of the Egyptian Centaurea scoparia. III. Phenolic constituents of the aerial parts. Planta Med. 1995;61:570–573. doi: 10.1055/s-2006-959378. [DOI] [PubMed] [Google Scholar]
- 76.Fullas F., Hussain R.A., Chai H.B., Pezzuto J.M., Soejarto D.D., Kinghorn A.D. Cytotoxic constituents of Baccharis gaudichaudiana. J Nat Prod. 1994;57:801–807. doi: 10.1021/np50108a017. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y.L., Ho D.K., Cassady J.M., Cook V.M., Baird W.M. Isolation of potential cancer chemopreventive agents from Eriodictyon californicum. J Nat Prod. 1992;55:357–363. doi: 10.1021/np50081a012. [DOI] [PubMed] [Google Scholar]
- 78.Rojas A., Villena R., Jiménez A., Mata R. Chemical studies on Mexican plants used in traditional medicine, XXI. Ratibinolide II, a new sesquiterpene lactone from Ratibida latipalearis. J Nat Prod. 1991;54:1279–1282. doi: 10.1021/np50077a006. [DOI] [PubMed] [Google Scholar]
- 79.Elema E.T., Schripsema J., Malingré T.M. Flavones and flavonol glycosides from Eupatorium cannabinum L. Pharm Weekbl Sci. 1989;11:161–164. doi: 10.1007/BF01959464. [DOI] [PubMed] [Google Scholar]
- 80.Merfort I. Methylated flavonoids from Arnica montana and Arnica chamissonis. Planta Med. 1984;50:107–108. doi: 10.1055/s-2007-969637. [DOI] [PubMed] [Google Scholar]
- 81.Hurabielle M., Eberle J., Paris M. Flavonoids of Artemisia campestris, ssp. glutinosa. Planta Med. 1982;46:124–125. doi: 10.1055/s-2007-970035. [DOI] [PubMed] [Google Scholar]
- 82.de Pascual-T J., González M.S., Vicente S., Bellido I.S. Flavonoids from Chenopodium botrys. Planta Med. 1981;41:389–391. doi: 10.1055/s-2007-971732. [DOI] [PubMed] [Google Scholar]
- 83.Csupor D., Widowitz U., Blazsó G. Anti-inflammatory activities of eleven Centaurea species occurring in the Carpathian Basin. Phytother Res. 2013;27:540–544. doi: 10.1002/ptr.4754. [DOI] [PubMed] [Google Scholar]
- 84.Maas M., Hensel A., Costa F.B., Brun R., Kaiser M., Schmidt T.J. An unusual dimeric guaianolide with antiprotozoal activity and further sesquiterpene lactones from Eupatoriumperfoliatum. Phytochemistry. 2011;72:635–644. doi: 10.1016/j.phytochem.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 85.Yu J., Liu H., Lei J., Tan W., Hu X., Zou G. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21:817–822. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- 86.Salah S.M., Jäger A.K. Two flavonoids from Artemisia herba-alba Asso with in vitro GABAA-benzodiazepine receptor activity. J Ethnopharmacol. 2005;99:145–146. doi: 10.1016/j.jep.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 87.Fu C.X., Cheng L.Q., Lv X.F., Zhao D.X., Ma F. Methyl jasmonate stimulates jaceosidin and hispidulin production in cell cultures of Saussurea medusa. Appl Biochem Biotechnol. 2006;134:89–96. doi: 10.1385/abab:134:1:89. [DOI] [PubMed] [Google Scholar]
- 88.Fu C.X., Xu Y.J., Zhao D.X., Ma F.S. A comparison between hairy root cultures and wild plants of Saussurea involucrate in phenylpropanoids production. Plant Cell Rep. 2006;24:750–754. doi: 10.1007/s00299-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 89.Fu C.X., Zhao D.X., Huang Y., Ma F.S. Cellular aggregate size as the critical factor for flavonoid production by suspension cultures of Saussurea medusa. Biotechnol Lett. 2005;27:91–95. doi: 10.1007/s10529-004-6934-1. [DOI] [PubMed] [Google Scholar]
- 90.Santos-Gomes P.C., Seabra R.M., Andrade P.B., Fernandes-Ferreira M. Determination of phenolic antioxidant compounds produced by calli and cell suspensions of sage (Salvia officinalis L.) J Plant Physiol. 2003;160:1025–1032. doi: 10.1078/0176-1617-00831. [DOI] [PubMed] [Google Scholar]
- 91.Patel K., Gadewar M., Tahilyani V., Patel D.K. A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect. 2012;2:46–52. [Google Scholar]
- 92.Patel D.K., Patel K., Dhanabal S.P. Development of quality control parameters for the standardization of Gymnema sylvestre. J Acute Dis. 2012;1:141–143. [Google Scholar]
- 93.Patel K., Gadewar M., Tripathi R., Patel D.K. Pharmacological and analytical aspects of gymnemic acid: a concise report. Asian Pac J Trop Dis. 2012;2:414–416. doi: 10.1016/S2221-1691(12)60239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patel D.K., Kumar R., Prasad S.K., Hemalatha S. Pharmacologically screened aphrodisiac plant-A review of current scientific literature. Asian Pac J Trop Biomed. 2011;1:S131–S138. [Google Scholar]
- 95.Patel D.K., Patel K., Kumar R., Gadewar M., Tahilyani V. Pharmacological and analytical aspects of bergenin: a concise report. Asian Pac J Trop Dis. 2012;2:163–167. doi: 10.1016/S2221-1691(12)60239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel K., Singh R.B., Patel D.K. Medicinal significance, pharmacological activities, and analytical aspects of solasodine: a concise report of current scientific literature. J Acute Dis. 2013;2:92–98. [Google Scholar]