Abstract
Hydro-distilled essential oil (EO) from the leaves of the western Mediterranean and Moroccan endemic plant Tetraclinis articulata was analyzed by GC/MS and examined for its acute toxicity on mice, in order to establish the safe doses. Furthermore, the anti-Inflammatory activity was evaluated based on carrageenan and trauma induced rats paw edema and the antioxidant potential has been investigated using different methods including DPPH radical-scavenging assay, Trolox equivalent antioxidant capacity (TEAC) and Ferric-reducing antioxidant power assay (FRAP). The major identified compounds in GC/MS analysis were bornyl acetate (26.81%), camphor (22.40%) and α-pinene (7.16%), with 25 other minor constituents. No mortalities in acute toxicity were observed, indicating that the LD50 of T. articulata essential oil is highest than 5 g/kg. In the anti-inflammatory test based on chemical and mechanical induced trauma, the EO demonstrated an effective reduce swelling by 64.71 ± 9.38% and 69.09 ± 6.02% respectively obtained 6 h after administration at the dose of 200 mg/kg when compared to the control groups. Moreover in the antioxidant testing battery, T. articulata essential oil showed a promising scavenging effect measured by DPPH, TEAC and ferric-reducing power assays with IC50 values of 12.05 ± 0.24 mg/mL, 8.90 ± 0.17 mg/mL and 0.15 ± 0.01 mg/mL respectively. These results suggest that, the EO from the leaves of T. articulata constitutes a valuable source of anti-inflammatory and antioxidant metabolites. These findings argue for the possible integration of this oil in pharmaceutical, cosmetic and food industries.
Keywords: T. articulata, Essential oil, GC–MS analysis, Toxicity, Anti-inflammatory activity, Antioxidant potential
Abbreviations: T. articulata, Tetraclinis articulata; EO, Essential oil; GC/MS, Gas chromatography/mass spectrometry; LD50, lethal dose of 50%; DPPH, 2,2-diphenyl-1-picrylhydrazyl radical-scavenging assay; TEAC, Trolox equivalent antioxidant capacity; FRAP, Ferric-reducing antioxidant power; BHT, Butylatedhydroxytoluene; IC50, Concentration providing 50% inhibition; SD, Standard deviation
Graphical abstract
1. Introduction
Tetraclinis articulata L. (Cupressaceae), commonly known as Sandarac tree and Barbary Thuya, is a native herbaceous plant of the south-western Mediterranean region, mainly North Africa. It is also considered as an important element of the Maghreb vegetation (Morocco, Algeria and Tunisia) and amongst the most used aromatic and medicinal plants worldwide due basically to its rich essential oils that impart many biological activities.1, 2
In Morocco, T. articulata, popularly known as “Al'Araar” or “Azouka” in Tachlhit, is one of the most popular herbs, used extensively in folk medicine.3 It is considered as an important flavoring agent, commonly used in the herbal tea, and very much appreciated for its smell as well as for its anti-inflammatory properties.4 Moreover, the specie is administered for treatment of severe diarrheas and gastric pains, as well as anti-diabetic traditional therapy.5, 6 In addition crushed leaves are used in poultices on both sides or on the top of the head against dizziness, headache, neck pain, insolation and fever.7 The honey from T. articulata is greatly appreciated for its nutritional and medical aspects. Moreover, the cade oil made by destructive distillation of T. articulata woods is used in medicine as a disinfectant, analgesic, and towards different skin diseases like psoriasis and dandruff.4, 8 T. articulata cade oil has been also used in cosmetics like soaps and shampoo, both for its disinfectant properties and as a perfume.9
Among several T. articulata extracts that may be useful as bioactive natural plant products, essential oil was reported to possess potent antibacterial,10, 11, 12, 13 antifungal14, 15 and antioxidant activities,16, 17 mediated by the major monoterpene component α-pinene, limonene, camphor, borneol and bornyl acetate.18, 19, 20, 21 The high monoterpenes content was also described as being responsible for antitumor activity of T. articulata essential oil.22, 23 Despite the interesting findings across data literature, it seems of interest to explore the in vivo relevant activity of T. articulata essential oil, since many factors can influence its metabolites bioavailability. These study outcomes bring evidence about T. articulata safety in oral treatments, and consolidate the traditionally observed anti-inflammatory effect. The antioxidant effect combined with safe high doses from this oil constitutes an interesting value in food additives, therapies or cosmetics.
2. Material and methods
2.1. Plant materials
The aerial parts of T. articulata were collected from the natural population located in the Marrakech region, Ait Aissi Ihahane (30°91′ N et 09°43′ W) on February 2014. In fact, it has been reported by many gatherers and traditional specialists that the great part of Arar traded in medicinal plant markets were collected from this region. The identification was done by Pr Fennane and vouchers specimens were deposited at the herbarium of Scientific Institute of Rabat, Morocco and referred as 79589. The leaves were separated, dried at room temperature and used for the extraction of the EO.
2.2. Animals
Female Swiss mice (18–35 g) and male Wistar rats (200–230 g) were used in the experiments. The animals were bred in the animal center at the Faculty of Medicine and Pharmacy, University Mohammed V in Rabat. All animals were housed in collective cages in temperature-controlled (23 °C ± 2 °C) and artificially lighted rooms on a 12-h light/12-h dark cycle with free access to water and standard diet.
2.3. Ethics approval
The study was conducted in accordance with the accepted principles outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health and all efforts were made to minimize animal suffering and the number of animals used. Ethics approval was obtained from the University Mohammed V in Rabat.
2.4. Essential oil extraction
Essential oil was hydrodistilled using a Clevenger-type apparatus for 4 h until total recovery of oil. The extraction of the EO was performed three times (3 × 200 g) and oil was stored in dark glass vials at 2–3 °C prior to analysis and bioactivity experiments. The yield of the essential oil (%) was calculated using the following formula (EO stands for essential oil):
2.5. Chemical analysis of EO
Gas chromatography/mass spectrometry (GC/MS) analysis was performed using a gas chromatograph (TRACE GC Ultra) fitted to a mass spectrometer (Polaris Q-Ion Trap MS). Fragmentation was performed by electron impact at 70 ev. The column used was a VB-5 (Methylpolysiloxane 5% phenyl) (30 m × 0.25 mm, film thickness 0.25 μm). The injection mode was split (1:50). The carrier gas was helium at a flow rate of 1.4 mL/min and the temperature program ramp from 40 to 300 °C with gradient of 4 °C/min (holding the initial and final temperature for 4 min). The identification of the individual constituents was based on comparison of their mass spectra with spectra and Kováts retention indices of authentic reference compounds stored in the NIST 98th edition library of mass spectral data. The individual components were also identified by their identical retention indices referring to the compounds known from the literature data.24
2.6. Acute oral toxicity
Acute toxicity study was carried out using the OECD (Organisation for Economic Co-operation and Development) – Guidelines 423.25
Healthy female Swiss mice (generally slightly more sensitive26) fasted 4 h before the experiments with water ad libitum. Animals were randomly divided into three groups (n = 3). The first group (control group) received orally corn oil (vehicle control). The second and third groups were treated with T. articulata essential oil at the doses of 2 and 5 g/kg respectively.
Animals were observed for their general behavioral symptoms, body weight changes, hazardous symptoms and mortality during the first 6 h and subsequently for the next two weeks after administration of T. articulata essential oil. The lethal dose of 50% (LD50) was estimated according to the method described by OECD Guidelines 423.27
2.7. Anti-inflammatory activity
In order to evaluate the anti-inflammatory activity of T. articulata essential oil in paw edema of rats, two experimental trials using different stimulus were used in this study (chemical and mechanical stimuli).28, 29
For each experimental protocol, 24 male Wistar rats were used and divided into different groups (n = 6). All animals were fasted 18 h before the test. The control group received (5 mL/kg of distilled water), the standard group received the reference drug (Indomethacin 10 mg/kg and 20 mg/kg), and the test groups received different concentration of T. articulata essential oil (100 and 300 mg/kg).
The right hind paw is not treated, and it is taken as a control. The percentages of inhibition in our anti-inflammatory tests were obtained for each group using the following ratio (formula):
where Vleft is the average volume of edema on the left hind paw and Vright is the average volume obtained for the right hind paw.
2.7.1. Carrageenan induced rat paw edema assay
Carrageenan-induced paw inflammation in rats was produced according to the method described previously.28 One hour after the oral administration of the EO (200 and 400 mg/kg), or the reference drug (Indomethacin, 10 mg/kg), the carrageenan solution (0.05 mL of 1% carrageenan suspended in 0.9% NaCl) was subcutaneously injected into the plantar surface of the left hind paw. The paw volumes of the animals were measured using a plethysmometer LE7500 (Ugo Basile – Italy) at several time-points; at 1 h 30 min, 3 h and 6 h after carrageenan administration.
2.7.2. Trauma induced rat paw edema assay
The effect of T. articulata oil on paw edema induced by trauma was evaluated according to the method described by Rieesterer and Jacques.29 One hour after oral administration of different substances dropping a weight of 50 g onto the dorsum of the left hind paw of all animals. The difference volume of two paws was also measured and taken as the edema value by using a digital plethysmometer at 1 h 30 min, 3 h and 6 h after induction of inflammation.
2.8. Antioxidant activity
Antioxidant activity is a complex process usually happening through several mechanisms. Because of its complexity, the determination of plant extracts antioxidant activity must be done by more than one test.30 In the present study, three complementary methods were used to assess the antioxidant capacity of T. articulata essential oil: DPPH free radical scavenging, ABTS free radical scavenging and ferric-reducing power assays.
2.8.1. DPPH free radical-scavenging activity
The ability of T. articulata oil to scavenge the DPPH radical was determined according to the method described by Sahin et al.31 Fifty microliters of various concentrations of the samples dissolved in methanol (EO and positive control) were added to 2 mL of a 60 μM of methanol solution of DPPH. The absorbance was recorded at 517 nm after 20 min in the dark at room temperature in the dark. Methanol was used as a blank, while methanol with DPPH solution was used as a negative control. Butylatedhydroxytoluene (BHT) and quercetin were used as positive controls. The percentage inhibition of the DPPH radical by the samples was calculated according to the formula:
where A0 is the absorbance of the blank sample, and At is the absorbance of the test sample. The sample concentration providing 50% inhibition (IC50) was calculated by plotting the inhibition percentages against the concentrations of the sample. The test was carried out in triplicate and IC50 values were expressed as mean values ± standard deviation (SD).
2.8.2. Trolox equivalent antioxidant capacity (TEAC) assay
The ABTS free radical-scavenging activity of T. articulata oils was estimated using the method described by Pukalskas et al.32 The ABTS radical cation was produced by reacting ABTS with potassium persulfate. The blue-green ABTS was produced through the reaction between 7 mM ABTS and 70 mM potassium persulfate in water. The mixture was stored at room temperature in the dark for 16 h prior use. The ABTS solution was then diluted with 80% methanol to obtain an absorbance of 0.700 ± 0.005 at 734 nm. One hundred microliters of appropriately diluted oil were added to 2 mL of ABTS solution and the absorbance was recorded at 734 nm after 1 min incubation at room temperature. This was compared to the blank where 100 μL of the methanol was added to 2 mL of ABTS solution. A standard curve was obtained by using Trolox standard solution at various concentrations (ranging from 0 to 0.24 μg/mL). The scavenging activity of different concentrations of extracts and fractions against ABTS•+ radical were also measured to calculate the IC50, and the procedure was similar to the DPPH scavenging method described above. The test was carried out in triplicate and IC50 values were reported as means ± SD.
2.8.3. Reducing power determination
The ferric-reducing capacity of T. articulata oils was investigated by using the potassium ferricyanide-ferric chloride method.33 Briefly, 0.2 mL of oils and positive control at different concentrations, 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide K3Fe(CN)6 (1%) were mixed and incubated at 50 °C for 20 min, to reduce ferricyanide into ferrocyanide. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 1000 rpm for 10 min. Finally, 2.5 mL of the upper layer was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%) and the absorbance was measured at 700 nm. The sample concentration providing 0.5 of absorbance (IC50) was calculated by plotting absorbance against the corresponding sample concentration. BHT and Quercetin were used as reference compounds. The test was carried out in triplicate and IC50 values were reported as means ± SD.
2.9. Statistical analysis
Data were expressed as the mean values ± standard deviation (SD) for each measurement. The data were also analyzed by one-way analysis of variance (one-way ANOVA). Post Hoc procedure was used for significance of difference (p < 0.05). Analysis was performed with Graph pad prism 6.0.
3. Results and discussion
3.1. Chemical constituents of EO
The hydro-distillation of the leaves of T. articulata gave light yellow oil with a yield of 2.68 ± 0.06% based on dry weight. This essential oil content is higher than those reported by Djouahri et al., (2013)17 (0.56%) and Larabi et al., (2015)34 (0.11%) from Algeria.
The GC–MS analysis resulted in the identification of 26 constituents, representing more than 95.0% of the total oil and comprising three classes: oxygenated monoterpenes (74.82%), monoterpene hydrocarbons (19.13%) and oxygenated sesquiterpenes (0.38%) (Table 1).
Table 1.
Chemical composition of essential oils obtained from leaves of the T. articulata.
| Name of the compounds | Formula | RI | Area (%) |
|---|---|---|---|
| Tricyclene | C10H16 | 920 | 2.33 |
| α-Pinene | C10H16 | 931 | 7.16 |
| Camphene | C10H16 | 945 | 2.73 |
| Verbenene | C10H14 | 960 | 0.49 |
| Sabinene | C10H16 | 968 | 0.24 |
| β-Pinene | C10H16 | 973 | 0.57 |
| p-Cymene | C10H14 | 1019 | 1.45 |
| Limonene | C10H16 | 1030 | 3.82 |
| γ-Terpinene | C10H16 | 1053 | 0.34 |
| Sabinene hydrate | C10H18O | 1067 | 0.33 |
| cis-Thujone | C10H16O | 1100 | 0.49 |
| α-Campholenal | C10H16O | 1126 | 1.71 |
| Camphor | C10H16O | 1140 | 22.40 |
| Camphene hydrate | C10H18O | 1144 | 2.08 |
| Pinocarvone | C10H14O | 1160 | 0.49 |
| Borneol | C10H18O | 1164 | 6.40 |
| Terpinen-4-ol | C10H18O | 1173 | 1.69 |
| p-Cymen-8-ol | C10H14O | 1178 | 1.40 |
| Myrtenol | C10H16O | 1193 | 2.34 |
| Verbenone | C10H14O | 1203 | 3.78 |
| trans-Carveol | C10H16O | 1214 | 2.12 |
| cis-Carveol | C10H16O | 1225 | 0.35 |
| Carvone | C10H14O | 1238 | 1.54 |
| Bornyl acetate | C12H20O2 | 1286 | 26.81 |
| α-Terpinenyl acetate | C12H20O2 | 1346 | 0.89 |
| Cubenol |
C15H26O |
1514 |
0.38 |
| Classes |
Identified compound |
Percentage % |
|
| Monoterpene hydrocarbons | 9 | 19.13% | |
| Oxygenated monoterpenes | 16 | 74.82% | |
| Sesquiterpenes hydrocarbons | 0 | 0% | |
| Oxygenated sesquiterpenes | 1 | 0.38% | |
| Total identified (%) | 26 | 94.33% | |
As shown in Table 1, bornyl acetate (20.81%), camphor (22.40%) and α-pinene (7.16%) were the major constituents. The chemical analysis of the leaves of T. articulata showed similarities in the identified compounds with the algerian variety,17, 34, 35 however different proportions were registered. Additionally, our results are in agreement with previous work of Bourkhiss et al., (2010),16 who found comparable concentrations of bornyl acetate (25.00–33.00%), camphor (15.10–22.20%) and α-pinene (15.2–18.5%), in Moroccan T. articulata harvested in the region of Khemisset at different seasons. Also, a study of Barrero et al., (2005)18 upon T. articulata from the region of Tetouan (Morocco) has reported the predominant presence of bornyl acetate (16.50%) and camphor (19.10%). Interestingly, Aitigri (1990)21 and Zrira et al. (2005)19 reported a rich content of carvacrol, cedrene and cedrol with low concentrations of bornyl acetate, camphor and α-pinene in T. articulata harvested in the region of Rabat and Aoulouz (Morocco). Moreover, it was noticed that our results are quite different from those reported in Tunisia, which contained caryophyllene oxide 4.24% and high amount of α-pinene 24.90% and linalool acetate 21.44%, with absence of bornyl acetate and camphor36.
Differences in essential oils composition results from various parameters and conditions such as the geographic allocation, the environmental conditions, the genetic factors and the time at which the plant was collected.37, 38
3.2. Acute oral toxicity
T. articulata is one of the most widely used herbal medicine in Morocco. Despite the interesting properties of the essential oil from T. articulata, frequently used alone, its safety has not been previously reported.4, 39 In this study, the acute toxicity of EO was conducted for evaluating the safety of EO at different doses (2 and 5 g/kg).
The acute effects of T. articulata essential oil after oral administration to mouse are reported in Table 2. When compared to the control group, different signs of convulsions, ptosis and reduced motor activity were observed following the administration of the EO at 5 g/kg (Table 2). Those toxicity manifestations can be explained by lipid solubility of EO monoterpenes, easily reaching the nervous system.40, 41 Many works42 have reported neurotoxic effect of EO compounds at very low doses. For instance, LD50 of thujone is 0.2 g/kg and the content from this element in our samples is 0.49%. Nevertheless, most of EO constituents such as camphor, bornyl acetate, borneol and α-pinene are safe (LD50 > 2 g/kg). It has been reported that medicinal plants rich in bornyl acetate and borneol e.g. valerian (Valeriana officinalis), chamomile (Matricaria chamomilla) and lavender (Lavandula officinalis) traditionally used to relieve anxiety, restlessness and insomnia, are characterized by a low acute toxicity and highly sedative effect. Furthermore, consumption of this kind of EO reduced the motor activity in animal and human experiments.43, 44 Similarly, α-pinene has been shown to have a low acute oral toxicity in the rat with LD50 of 3.7 g/kg. However high doses can generate central nervous system depression and respiratory failure in rats.45
Table 2.
Acute effects of T. articulata essential oil after oral administration to Swiss mice (n = 6 female per group).
| Toxic signs | Control | EO (2000 mg/kg) % | EO (5000 mg/kg) % |
|---|---|---|---|
| Reduction of locomotor activity | 0 | 33.33 | 100 |
| Occurrence interval (h) | – | 0–1 | 0–6 |
| Ptosis | 0 | 0 | 100 |
| Occurrence interval (h) | – | – | 0–4 |
| Tremors | 0 | 33.33 | 100 |
| Occurrence interval (h) | – | 0–1 | 0–4 |
| Increased respiration rate | 0 | 33.33 | 100 |
| Occurrence interval (h) | – | 0–1 | 0–2 |
| Salivation | 0 | 66.66 | 66.66 |
| Occurrence interval (h) | – | 0–2 | 0–5 |
%: The percentage refers to the proportion of animals in the group that expressed the respective signals at some point during the observational period (up to 6 h).
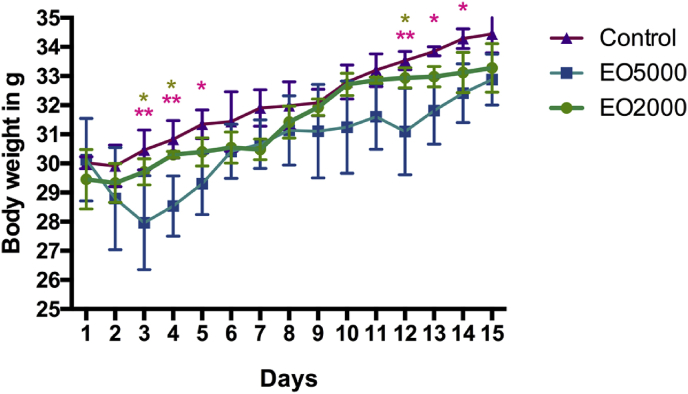
It is known that the changes in body weight (Fig. 1) are sensitive indicators for the detection of adverse effects of drugs and chemicals.46, 47 Since there was no visible changes in the general behavior and body weight of animals in the treated group with dose of 2 g/kg as compared to the control group after EO administration for 15 days; it could be concluded that oral administration of T. articulata EO had no effect on the growth and functions of mouses at this concentration. However, the group treated with 5 g/kg showed less activities and induced significant decrease in the final body weight compared to the control group. Those observations were highly detected during the first 5 days after administration, which is probably the result of the disturbances in the central nervous system functions (see Fig. 1 and Table 2).
Fig. 1.
Effect of acute oral toxicity of T. articulata essential oil on body weight changes of female mouses during the 14 days. All data are mean ± SD for 3 mouse/group. The (*) indicate significant differences at p < 0.05, while ** is for p < 0.001. *: diferences between EO5000 and control, and *: differences between E2000 and E5000.
In the other hand, the administration of EO at different doses (2 and 5 g/kg) did not produce any mortality. Thus, T. articulata EO falls in Class 5 (a substance with LD50 higher than 5000 mg/kg), hence the EO can be considered with low toxicity.25 Similar results were found by Teke et al., (2013),48 who did not observe any mortality after administration of the EO from Cupressus lusitanica an other member of the Cupressaceae family.
3.3. Anti-inflammatory activity
The inflammatory response is a multi-mediated phenomenon. During the progression of edema there is a release of some mediators produced from arachidonic acid metabolism through cyclo-oxygenase activity.28, 49, 50, 51 The first phase of inflammation, which lasts up to 2 h, involves the participation of histamine, serotonin, and bradykinin; while the second phase, that is from 3 to 4 h, is mainly sustained by prostaglandins, cytokines and nitric oxide release.52, 53, 54, 55
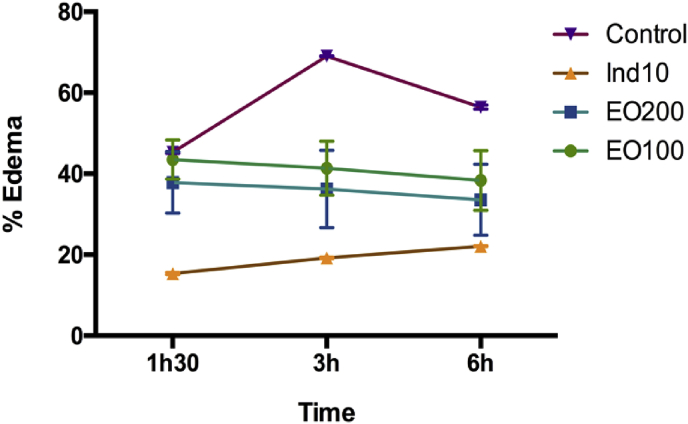
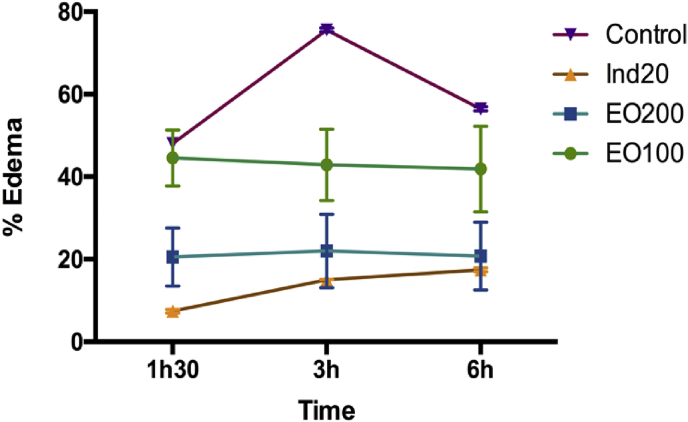
In this study, as shown in Table 3, Fig. 2, Fig. 3, the injection of carrageenan into the sub-plantar tissue and mechanical trauma of the right hind paw of rats in the control groups caused edema development, which peaked (0.57 ± 0.01 and 0.71 ± 0.02 mL in paw volume respectively) 3 h after the experimental induced paw edema. This result confirms that experimental trauma and carrageenan injection into the rat paw provokes a local and acute inflammatory reaction.56
Table 3.
Percentage of inhibition of induced inflammation by T. articulata essential oil.
| Treatments | Percentage of inhibition of induced inflammation |
||
|---|---|---|---|
| 1 h 30 min | 3 h | 6 h | |
| Carrageenan induced inflammation | |||
| Indomethacin (10 mg/kg) | 66.83 | 72.63 | 62.37 |
| EO (100 mg/kg) | 45.26 ± 6.42 | 64.91 ± 4.96 | 60.90 ± 7.36 |
| EO (200 mg/kg) | 50.43 ± 9.17 | 68.42 ± 8.00 | 64.71 ± 9.38 |
| Trauma induced inflammation | |||
| Indomethacin (20 mg/kg) | 86.89 | 84.08 | 75.45 |
| EO (100 mg/kg) | 50.18 ± 7.61 | 69.01 ± 4.18 | 48.79 ± 6.37 |
| EO (200 mg/kg) | 77.45 ± 6.57 | 84.51 ± 5.70 | 69.09 ± 6.02 |
Each value represents the mean ± SD (n = 6).
Fig. 2.
Effects of essential oil of T. articulata on carrageenan induced rat paw edema. Each value represents the mean ± SEM (n = 6) p < 0.001, statistically significant relative to control at 3 h.
Fig. 3.
Effect of essential oil of T. articulata on experimental trauma-induced rat paw edema. Each value represents the mean ± SEM (n = 6) p < 0.001, statistically significant relative to control at 3 h.
The effect of EO on carrageenan induced rat paw edema (Table 3) was dose-dependent from the first hour and 3 h after carrageenan injection with a peak effect (68.42 % inhibition) produced at 200 mg/kg. This effect was not statistically different (p < 0.05) from that produced by 10 mg/kg of indomethacin (72.63 % inhibition) (Table 3). The effect of the essential oil on experimental trauma induced rat paw edema was also time and dose dependent (Table 3) with a peak effect (84.51% inhibition after 3 h) produced at 200 mg/kg. Similarly this effect was comparable to that produced by 20 mg/kg of indomethacin (84.08 % inhibition) with no statistical differences (p < 0.05).
Considering that the production of arachidonic acid metabolites is the main factor responsible for both the first and the second phase of the inflammatory response, our results suggest that the anti-inflammatory effect of the T. articulata essential oil could be related to the inhibition of the release or synthesis of cyclo-oxygenase products. This might be attributed to its oxygenated monoterpenes (bornyl acetate, camphor and borneol) and monoterpene hydrocarbons (α-pinene, camphene and isolimonene), which possess strong anti-inflammatory activities.57, 58, 59, 60 It was demonstrated, that bornyl acetate, the main ingredient of Amomum villosum volatile oil, has been shown to possess anti-inflammatory activity as it was able to suppress ear swelling caused by dimethylbenzene.61 Moreover, borneol is able to exert inhibitory effects on the release of histamine from abdominal mast cells by 40.4%.62 It is also known that α-pinene presents important anti-inflammatory effects.59 Other monoterpenes as cis-verbenol, γ-terpinene and terpineol have been reported to possess anti-inflammatory activity in the animal models by TNFα inhibition and IL-2 production (intercellular chemical messengers, produced by macrophages and involved in the inflammatory process).63
Similar results were found by Djouahri et al. (2014)10 and Djouahri et al. (2013)17, which revealed that Algerian T. articulata essential oil had an excellent inflammatory activity using the Lipoxygenase inhibition and Xanthine oxidase inhibition assays.
The inflammatory response is also related to the neutrophil infiltration and production of the reactive free radical species derived from them, indicating that the T. articulata essential oil tested may exert its anti-inflammatory activity, at least partly, through the inhibition of neutrophil infiltration as well as the free radical scavenging. It has been shown that some of the anti-inflammatory agents including a terpenic compound of plant origin, exert their effect on inflammation through the inhibition of free radical generation by the activated neutrophils.64 Taking into consideration the above mentioned implication of antioxidants in anti-inflammatory process, it is suggested that the anti-inflammatory effect of the T. articulata leaves essential oil can be ameliorated and consolidated by its antioxidant activity.
3.4. Antioxidant activity
The antioxidant activity of Moroccan T. articulata essential oil was assessed by three complementary in vitro antioxidant assays (Table 4): i) the FRAP assay that estimates the ferric-reducing capacity of our extract. ii) the DPPH test and iii) TEAC assay, evaluating the H-donating or radical-scavenging ability of the oil using the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ABTS radical cation, respectively. The concentrations providing 50% inhibition (IC50) are listed in Table 4. As summarized in Table 4, a high ferric reductive capacity was obtained with the oil from Moroccan T. articulata (IC50 = 0.15×103 ± 0.01 μg/mL), but it's still lower compared to the positive controls namely the flavonol quercetin (IC50 = 2.06 ± 0.01 μg/mL) and synthetic BHT (IC50 = 7.02 ± 0.02 μg/mL). In the DPPH assay, the oil present IC50 with 12.05.103 ± 0.24 μg/mL which means low activity compared to Quercetin (IC50 = 1.29 ± 0.01 μg/mL) and BHT (IC50 = 4.20 ± 0.02 μg/mL). Similarly, in ABTS test IC50 of the EO is 8.90.103 ± 0.17 μg/mL, the synthetics antioxidant Trolox, as references showed higher activity (IC50 = 1.93 ± 0.00 μg/mL).
Table 4.
IC50 values (μg/mL) of T. articulata essential oil and of BHT, Quercetin and Trolox.
| Assays | T. articulata EO | Positive control |
||
|---|---|---|---|---|
| BHT | Quercetin | Trolox | ||
| DPPH | 12.05×103 ± 0.24 | 4.20 ± 0.02 | 1.29 ± 0.01 | – |
| ABTS | 8.90×103 ± 0.17 | – | – | 1.93 ± 0.05 |
| FRAP | 0.15×103 ±0 01 | 7.02 ± 0.02 | 2.06 ± 0.01 | – |
Values represent means ± SD (standard deviations) for triplicate experiments.
The antioxidant potential of the T. articulata EO might be explained partly by its high content of bornyl acetate, camphor and α-pinene. The antioxidant activities of this monoterpenes have been previously reported65 and have been demonstrated to be higher than that of camphene and camphor,66 however their antioxidant activity is less important than that of carvacrol and thymol which are absent in our plant extract66, 67.
4. Conclusions
The essential oil tested in the present work exhibited a considerable antioxidant and anti-inflammatory activities, with low toxicity, since the oral route at dose of 5.0 g/kg did not caused lethality in mouses during 15 days of observation. Those results indicate the promising pharmaceutical potential of T. articulata. Accordingly, the pharmacological actions of T. articulata in popular medicine practices may be related to the presence of bornyl acetate, camphor and α-pinene as major component in the essential oil.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
The author Meryem El Jemli is thankful to Pr Jamal Taoufik (Director of CeDoc Faculty of Medecine and Pharmacy Rabat) for his kind support, encouragement and interest in this research work. Also to Dr Abdelmounaim El Hassani engineer in the Regional Directorates of Water and Forests and Desertification Control for his help to collect the plant material. Moreover, many thanks to Pr Fennane botanist of scientific institute, Rabat for his help in identification of the species. Special thanks to Yousra El Jemli from Laboratory of Research and Innovation, Sustainable Development and Green Chemistry Expertise, University of Cadi Ayyad, Faculty of Sciences Semlalia, Marrakech, Morocco, who helped to perform the statistical analysis and to draft the manuscript. We also would like to thank all colleagues and professors at the Laboratory of Pharmacology and Toxicology, Faculty of Medicine and Pharmacy, Mohammed V University, Rabat, Morocco, especially Pr Amina Zellou for her kind advice during our experimental work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Meryem El Jemli, Email: meryem.jemli@um5s.net.ma.
Katim Alaoui, Email: k.alaoui@um5s.net.ma.
References
- 1.Polunin O., Huxley A. 1967. Fleurs du bassin méditerranéen, Paris. [Google Scholar]
- 2.Nicolás M.J., Esteve M.A., Palazón J.A., López Hernández J.J. Modelo sobre las preferencias de hábitat a escala local de Tetraclinis articulata (Vahl) Masters en una población del límite septentrional de su área de distribución. An Biol. 2004;26:157–167. [Google Scholar]
- 3.Montanari B. Aromatic, medicinal plants and vulnerability of traditional herbal knowledge in a berber community of the high atlas mountains of Morocco. Plant Divers Resour. 2014;36:388–402. [Google Scholar]
- 4.Bellakhdar J. Ibis Press; 1997. La pharmacopée marocaine traditionnelle. Médecine arabe ancienne et savoirs populaires – Saint–Etiew; pp. 272–273. [Google Scholar]
- 5.Ziyyat A., Legssyer A., Mekhfi H., Dassouli A., Serhrouchni M., Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol. 1997;58:45–54. doi: 10.1016/s0378-8741(97)00077-9. [DOI] [PubMed] [Google Scholar]
- 6.Patel D.K., Kumar R., Laloo D., Hemalatha S. Natural medicines from plant source used for therapy of diabetes mellitus: an overview of its pharmacological aspects. Asian Pac J Trop Dis. 2012;2:239–250. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batanounmy K.H., Hammouda F.M., Ismail S.I., Abdel-Azim N.S., Shams K.A. IUCN Centre for Mediterranean Cooperation; Malaga (Spain): 2005. Citrullus colocynthis. A guide to medicinal plants in North Africa; pp. 235–237. [Google Scholar]
- 8.Anon Final report on the safety assessment of Juniperus communis extract, Juniperus oxycedrus extract, Juniperus oxycedrus Tar, Juniperus phoenicea extract, and Juniperus virginiana extract. Int J Toxicol. 2001;20:41–56. doi: 10.1080/10915810160233758. [DOI] [PubMed] [Google Scholar]
- 9.Ciesla W.M. FAO. Non-Wood Forest 12; Roma, Italy: 1998. Non-Wood Forest Products from Conifers. Food and Agriculture Organization of the United Nations; p. 124. [Google Scholar]
- 10.Djouahri A., Sakaa B., Boudarene L. In vitro synergistic/antagonistic antibacterial and anti-inflammatory effect of various extracts/essential oil from cones of Tetraclinis articulata (Vahl) Masters with antibiotic and anti-inflammatory agents. Ind Crops Prod. 2014;56:60–66. [Google Scholar]
- 11.Abi-Ayada F.Z., Abi-Ayada M., Lazzounia H.A., Rebiahib S.A., Bessierec C. Antibacterial activity of essential oil extracted from leaves of Tetraclinis articulata (Vahl) Masters from Algeria flora. J Microbiol Biotechnol Res. 2011;1:1–6. [Google Scholar]
- 12.Bourkhiss B., Ouhssine M., Hnach M., Amechrouq A., Chaouch A., Satrani B. Chemical composition of the essential oil of Tetraclinis articulata (Vahl) from Maroc. Phys Chem News. 2007;35:128–132. [Google Scholar]
- 13.Bourkhiss M., Hnach M., Bourkhiss B., Ouhssine M., Chaouch A. Chemical composition and antimicrobial properties of the essential oil extracted the leaves of Tetraclinis articulata (Vahl) from Morocco. Afr Sci. 2007;3:232–242. [Google Scholar]
- 14.Abi-Ayad F.Z., Abi-Ayad M., Lazouni A.H., Rebiahi S.A. Evaluation of Tetraclinis articulata essential oil from Algeria flora as a potential source of antifungal activity and study of its chemical composition. J Indian Acad Wood Sci. 2013;10:9–15. [Google Scholar]
- 15.Tekaya-Karoui A., Boughalle N., Hammami S., Ben Jannet H., Mighri Z. Chemical composition and antifungal activity of volatile components from woody terminal branches and roots of Tetraclinis articulata (Vahl) Masters growing in Tunisia. Afr J Plant Sci. 2011;5:115–122. [Google Scholar]
- 16.Bourkhiss M., Hnach M., Paolini J., Costa J., Farah A., Satrani B. Antioxidant and anti-inflammatory properties of essential oils of the various parts of Tetraclinis articulata (Vahl) masters from Morocco. Bull Soc R Sci Liège. 2010;79:141–154. [Google Scholar]
- 17.Djouahri A., Boudarene L., Meklati B.Y. Effect of extraction method on chemical composition, antioxidant and anti-inflammatory activities of essential oil from the leaves of Algerian Tetraclinis articulata (Vahl) Masters. Ind Crops Prod. 2013;44:32–36. [Google Scholar]
- 18.Barrero A.F., Herrador M.M., Arteaga P. Chemical composition of the essential oil of leaves and wood of Tetraclinis articulata (Vahl) Masters. J Essent Oil Res. 2005;17:166–168. [Google Scholar]
- 19.Zrira S., Benjilali B., Elmrani A. Chemical composition of the sawdust oil of Moroccan Tetraclinis articulata (Vahl) J Essent Oil Res. 2005;17:96–97. [Google Scholar]
- 20.Buhagiar J., Podestat M.T.C., Cioni P.L., Flamini G., Morelli L. Essential oil composition of different parts of Tetraclinis articulata. J Essent Oil Res. 2000;12:29–32. [Google Scholar]
- 21.Ait Igri M., Holeman M., Ilidrissi A., Berrada M. Contribution to the chemical study of branches and wood essential oils of Tetraclinis articulata (Vahl) Masters. Plant Med Phytother. 1990;24:36–43. [Google Scholar]
- 22.Buhagiar J.A., Podesta M.T.C., Wilson A.P., Micallef M.J., Ali S. The induction of apoptosis in human melanoma, breast and ovarian cancer cell lines using an essential oil extract from the conifer Tetraclinis articulata. Anticancer Res. 1999;19:5435–5443. [PubMed] [Google Scholar]
- 23.Gautam N., Mantha A.K., Mittal S. Essential oils and their constituents as anticancer agents: a mechanistic view. BioMed Res Int. 2014:2014. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams R.P. Identification of essential oil components by gas chromatography/mass spectroscopy. J Am Soc Mass Spectrom. 1997;6:671–672. [Google Scholar]
- 25.Organisation for Economic Co-operation and Development (OECD) 2008. Guidelines for Testing of Chemical, Guideline 425. Acute Oral Toxicity-up-and-Down-procedure (UDP) (Paris) [Google Scholar]
- 26.Lipnick R.L., Cotruvo J.A., Hill R.N. Comparison of the up-and-down, conventional LD50 and fixed dose acute toxicity procedures. Food Chem Toxicol. 1995;33:223–231. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- 27.Organisation for Economic Co-operation and Development (OECD) 2002. Guidelines for Testing of Chemical, Guideline 423. Acute Oral Toxicity – Acute Toxic Class Method (Paris) [Google Scholar]
- 28.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema hind paw of the rat as an easy for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 29.Riesterer L., Jacques R. 1970. Pharmacology; pp. 3–243. [DOI] [PubMed] [Google Scholar]
- 30.Aruoma O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant food. Mutat Res. 2003;9:523–524. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 31.Sahin F., Cakmakci R., Kantar F. Sugar beet and barley yields in relation to inoculation with N2 fixing and phosphate solubilizing bacteria. Plant Soil. 2004;265:123–129. [Google Scholar]
- 32.Pukalskas A., Van Beek T.A., Venskutonis R.P., Linssen J.P.H., Van Veldhuizen A., Groot A. Identification of radical scavengers in sweet grass (hierochloeodorata) J Agric Food Chem. 2002;50:2914–2919. doi: 10.1021/jf011016r. [DOI] [PubMed] [Google Scholar]
- 33.Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. [Google Scholar]
- 34.Larabi F., Benhassaini H., Bennaoum Z. Essential oil composition of Tetraclinis articulata (Vahl.) Masters. Leaves from Algeria. Int J Herb Med. 2015;2:31–33. [Google Scholar]
- 35.Toumi F.B., Benyahia M., Hamel L., Mohamedi H., Boudaghen L. Etude comparative de la composition chimique des huiles essentielles de Tetraclinis articulata (Vahl) Masters originaire d'Algérie. Acta Bot Gallica. 2011;158:93–100. [Google Scholar]
- 36.Herzi N., Bouajila J., Camy S., Romdhane M., Condoret J.S. Comparison of Extraction techniques for essential oil from Tetraclinis articulata: yield, chemical composition and antioxidant activity. Food Chem. 2013;141:3537–3545. doi: 10.1016/j.foodchem.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 37.Abbad A., Sfairi Y., Hassani L. Intraspecific chemical variability of essential oil from leaves of Cupressus atlantica Gaussen, an endemic and endangered coniferous species in Morocco. Nat Prod Res. 2013;27:579–582. doi: 10.1080/14786419.2012.673609. [DOI] [PubMed] [Google Scholar]
- 38.Sivropoulou A., Nikolaou C., Papanikolaou E., Kokkini S., Lanaras T., Arsenakis M. Antimicrobial, cytotoxic and antiviral activities of Salvia fructicosa essential oil. J Agric Food Chem. 1997;45:3197–3201. [Google Scholar]
- 39.Fakchich J., Elachouri M. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J Ethnopharmacol. 2014;154:76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 41.Teuscher E., Melzig M., Villmann E., Möritz K.U. Untersuchungen zum wirkungsmechanismus ätherischer öle. Z Phytother. 1990;11:87–92. [Google Scholar]
- 42.Teke G.N., Kuete V. Acute and subacute toxicities of African medicinal plants. Toxicol Surv Afr Med Plants. 2014:63. [Google Scholar]
- 43.Assemi M. Herbs affecting the central nervous system: gingko, kava, St. John's wort, and valerian. Clin Obstet Gynecol. 2001;44:824–835. doi: 10.1097/00003081-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 44.Buchbauer G., Jirovetz L., Jager W., Plank C., Dietrich H. Fragrance compounds and essential oils with sedative effects upon inhalation. J Pharm Sci. 1993;82:660–664. doi: 10.1002/jps.2600820623. [DOI] [PubMed] [Google Scholar]
- 45.Da Silva A.C., Lopes P.M., de Azevedo M.M., Costa D.C., Alviano C.S., Alviano D.S. Biological activities of a-pinene and ß-pinene enantiomers. Molecules. 2012;17:6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tofovic S.P., Jackson E.K. Effects of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J Cardiovasc Pharmacol. 1999;33:360–366. doi: 10.1097/00005344-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Bailey J.K., Schweitzer J.A., Rehill B.J., Lindroth R.L., Martinsen G.D., Whitham T.G. Beavers as molecular geneticists: a genetic basis to the foraging of an ecosystem engineer. Ecology. 2004;85:603–608. [Google Scholar]
- 48.Teke G.N., Elisée K.N., Roger K.J. Chemical composition, antimicrobial properties and toxicity evaluation of the essential oil of Cupressus lusitanica Mill. leaves from Cameroon. BMC Complement Altern Med. 2013;13:130. doi: 10.1186/1472-6882-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Della Loggia R., Tubaro A., Dri P., Zilli C., Del Negro P. Plant Flavonoids in Biology and Medicine, Buffalo, New York (USA) Liss; 1986. The role of flavonoids in the antiinflammatory activity of Chamomilla recutita. 22–26 Jul 1985. [PubMed] [Google Scholar]
- 50.Alcaraz M.J., Jimenez M.J. Flavonoids as anti-inflammatory agents. Fitoterapia. 1988;59:25–38. [Google Scholar]
- 51.Cuzzocrea S., Chatterjee P.K., Mazzon E. Role of induced nitric oxide in the initiation of the inflammatory response after postischemic injury. Shock. 2002;18:169–176. doi: 10.1097/00024382-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Vinegar R., Schreiber W., Hugo R. Biphasic development of carrageenin edema in rats. J Pharmacol Exp Ther. 1969;166:96–103. [PubMed] [Google Scholar]
- 53.Di Rosa M., Sorrentino L., Parente L. Non-steroidal anti-inflammatory drugs and leucocyte emigration. J Pharm Pharmacol. 1972;24:575–577. doi: 10.1111/j.2042-7158.1972.tb09059.x. [DOI] [PubMed] [Google Scholar]
- 54.Di Rosa M. Piccin Medical Books; Padua: 1974. Effect of Non-steroidal Anti-inflammatory Drugs on Leukocyte Migration. Future Trends in Inflammation; pp. 143–152. [Google Scholar]
- 55.Seibert K., Zhang Y., Leahy K. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boughton-Smith N.K., Deakin A.M., Follenfant R.L., Whittl B.J.R., Coarland L.G. Role of oxygen radicals and arachidonic acid metabolites in the reverse passive arthus reaction and carrageenan paw oedema in the rat. Br J Pharmacol. 1999;110:896–902. doi: 10.1111/j.1476-5381.1993.tb13897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tung Y.T., Chua M.T., Wang S.Y., Chang S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol. 2008;99:3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 58.Tung Y.T., Yen P.L., Lin C.Y., Chang S.T. Anti-inflammatory activities of essential oils and their constituents from different provenances of indigenous cinnamon (Cinnamomum osmophloeum) leaves. Pharm Biol. 2010;48:1130–1136. doi: 10.3109/13880200903527728. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J.Y., Tang F.D., Mao G.G., Bian R.L. Effect of a-pinene on nuclear translocation of NF-kB in THP-1 cells. Acta Pharmacol Sin. 2004;25:480–484. [PubMed] [Google Scholar]
- 60.Cheng C.Y., Ho T.Y., Lee E.J., Su S.Y., Tang N.Y., Hsieh C.L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008;36:1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 61.Wu X., Li X., Xiao F., Zhang Z., Xu Z., Wang H. Studies on the analgesic and anti-inflammatory effect of bornyl acetate in volatile oil from Amomum villosum. Zhong Yao Cai. 2004;27:438–439. [PubMed] [Google Scholar]
- 62.Watanabe K, Yano S, Horie T, et al. Borneol as allergy inhibitor. Japan Patent No. 06211713. 1994.
- 63.Ahn GS, Jang HU, Jung GY, et al. Essential oil component having inhibition activity of leukotriene production. Korea Patent. 2001; 2102837321:12.
- 64.Halliwell B. Drug antioxidant effects. A basis for drug selection. Drugs. 1991;42:596–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W., Wu N., Zu Y.G., Fu Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108:1019–1022. doi: 10.1016/j.foodchem.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 66.Ruberto G., Baratta M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69:167–174. [Google Scholar]
- 67.Kulisic T., Radonic A., Katalinic V., Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. [Google Scholar]