Abstract
Objective
To assess the compliance of community pharmacies with the regulations that prohibit the dispensing of prescription-only medications in the absence of a physician prescription in Saudi Arabia.
Method
A cross-sectional study was conducted in the period between October 2014 and January 2015. A list of 10 prescription-only medications were selected to be studied. 150 community pharmacies were visited across 6 major regions in Saudi Arabia to assess the prevalence of non-compliance among community pharmacies. Pharmacies were selected in random and researchers (disguised as patients) requested to purchase prescription-only medications in the absence of a prescription. Not all medications were purchased at once. Data were recorded per pharmacy, where pharmacies that approved dispense of the selected drug were scored as non-compliant and the pharmacies that rejected dispense of the selected drug were scored as compliant. Compliance rate was calculated per region per drug. Pharmacies based in governmental hospitals were visited in parallel. A total of 20 were visited. Data and statistical analysis were performed using Statistical Analyses Software (SAS 9.3).
Results
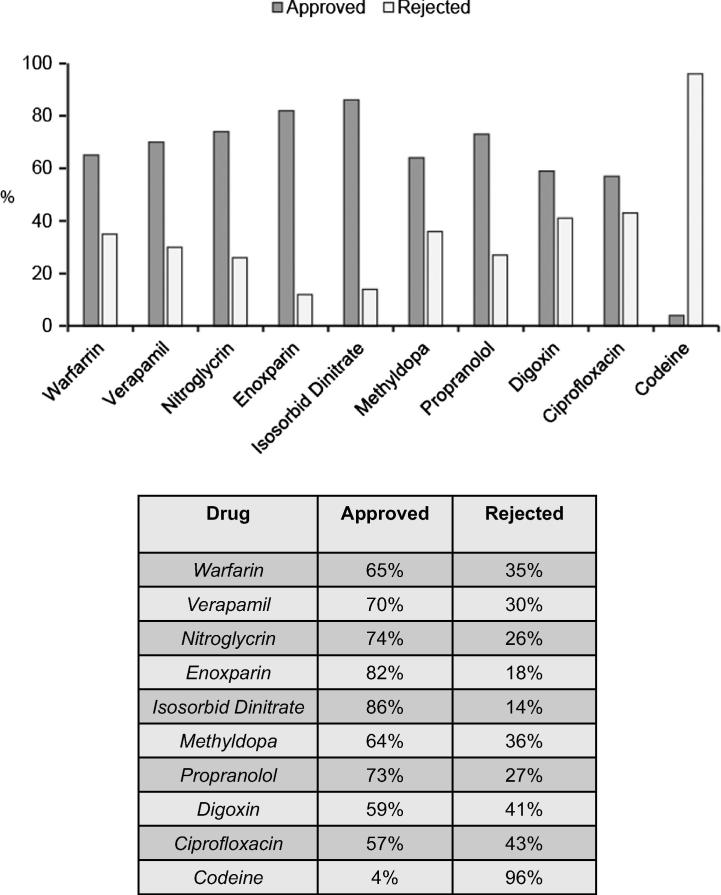
A total of 150 pharmacies were visited over a period of 3 months. On average, the percent approved dispense of prescription-only drugs across 6 regions in Saudi Arabia is 63% and the percent rejected dispense is 37% representing a significant non-compliance rate regarding the selected list of medications in this study. The frequency of dispense per medication across 6 major regions in Saudi Arabia is as follows: Isosorbide dinitrate (86%), Enoxaparin (82%), nitroglycerin (74%), Propranolol (73%), Verapamil (70%), Warfarin (65%), Methyldopa (64%), Ciprofloxacin (57%) and Codeine (4%).
Conclusions
Non-compliance of community pharmacies with the law of pharmaceutical practice is at an alarming rate in the Kingdom of Saudi Arabia and authoritative figures must intervene to impede and combat such activities.
Keywords: Pharmacy compliance, Prescription, Drug dispensing
1. Introduction
In Saudi Arabia, patients perceive community pharmacies as a first line of contact prior to a physician visit, as is the case with many developing countries (Sabry et al., 2014, Hussain et al., 2012). This is a concern to the public, in particular, as medications are dispensed for inappropriate indications (Sabry et al., 2014). Such practices are threatening to patients’ safety and to the potential development of drug resistant pathogens, especially with the uncontrolled dispense of antibiotics (Al-Ghamdi, 2001, Zowawi et al., 2013). Under the Saudi law, only licensed pharmacists are allowed to practice the profession of pharmacy. This law requires pharmacists to dispense drugs with a valid physician prescription only (Al-Mohamadi et al., 2013). Despite having a clear law that regulated the dispense of prescription-only medications, several studies have documented breaches of the current law SA Bawazir, 1992 (Al-Mohamadi et al., 2013).
In 1987, 10 years after the Saudi law of pharmacy practice was passed, 85.4% of pharmacies in Saudi Arabia dispensed antibiotics promptly (Al-Mohamadi et al., 2013). In 1992, the percentage of dispensing prescription drugs without a prescription was 35% (SA Bawazir, 1992), an alarming percentage after 15 years. The percentage of non-compliance to the law was as high as 98.9% in 2001 in the Eastern Province, a large region of Saudi, where only 1 pharmacist out of 88 community pharmacies surveyed refused to dispense an antibiotic without a prescription (Al-Ghamdi, 2001) illustrating that the vast majority of pharmacists do not comply to current pharmaceutical law in Saudi Arabia.
Dispensing medications without a valid prescription can have devastating consequences in the long run. For instance, it can impact patient compliance to prescribed medications, which is a worldwide concern (Al-Ghamdi, 2001, Brown and Bussell, 2011). It will be more likely that patients do not take their medications if they do not get the proper diagnosis or education from a physician; and simply suffice by managing symptoms with prescription medications from pharmacies. Yet of more concern is prescription drug abuse, addiction and overdose, as controlled substances are dispensed without a valid prescription.
In the present study, we assess the magnitude of pharmacy compliance with local laws and regulations that govern dispensing of prescription-only drugs in the Kingdom of Saudi Arabia. These results represent the first true reflection of the current situation of pharmacy compliance with appropriate dispensing practice of prescription-only medications.
2. Method
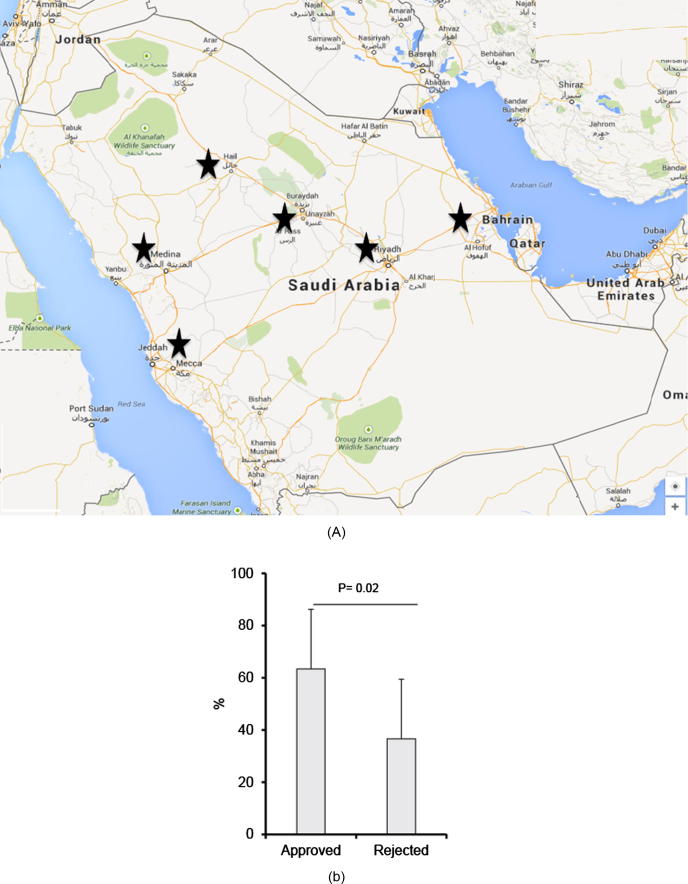
In the current study, 150 community pharmacies across Saudi Arabia were randomly selected to assess the magnitude of compliance/noncompliance across the country The pharmacies distribution is as follows: Riyadh (36 pharmacies), Jeddah (24 pharmacies), Madinah (20 pharmacies), Hail (15 pharmacies), Qassim (33 pharmacies) and Sharqiah (22 pharmacies) (Fig. 1A). All selected cities represent major regions in the country. The study was performed over a period of 3 months (between Oct 2014 and Jan 2015). A total of 10 prescription-only medications for various indications and with a high risk profile of adverse effects were selected for the study. The researchers posed as patients and requested to purchase one or two of the selected medications.
Figure 1.
Non-compliance rate with prescription-only medications in Saudi Arabia. (A) Major regions where both community and hospital based-pharmacies were visited. (B) Overall non-compliance rate with the law of pharmaceutical practice in community pharmacies in Saudi Arabia. The bars represent the average of approved and rejected rates of dispense for the prescription-only medications across the major regions in Saudi Arabia. Statistical analysis was performed using the unpaired t test, and error bars represent the standard deviation among the medications surveyed.
The list of drugs is presented in Table 1. The same requests were submitted to hospital pharmacies, the government sector, for comparison between the two settings.
Table 1.
List of requested medications without prescriptions.
| Medication name | Indication |
|---|---|
| Methyldopa | Antihypertension (Zowawi et al., 2013) |
| Codeine | Pain Management (Wolff et al., 1940) |
| Isosorbide Dinitrate | Heart Failure (Thadani and Jacob, 2008) |
| Enoxaparin | Antithrombotic (Meneveau, 2009) |
| Nitroglycerin | Stable Angina Pectoris (Todd et al., 1990) |
| Ciprofloxacin | Complicated UTI and other infections (Finch, 2000) |
| Verapamil | Antiarrhythmic (Baky and Singh, 1982) |
| Warfarin | Primary and secondary prevention of VTE (Alquwaizani et al., 2013) |
| Digoxin | Permanent atrial fibrillation (AF), heart failure and left ventricular dysfunction (Tatlisu et al., 2015) |
| Propranolol | Antihypertension (Hayton, 1975) |
3. Statistical analyses
Descriptive statistics were produced for requested medications. Surveyed community pharmacies were scored as per their response to each requested medication. Responses were calculated as percentage of non-compliance with laws and regulations per drug per region (Figs. 2). Also, we calculated the overall percentage of non-compliance for each requested drug (Fig. 3). Data were analyzed using SAS version 9.3. A chi-square test or Fisher’s exact tests were used to analyze the categorical data. All statistical tests were conducted with a 2-tailed alpha of 0.05.
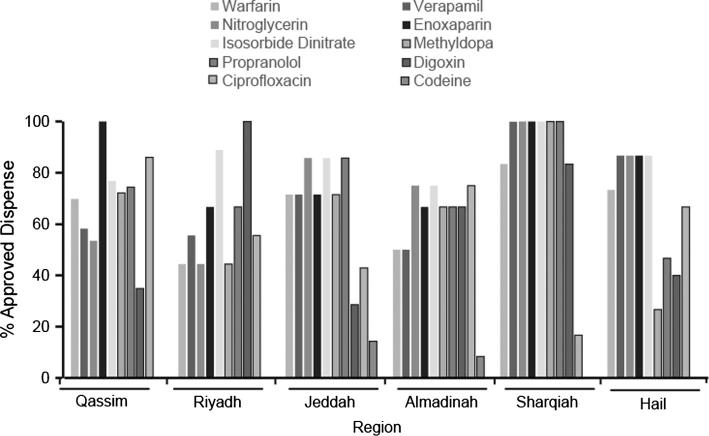
Figure 2.
Prescription-only medications approved dispense rate in 6 major regions in Saudi Arabia. Pharmacies were scored per region and per medication. The percentage of approved or rejected dispense was calculated based on the number of pharmacies surveyed per region. Bars represent the percentage of approved dispense only.
Figure 3.
Incidence of dispense for prescription-only medications in Saudi Arabia. Incidence of dispense was calculated per medication. The average of approved dispense per medication was calculated from the 6 regions as well as the average of the rejected dispense per medication. Statistical analysis was performed between the groups and a significant difference between approved and rejected rates was calculated (p = 0.09).
4. Results
4.1. Prevalence of non-compliance with the pharmaceutical professional standards regarding the dispense of prescription-only drugs
We assessed the prevalence of noncompliance across major cities in the Kingdom (Fig. 1A). On average, the percent approved dispense of prescription-only drugs across 6 regions in Saudi Arabia is 63% and the percent rejected dispense is 37% (Fig. 1B).
4.2. Percent distribution of approved dispense of prescription-only drugs across Saudi Arabia
Non-compliance was scored as percentage of approved dispense (Fig. 2) and as percentage of rejected dispense per region. Across all regions, the only two drugs that were rejected to be dispensed at some pharmacies are as follows: Enoxaparin in the regions of Qassim and Sharqiah only and codeine in the regions of Qassim, Riyadh, Sharqiah and Hail. Interestingly, the percent of approved dispense was on average above 50 percent for the remaining 8 drugs (Fig. 2).
4.3. Codeine is the least dispensed prescription-only medication in Saudi Arabia
We further analyzed the data collected from the major 6 regions in Saudi Arabia to assess which prescription –only drug is most likely to be dispensed and vice versa. Among the 10 studied classified prescription-only drugs, Isosorbide Dinitrate had the highest incidence of dispense, at an 86% approved rate of dispense, followed by Enoxaparin at 82%. The prescription-only drug that had the lowest incidence of dispense was Codeine, with 4% approved rate of dispense, followed by Ciprofloxacin at a 57% approved rate of dispense (Fig. 3). Collectively, these results illustrate an alarming rate of dispense of prescription-only medications in Saudi Arabia.
The same medication requests were made to pharmacy departments in governmental hospitals in each of the investigated cities [data not shown]. Unlike community pharmacies, requests were rejected in the absence of valid prescriptions, demonstrating that laws and work ethics are well observed in the governmental settings. While there are many explanations for the observed violations of drug dispensing laws, financial gain cannot be ruled out as the main reason.
5. Discussion
Non-compliance to the pharmaceutical law governing dispense of prescription-only medications is a common malpractice in Saudi Arabia and other developing countries (Sabry et al., 2014, Al-Mohamadi et al., 2013). Such practices do not only reflect the pharmacist’s misconduct, but it also puts a patient’s life in danger (Khan, 2014).
While a number of studies have previously investigated the dispensing malpractice in confined cities in Saudi Arabia, the present study, however, is the first of its kind to rather assess pharmacy compliance over six major cities in Saudi Arabia. In the current study we assessed the community pharmacy compliance with dispensing regulations that prohibit the dispensing of medications in the absence of a physician prescription.
The profession of pharmacy mandates a pharmacist to comply with international ethical standards of care and it holds the pharmacist responsible for malpractice (Sveska, 1993). A study by Bawazir and colleagues more than two decades ago, reported that more than 35% of medications dispensed without prescription in Saudi Arabia were prescription only drugs [SA Bawazir, 1992]. More recently, a study conducted in the region of Riyadh reported 49% of the medications being dispensed was prescription-only (Aljadhey et al., 2015), indicating an unfavorable increase in the malpractice over a period of more than 20 years.
The rate of non-compliance across community pharmacies in Saudi Arabia is alarming; however, the extent of non-compliance to dispensing high risk prescription-only medications has not been thoroughly investigated. We report that the non-compliance rate with dispense of prescription-only medications across 150 community pharmacies across 6 regions in Saudi Arabia is as high as 63%, a rate of appreciable concern.
The relatively narrow therapeutic index of digoxin and warfarin makes them toxic without appropriate use (Tatlisu et al., 2015, Alquwaizani et al., 2013). Digitalis toxicity is a well-documented problem for many years due to their narrow therapeutic index, and thus prescription of digoxin usually requires through estimation of renal and hepatic functions as well as close monitoring (Kanji and MacLean, 2012, Tatlisu et al., 2015). However, surprisingly the rate of approved dispense for digoxin in community pharmacies without a valid prescription is as high as 59%. Warfarin adverse effects hospitalization accounts for 33.3% of hospitalized cases (Budnitz et al., 2011), yet the approved dispense rate across community pharmacies in Saudi Arabia is at a high of 63%. Additionally, having prescription-only drugs with narrow therapeutic indices readily dispensed will likely increase hospital related visits due to potential overdose or toxicity. Although 66.6% of pharmacists in a previous study perceived unwanted professional behavior about controlled drugs, as an ethical problem (Al-Arifi, 2014), it is intriguing that the rate of non-compliance is well above 65% with the vast majority of the prescription-only medications surveyed in the current study.
The likely outcome for patients of non-complied pharmacists includes increased risks of adverse drug reaction and its consequences such as death, reduced functional abilities or long-term disability that may require use of medical resources such as nursing homes, hospital visits and hospital admissions, which as a result increases the health cost and expenditure (Col et al., 1990, Sullivan et al., 1990). Reports of Chronic Obstructive Pulmonary Disease patients indicated that poor adherence to drug therapy and disease management leads to emergency hospitalization (Fuso et al., 1995). Dispensing malpractice can also be viewed as an ethical issue, where privately run pharmacies demonstrated their preference of financial gain over patient’s safety. Dispensing malpractice does not only affect the patients, but also the provider, the physician and the significant healthcare consequences that dispensing malpractice possesses, makes it an imperative public health concern.
In conclusion, dispensing malpractice remains a major threat to public health. The vast majority of pharmacists do not adhere to the law that governs dispense of prescription-only medications, thus neglecting the harms that such practices would cause to the community. We suggest that in addition to the necessity of establishing a code of ethics for pharmacists in Saudi Arabia (Al-Arifi, 2014), a close and constant monitoring of pharmacies by the responsible authority figures such as the Saudi FDA and the Ministry of Health will minimize and reduce potential misconduct. Also, the authorities should provide further education and training for pharmacists to emphasize the potential devastating and detrimental consequences of negligence in pharmacy practice to the public health.
Acknowledgment
This research was supported by the Deanship of Research (grant 0150040 to M.A.) at the University of Hail, Kingdom of Saudi Arabia.
The data from Al-Qassim region were kindly collected and supplied by Mohammed Almutairi and Mased Almutairi.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Arifi M.N. Community pharmacist perception and attitude toward ethical issues at community pharmacy setting in central Saudi Arabia. Saudi Pharm. J. 2014;22(4):315–325. doi: 10.1016/j.jsps.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi M.S. Empirical treatment of uncomplicated urinary tract infection by community pharmacist in the Eastern province of Saudi Arabia. Saudi Med. J. 2001;22(12):1105–1108. [PubMed] [Google Scholar]
- Aljadhey H. Self-medication in Central Saudi Arabia. Community pharmacy consumers’ perspectives. Saudi Med. J. 2015;36(3):328–334. doi: 10.15537/smj.2015.3.10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mohamadi A. Dispensing medications without prescription at Saudi community pharmacy: extent and perception. Saudi Pharm. J. 2013;21(1):13–18. doi: 10.1016/j.jsps.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alquwaizani M. Anticoagulants: a review of the pharmacology, dosing, and complications. Curr. Emerg Hosp. Med. Rep. 2013;1(2):83–97. doi: 10.1007/s40138-013-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baky S.H., Singh B.N. Verapamil hydrochloride: pharmacological properties and role in cardiovascular therapeutics. Pharmacotherapy. 1982;2(6):328–353. doi: 10.1002/j.1875-9114.1982.tb03210.x. [DOI] [PubMed] [Google Scholar]
- Brown M.T., Bussell J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnitz D.S. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- Col N., Fanale J.E., Kronholm P. The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly. Arch. Intern. Med. 1990;150(4):841–845. [PubMed] [Google Scholar]
- Finch R.G. Ciprofloxacin: efficacy and indications. J. Chemother. 2000;12(Suppl 1):5–7. doi: 10.1080/1120009x.2000.11782289. [DOI] [PubMed] [Google Scholar]
- Fuso L., Incalzi R.A., Pistelli R., Muzzolon R., Valente S., Pagliari G. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am. J. Med. 1995;98(3):272–277. doi: 10.1016/s0002-9343(99)80374-x. [DOI] [PubMed] [Google Scholar]
- Hayton R.C. Use and abuse of propranolol. Can. Fam. Physician. 1975;21(11):71–73. [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Ibrahim M.I., Baber Z.U. Compliance with legal requirements at community pharmacies: a cross sectional study from Pakistan. Int. J. Pharm. Pract. 2012;20(3):183–190. doi: 10.1111/j.2042-7174.2011.00178.x. [DOI] [PubMed] [Google Scholar]
- Kanji S., MacLean R.D. Cardiac glycoside toxicity: more than 200 years and counting. Crit. Care Clin. 2012;28(4):527–535. doi: 10.1016/j.ccc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Khan T.M. The consequences of nonprescription medication sales in Saudi Arabia’s community pharmacies: regulations without implementation. Ther. Adv. Drug Saf. 2014;5(4):173–174. doi: 10.1177/2042098614526770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneveau N. Safety evaluation of enoxaparin in currently approved indications. Exp. Opin. Drug Saf. 2009;8(6):745–754. doi: 10.1517/14740330903352498. [DOI] [PubMed] [Google Scholar]
- Sabry N.A., Farid S.F., Dawoud D.M. Antibiotic dispensing in Egyptian community pharmacies: an observational study. Res. Soc. Adm. Pharm. 2014;10(1):168–184. doi: 10.1016/j.sapharm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Sullivan S., Kreling D., Hazlet T. Noncompliance with medication regimens and subsequent hospitalizations: a literature analysis and cost of hospitalization estimate. J. Res. Pharm. Econ. 1990;2:19–33. [Google Scholar]
- Sveska K.J. Pharmacist liability. Am. J. Hosp. Pharm. 1993;50(7):1429–1436. [PubMed] [Google Scholar]
- Tatlisu M.A. Inappropriate use of digoxin in patients presenting with digoxin toxicity. J. Geriatr. Cardiol. 2015;12(2):143–146. doi: 10.11909/j.issn.1671-5411.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani U., Jacob R.G. Isosorbide dinitrate/hydralazine: its role in the treatment of heart failure. Drugs Today (Barc.) 2008;44(12):925–937. doi: 10.1358/dot.2008.44.12.1131826. [DOI] [PubMed] [Google Scholar]
- Todd P.A., Goa K.L., Langtry H.D. Transdermal nitroglycerin (glyceryl trinitrate). A review of its pharmacology and therapeutic use. Drugs. 1990;40(6):880–902. doi: 10.2165/00003495-199040060-00009. [DOI] [PubMed] [Google Scholar]
- Wolff H.G., Hardy J.D., Goodell H. Studies on pain. Measurement of the effect of morphine, codeine, and other opiates on the pain threshold and an analysis of their relation to the pain experience. J. Clin. Invest. 1940;19(4):659–680. doi: 10.1172/JCI101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zowawi H.M. Beta-Lactamase production in key gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013;26(3):361–380. doi: 10.1128/CMR.00096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]