Abstract
Mechanisms of antioxidant and apoptosis induction may be involved in the management of cancer by medicinal plants. Aim of the study was designed to evaluate anticancer activity of the methanolic extract of Cordia dichotoma leaves (MECD) against a human prostate carcinoma cell line, PC3. Flavonoid content was determined by colorimetric principle and antioxidant activity by various in vitro assays. MTT, DCFH-DA and DAPI staining assays were performed for the evaluation of cytotoxicity, analysis of induction of apoptosis and intracellular reactive oxygen species (ROS) activity level by MECD against human prostate carcinoma cell line, PC3. Flavonoid content was found to be 160 mg QE/g extract. IC50 values for MECD treatment in various assays based on scavenging of 2,2-diphenyl-1-picrylhydrazyl, 2,2-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid), nitric oxide, peroxy radical, superoxide anion, hydroxy radical were found to be 315.5, 38, 476, 523, 197, 82 μg/ml respectively. MECD exposure to PC3 cells significantly increased the cell death (p < 0.001, IC50 = 74.5 μg/ml), nuclear condensation, apoptosis (p < 0.001) and induced production of ROS (p < 0.001) initiating apoptotic cascade in a dose dependent manner. This study confirms that MECD possesses antioxidant property and can prevent carcinogenesis by reducing oxidative stress. MECD possesses anticancer activity and lead to PC3 cell death via induction of apoptosis mediated through excessive ROS generation. Flavonoids in MECD may be responsible for these activities due to dual antioxidant and pro-oxidant properties.
Keywords: Anticancer, Antioxidant, Apoptosis, Cordia dichotoma, Prostate cancer
Graphical abstract
1. Introduction
Oxidative stress initiated by reactive oxygen species (ROS) such as nitric oxide (NO), superoxide anion (), peroxy radical (ROO•) and hydroxy radical (•OH) has a major role in the pathogenesis of several diseases such as liver cirrhosis, atherosclerosis including cancer.1 Deficient DNA repair or DNA damage by it is reported to have a prognostic or etiological role in cancer, the disease which is one of the leading causes of death.2, 3 Chemotherapy is an important choice for the cancer management clinically apart from the utilities of irradiation and surgical operations. Medicinal plants are one of the major sources of chemotherapy drugs in traditional as well as modern systems of medicine throughout the world.4 Their phytochemicals or extracts have positive effects against cancer as compared with chemotherapy or recent hormonal treatments.5 The phytochemicals such as flavonoids, alkaloids and terpenes have received major attention in recent years due to their various pharmacological properties including cancer chemopreventive and cytotoxic effects.6 Flavonoids are among the most widely occurring phytochemicals having antioxidant activity. They scavenge the ROS due to their antioxidant property and prevent carcinogenesis. They are known to have anticarcinogenic or anticancer properties and induce programmed cell death called as apoptosis in several cancer cell lines due to their pro-oxidant property.7, 8
The inactivation of enzymes by ROS and the accumulation of oxidized proteins play a critical part in the alteration of cellular function and cell death.9 Apoptosis plays a crucial role in the normal development and pathology of a wide variety of tissues.10 Though, most cancer cells do not undergo it due to impairment of apoptotic signal transmission.11 Triggering it in these cancer cells can therefore, be an effective strategy in anticancer therapy. Many stimuli such as anticancer agents prompt these cells to produce ROS which induces apoptosis.12, 13
The medicinal plant Cordia dichotoma (Boraginaceae) is practiced in several indigenous systems of medicine and popular among the various ethnic groups in India for the cure of variety of ailments as demulcent, astringent, anthelmentic, diuretic, expectorant and anti-diabetic.14, 15 Phenolics, flavonoids and carotenoids are mainly present in their leaves which have potent antioxidant activity and can show anticancer activity too.16, 17, 18 The aim of study was thus designed for the exploration purpose to evaluate anticancer potential and apoptosis inducing effect of methanolic extract of C. dichotoma leaves on human prostate carcinoma cell line, and to determine total flavonoid content and antioxidant activity.
2. Materials and methods
2.1. Authentication of collected plant specimen
Plant specimen of C. dichotoma Linn was collected from Kukrail forest, Lucknow, Uttar Pradesh, India on midday before fruiting in the month of April from the mature tree and authenticated by Dr. A K S Rawat, Botanist, National Botanical Research Institute, Lucknow, Uttar Pradesh, India (NBRI/CIF/306/2012).
2.2. Preparation of dry extract
Collected leaves of C. dichotoma Linn was shade dried and powdered. 250 gm of the powdered material was subjected to continuous Soxhlet extraction by methanol (2.5 L) to get the crude extract. Appearance of colorless methanol (after 12 h of continuous extraction) in the siphon tube was considered as the termination of Soxhlet extraction. Crude extract obtained was filtered and then concentrated to dryness in a rotary evaporator (Buchi Rotavapor-R, Labco, India) under controlled temperature and reduced pressure.19
2.3. Instruments and reagents
Microplate reader (BIORAD-680), multiwell micro-plate reader (Synergy H1 hybrid multi-mode microplate reader, BioTek), inverted fluorescent microscope (Nikon Eclipse Ti-S, Japan), CO2 incubator (Excella ECO-170, New Brunswick), inverted phase contrast microscope (Nikon Eclipse Ti-S, Japan), fetal calf serum (Himedia), Dulbecco's modified Eagle's medium (DMEM, Himedia), 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA), methyl-thiazolyl-tetrazolium dye (MTT, Himedia), 4′,6-diamidino-2-phenylindole (DAPI, Himedia), Triton X-100 (Merck, India), Phenazine methosulphate (PMS, Himedia), nicotinamide adenine dinucleotide reduced (NADH, Himedia), nitro-blue tetrazolium chloride (NBT, Himedia), 2,4,6-tripyridyl-triazine (TPTZ, Himedia), 2,2-diphenyl-1-picrylhydrazyl (DPPH, Himedia), 2,2-Azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, Himedia).
2.4. Cell line culture
Human prostate carcinoma cell line, PC3 was obtained from National Centre for Cell Sciences, Pune, India. The cells were cultured in DMEM medium supplemented with 10% (v/v) fetal calf serum, 0.1 mM non-essential amino acids, 2 mM l-glutamine, 1 mM sodium pyruvate, 1.5 g/l sodium bicarbonate and 1% antibiotic solutions. The cells were grown at 37 °C with 5% CO2 in a humidified air.
2.5. Determination of total flavonoid content
1 ml of MECD solution (1000 μg/ml) was added into a tube containing 2 ml of double-distilled water. Then, 3 ml of 0.5% sodium nitrite, 0.30 ml of 10% aluminum chloride and 2 ml of 1 M sodium hydroxide were added at 0, 5 and 6 min sequentially. Finally, the volume of reacting solution was adjusted to 10 ml with double-distilled water. It was thoroughly mixed and allowed to stand for 15 min. The absorbance was read at 510 nm against blank sample using UV-spectrophotometer. The flavonoid content was then, obtained from a standard curve prepared with quercetin and expressed in the unit of ‘mg of quercetin equivalents per gram of dried extract’ (mg QE/g extract). All the determinations were performed thrice.20
2.6. Evaluation of antioxidant activity
2.6.1. 2,2-Diphenyl-1-picrylhydrazyl scavenging assay
MECD was measured in terms of hydrogen donation or radical scavenging ability using the stable radical DPPH, which is purple at room temperature.21 5.5 ml of 120 μM DPPH in methanol was added to 0.6 ml of different concentrations of MECD (50–1000 μg/ml) and then incubated in dark at room temperature for 15 min. The absorbance was read at 517 nm against blank sample. Butylated hydroxytoluene (BHT) was used as standard.
2.6.2. 2,2-Azinobis-(3-ethylenebenzothiazoline-6-sulfonic acid) radical cation scavenging assay
The ABTS•+ was prepared by reacting 7 mM ABTS solution and 140 mM potassium persulphate solution in the ratio of 5:0.08 and allowing the mixture to stand in the dark at room temperature for 14 h before use. 1 ml of the mixture ABTS•+ was added to the separate test tubes containing 50 μl of methanolic solution of MECD (25–1000 μg/ml) and mixed by vortex mixer for 30 s. The absorbance was read at 734 nm against blank sample after 2 min of reaction. BHT was used as standard. The extent of decolorization was calculated as percentage reduction of absorbance.22
2.6.3. Nitric oxide scavenging assay
The 200 μl of 25 mM sodium nitroprusside was mixed with 800 μl of MECD (25–1000 μg/ml) dissolved in 25 mM PBS (pH 7.4) in the separate test tubes. The tubes were then, incubated at room temperature for 2 h under normal light exposure. After incubation, the tubes were kept in the dark at room temperature for 30 min. Thereafter, 300 μl of Griess's reagent [1% sulfanilamide in 5% phosphoric acid and 0.1% N-(1-naphtyl) ethylenediamine dihydrochloride] was added to each tube. Absorbance of the chromophore that formed during diazotization of the nitrite with sulfanilamide and subsequent coupling with N-(1-naphtyl) naphthylethylenediamine dihydrochloride was immediately read at 546 nm against blank sample. Ascorbic acid was used as standard.23
2.6.4. Peroxide radical scavenging assay
The 0.2804 g of linoleic acid and 0.2804 g of Tween 20 were mixed in 50 ml of 0.2 M phosphate buffer (pH 7.0) and homogenized to prepare linoleic acid emulsion. Then, 0.5 ml of methanolic solution of MECD (25–1000 μg/ml) was mixed with 2.5 ml of linoleic acid emulsion and 2 ml of 0.2 M phosphate buffer (pH 7.0) in separate test tubes, and incubated in dark at 37 °C to accelerate the peroxidation process. After incubation, 0.1 ml each of 20 mM ferrous chloride in 3.5% hydrochloric acid and 30% ammonium thiocyanate were added to the mixture for color development. The absorbance was read at 500 nm against blank sample. Ascorbic acid was used as standard.24
2.6.5. Superoxide radical anion scavenging assay
The reaction mixture containing 1 ml each of 60 μM PMS, 468 μM NADH and 150 μM NBT (prepared in 0.1 M sodium phosphate buffer with pH 7.4) was mixed with 1 ml of methanolic solution of MECD (25–1000 μg/ml) in separate test tubes.25 The mixture was then incubated at an ambient temperature for 5 min. The absorbance was read at 560 nm against blank sample. Quercetin was used as standard.
2.6.6. Hydroxyl radical scavenging assay
The reaction mixture containing 3.6 mM 2-deoxy-d-ribose, 0.1 mM ferric chloride, 0.1 mM ethylenediamine tetraacetate, 0.1 mM ascorbic acid and 1 mM hydrogen peroxide in 25 mM phosphate buffer pH 7.4 was mixed to MECD (25–1000 μg/ml) in separate test tubes to make the total volume upto 1 ml and incubated at 38 °C for 60 min. After incubation, 1 ml each of 1% thiobarbituric acid in 0.05 M sodium hydroxide and 10% trichloroacetic acid were added to the reaction mixture to yield a final volume of 3 ml. It was heated in boiling water bath for 15 min, cooled and the absorbance was read at 532 nm against blank sample. α-tocopherol was used as standard.26
Determinations in all the scavenging assays were performed thrice. The percentage scavenging was calculated according to the equation [% scavenging = {1 − (AT/AC)} × 100], where AC is the absorbance value of the control and AT is the absorbance value of treated. The plot of % scavenging versus different concentrations of MECD was used to calculate the concentration inhibiting 50% of ROS (IC50).
2.6.7. Ferric-reducing antioxidant power (FRAP) assay
0.02 M ferric chloride solution was prepared by adding 1 ml of 1 M hydrochloric acid and dissolving it in 50 ml water. 0.01 M TPTZ solution was prepared in 50 ml 96% ethanol. Fresh FRAP reagent was prepared by mixing 0.3 M acetate buffer, 0.01 M TPTZ and 0.02 M ferric chloride in a volume ratio of 10:1:1. 0.2 ml MECD solution (50–500 μg/ml) and 0.6 ml water were added to 6 ml of the FRAP reagent and the absorbance was read at 595 nm against blank sample after 6 min.27 BHT was used as standard. All determinations were performed thrice.
2.7. Evaluation of anticancer activity
2.7.1. MTT assay for cytotoxicity in PC3 cells
PC3 cells (1 × 104) were seeded in 100 μL complete medium in each well of the 96-well culture plate for 24 h at 37 °C and 5% CO2 in a humidified air. MECD was dissolved in minimum amount (non-toxic to cells) of DMSO and then diluted to the desired concentrations (25, 50, 75, 100 μg/ml) in the medium and added to the wells with exponentially growing cells in triplicate as per experimental design. After 21 h of treatment, 10 μL of MTT (5 mg/ml of media without phenol red and serum) solution was added in each well and the plate was further incubated for another 3 h at 37 °C until purple formazan crystals developed. Then, the supernatant was discarded from each well and 100 μL of DMSO was added to each well for 10 min at 37 °C. The absorbance was read at 540 nm by a microplate Elisa reader using the wells without MECD as control. All the plates were normally read within 1 h of adding the DMSO. The percent cytotoxicity was calculated according to the equation [% cytotoxicity = {1 − (AT/AC)} × 100], where AC is the absorbance value of the control and AT is the absorbance value of treated.28
The plot of % cytotoxicity versus different concentrations of MECD was used to calculate the concentration lethal to 50% of the cells (IC50). The cellular morphology was also observed by inverted phase contrast microscopy.
2.7.2. DAPI staining for analysis of induction of apoptosis
PC3 cells (1 × 104) were seeded in 100 μL complete medium in each well of the 96-well culture plate for 24 h at 37 °C and 5% CO2 in a humidified air. The cells in triplicate were then treated with two different concentrations of MECD (25 and 75 μg/ml) for 12 h. After treatment, extract plus media was removed. The cells were washed with PBS and fixed in 4% paraformaldehyde for 10 min. Subsequently, the cells were permealized with the buffer (0.5% Triton X-100 and 3% paraformaldehyde) and stained with 50 μl of fluorescent nuclear dye DAPI with a final concentration of 1 μg/ml. After 1 h, the cells were observed for the fluorescence intensity and apoptotic cells. The images were captured and number of cells was quantified by using a fluorescent microscope. The percent apoptotic cell was calculated according to the equation [% apoptotic cell = {(apoptotic cells + late apoptotic cells)/(total no of cells)} × 100].29
2.7.3. DCFH-DA staining for analysis of intracellular ROS activity level
Microscopic fluorescence imaging and quantitative fluorometric analysis were used to analyze ROS generation in PC3 cells exposed to MECD.30 The cells (1 × 104 per well) were seeded in 96-well culture plates and allowed to adhere for 24 h in a CO2 incubator at 37 °C. The cells in triplicate were then treated with two different concentrations of MECD (25 and 75 μg/ml) for 12 h. After treatment, cells were incubated with 10 mM DCFH-DA for 30 min at 37 °C. The mixture was aspirated and replaced by 200 μL of PBS in each well. The plates were kept on a shaker for 10 min at room temperature in the dark. An inverted fluorescence microscope with a CCD cool camera was used to analyze intracellular fluorescence of cells. For quantitative fluorometric analysis, cells (1 × 104 per well) were re-seeded in black bottomed 96-well culture plate and allowed to adhere for 24 h in a CO2 incubator at 37 °C. Cells in triplicate were then exposed to two different concentrations of MECD (25 and 75 μg/ml) for 12 h. After exposure, cells were incubated with 10 mM DCFH-DA for 30 min at 37 °C. Fluorescence intensity was measured with a multi-well microplate reader at an emission wavelength of 528 nm and at an excitation wavelength of 485 nm. All the values were expressed as percentage fluorescence intensity relative to the control. Increased intensity of intracellular fluorescence was indicative of increased intracellular ROS activity level.
2.8. Statistical analysis
All the data were expressed as mean ± SD of pooled results obtained from three independent experiments. Levels of statistical significance were determined by one-way ANOVA followed by Dunnett's using the GraphPad Prism program. P < 0.05 was considered as statistically significant.
3. Results
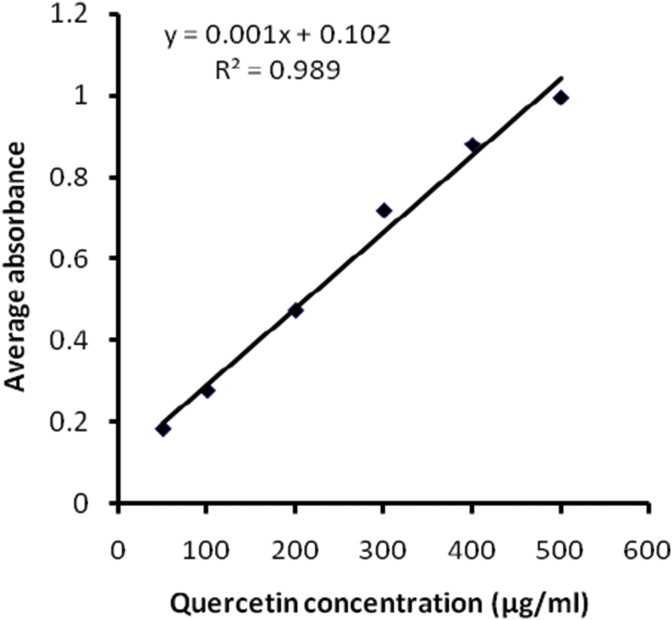
Total flavonoid content in MECD was found to be 160 mg QE/g dried extract (Fig. 1).
Fig. 1.
Standard plot for the determination of total flavonoid content in methanolic extract of C. dichotoma leaves.
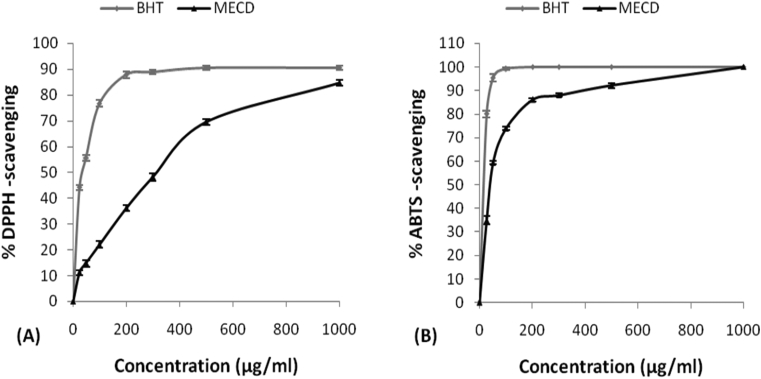
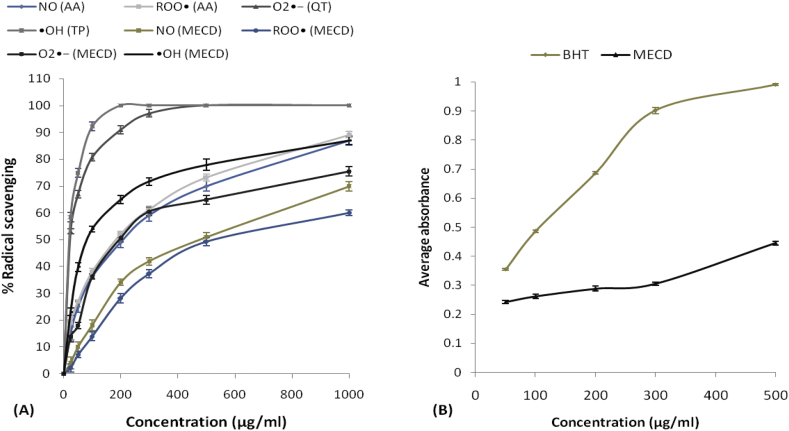
Antioxidant activity of MECD was found to be increasing in dose dependent manner. IC50 values for the scavenging by BHT and MECD were found to be 37.2 and 315.5 μg/ml respectively in DPPH scavenging assay, 14 and 38 μg/ml respectively in ABTS•+ scavenging assay (Fig. 2). IC50 values for the scavenging by ascorbic acid and MECD were found to be 212 and 476 μg/ml respectively in nitric oxide scavenging assay, 184 and 523 μg/ml respectively in peroxy radical scavenging assay. IC50 values for the scavenging by quercetin and MECD were found to be 23 and 197 μg/ml respectively in superoxide anion scavenging assay. IC50 values for the scavenging by tocopherol and MECD were found to be 20 and 82 μg/ml respectively in •OH radical scavenging assay. The IC50 values of MECD in all scavenging assays were always higher than the corresponding standard used indicative of lesser antioxidant activity compared to the standard. Total ferric-reducing antioxidant power of MECD in FRAP assay was found to be increasing in dose dependent manner and 0.34 times that of BHT at the concentration of 300 μg/ml (Fig. 3).
Fig. 2.
(A) Percentage of DPPH-scavenging, and (B) percentage of ABTS•+-scavenging, by different concentrations of methanolic extract of C. dichotoma leaves and different standards.
Fig. 3.
(A) Percentage of nitric oxide (NO), peroxy radical (ROO•), superoxide radical anion (), hydroxy radical (•OH) scavenging, and (B) Ferric-reducing antioxidant power, FRAP, by different concentrations of methanolic extract of Cordia dichotoma leaves (MECD) and different standards.
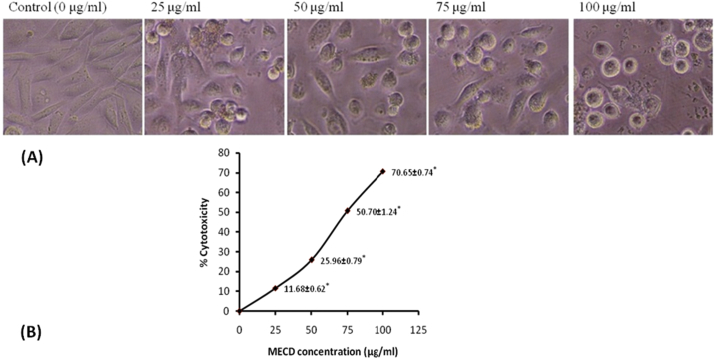
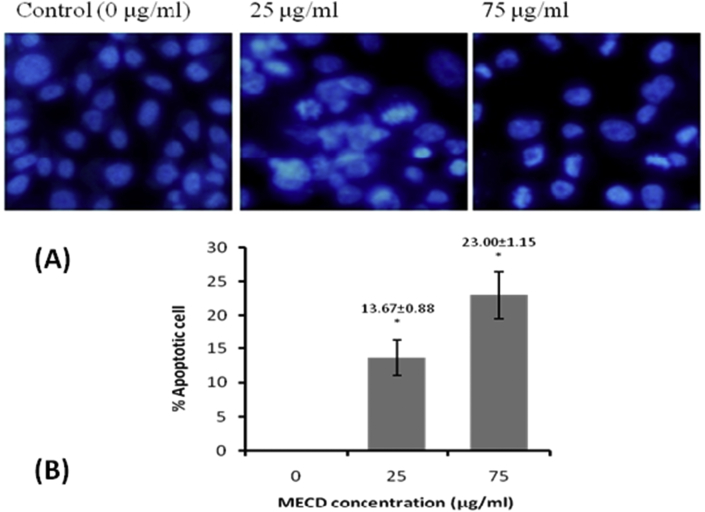
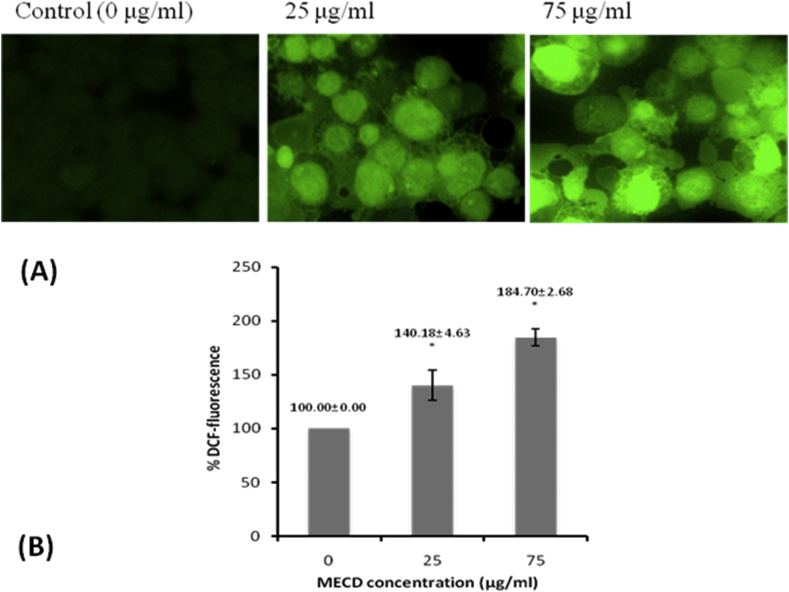
MTT assay for cytotoxicity in PC3 cells demonstrated the anticancer effect of extract MECD in prostate carcinoma, PC3 cell line. It was found that morphological shapes of the cells were drastically changed in dose dependent manner. Photomicrographs (Fig. 4) clearly revealed that cells were detaching themselves from the surface and changing to round in shape in the treatment groups. Significant cell death was also observed which was characterized by surface detachment, cellular shrinkage and deformation of cell bodies at higher concentrations. From the sigmoidal curve obtained, IC50 value was found to be 74.5 μg/ml of the extract MECD. Supplementary photomicrographs (Fig. 5) depict the details of MECD induced nuclear apoptosis observed by using fluorescent DAPI staining. The cells with condensed and fragmented nuclei were regarded as apoptotic cells. As observed from the graph, MECD induced significant nuclear condensation in a dose dependent manner depicting the induction of apoptosis in PC3 cells. Fluorescent photomicrographs (Fig. 6) of PC3 cells stained with DCFH-DA clearly depict the effect of MECD-induced intracellular ROS activity level. The photomicrographs and the graph of percent DCF-fluorescence suggest that MECD elevated the significant ROS activity level and fluorescence intensity in a dose dependent manner as compared to control in PC3 cells treated with MECD. It indicates that increased ROS activity level in PC3 cells is involved in the induction of apoptosis through various pathways.
Fig. 4.
(A) Morphological view of live and dead cells, and (B) Percent cytotoxicity of PC3 cells treated with different concentrations (0–100 μg/ml) of methanolic extract of C. dichotoma leaves, MECD as measured by MTT assay at 24 h.
Fig. 5.
(A) Apoptosis (chromatin condensation) showing fragmented and condensed nuclei, and (B) percent apoptotic cells (chromatin condensation), of PC3 cells treated with different concentrations of methanolic extract of C. dichotoma leaves, MECD (0–75 μg/ml) as measured by DAPI staining at 12 h.
Fig. 6.
(A) Intracellular ROS activity level, and (B) Percent DCF-fluorescence, in PC3 cells treated with different concentrations of methanolic extract of C. dichotoma leaves, MECD (0–75 μg/ml) as measured by DCFH-DA staining at 12 h.
[The values in all the figures were expressed as mean ± SD (n = 3). * indicates p < 0.001 as compared with control group (0 μg/ml)].
4. Discussion
Cancer is one of the leading causes of death.3 Prostate cancer is the most often diagnosed cancer and the second foremost cause of cancer death in Western males, the progression of which may be a consequence of defect in apoptotic machinery.31 Thus, the agents who can modulate apoptosis in PC3 cells may be valuable in the therapy of prostate cancer. Hence, there is a need to develop agents for the therapy of prostate cancer. Phyto-chemicals are playing an important role nowadays to cure various ailments and have been demonstrated significant potential as anticancer therapeutic agents without any side effects.32 Hence, anticancer activity of the methanolic extract of C. dichotoma leaves (MECD) along with apoptotic machinery has been evaluated here against PC3 cell lines. Also, it was not evaluated for toxicity concern on normal cell line as MECD has shown in vitro antioxidant activity in the present study and phyto-chemicals have been demonstrated significant potential as anticancer therapeutic agents without any side effects.32 Phenolics and flavonoids are well-known to have anticancer activity on various cancer cell lines and induce apoptosis.16 Methanolic extract of C. dichotoma leaves contain steroid, carbohydrate, alkaloid, saponins, cardiac glycosides, flavonoids and phenolics.18 Results of the study demonstrated that total flavonoid content in MECD was 160 mg QE/g dried extract. All these findings prompted us to explore C. dichotoma as a new potential antioxidant and anticancer agent.
Flavonoids (phenolics) are potent antioxidants and free radical scavengers.33 The MECD contains flavonoids (phenolics).18 Results of the study demonstrated that MECD possesses natural flavonoids about 160 mg QE/g dried extract and possesses antioxidant activity too. Hence, non-purified flavonoids (phenolics) present in MECD may be responsible for the antioxidant activity of the extract and can prevent carcinogenesis by its antioxidant action which scavenges the ROS. Results of the study also demonstrated that among all, the lowest IC50 value for the scavenging by MECD was found to be 38 μg/ml in ABTS•+ scavenging assay while second lowest 82 μg/ml in •OH radical scavenging assay. The guidelines of American National Cancer Institute set the activity limit for crude extracts at 50% inhibition (IC50) of proliferation of less than 30 μg/ml after the exposure time of 72 h i.e., IC50 may be upto 100 μg/ml after the exposure time of 24 h.34 Hence, keeping in mind that IC50 in MTT assay may be near to lowest value and the guidelines of American National Cancer Institute, concentrations of 0–100 μg/ml of MECD were chosen for the treatment of PC3 cell lines.
MTT assay for cytotoxicity in PC3 cells demonstrated the anticancer effect of extract MECD in prostate carcinoma, PC3 cell line. Significant cell death was also observed which was characterized by surface detachment, cellular shrinkage and deformation of cell bodies at higher concentrations. From the sigmoidal curve obtained, IC50 value was found to be 74.5 μg/ml of the extract MECD (Fig. 4). MECD induced nuclear apoptosis observed by using fluorescent DAPI staining. It also induced significant nuclear condensation in a dose dependent manner depicting the induction of apoptosis in PC3 cells (Fig. 5). It elevated the significant ROS activity level and fluorescence intensity in a dose dependent manner as compared to control in PC3 cells treated with MECD (Fig. 6). The experiments conducted here for the qualitative and quantitative analysis of ROS production suggest that MECD induced increased ROS activity level in PC3 cells and is involved in the induction of apoptosis through various pathways.13 Results of the MTT assay suggested that the treatment of MECD significantly increases cytotoxicity of cancer cell line in a dose dependent manner.
Flavonoids (phenolics) are known to have anticarcinogenic or anticancer properties and induce apoptosis in various cancer cell lines due to their pro-oxidant property.7, 8 The MECD contains flavonoids (phenolics).18 Results of the study demonstrated that MECD possesses natural flavonoids about 160 mg QE/g dried extract too. Hence, non-purified flavonoids (phenolics) present in MECD may be responsible for the anticancer activity of the extract.
5. Conclusion
MECD contains flavonoid in a quantity of 160 mg QE/g extract. It possesses anticancer activity and leads to PC3 cell death via induction of apoptosis mediated through excessive ROS generation. It possesses antioxidant property also and can prevent carcinogenesis by its scavenging action and reducing oxidative stress. Hence, the extract MECD may be an important cancer chemopreventive or chemotherapeutic agent for prostate cancer due its promising activity and may be considered for further studies in drug development.
Conflicts of interest
None to declare.
Acknowledgments
The study did not receive any financial support from any funding bodies or organizations. The authors express their sincere thanks to Faculty of Pharmacy, Integral University, Lucknow to encourage them by its research atmosphere and its all valuable moral supports.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin C.T., Lin W.H., Lee K.D., Tzeng P.Y. DNA mismatch repair as an effector for promoting phorbol ester-induced apoptotic DNA damage and cell killing: implications in tumor promotion. Int J Cancer. 2006;119:1776–1784. doi: 10.1002/ijc.22068. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Suri R.K., Chaudhari D.C., Jaffer R. Commercially important medicinal plants from forest. J Ecol Bot Phytochem. 1992;3:129–140. [Google Scholar]
- 5.Wu J., Wu Y., Yang B.B. Anticancer activity of Hemsleya amabilis extract. Life Sci. 2002;71:2161–2170. doi: 10.1016/s0024-3205(02)02013-1. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Kumar R., Dwivedi A., Pandey A.K. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. Biomed Res Int. 2014;2014:1–11. doi: 10.1155/2014/459452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher L., Ianiro T., Lau F., Wang H., Daggy B. Synergistic effects of phenolic mixtures in human cell models of aging. FASEB J. 2015;29:608–636. [Google Scholar]
- 8.Prochazkova D., Bousova I., Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Guo C., Sun L., Chen X., Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayakiran M. Apoptosis-biochemistry: a mini review. J Clin Exp Pathol. 2015;5:1–4. [Google Scholar]
- 11.Wang J., Jenkins S., Lamartiniere C.A. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer. 2014;14:379. doi: 10.1186/1471-2407-14-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simizu S., Takada M., Umezawa K., Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273:26900–26907. doi: 10.1074/jbc.273.41.26900. [DOI] [PubMed] [Google Scholar]
- 13.Aggeli I.K., Gaitanaki C., Beis I. Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell Signal. 2006;18:1801–1812. doi: 10.1016/j.cellsig.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Mishra A., Garg G.S. Antidiabetic activity of fruit pulp of Cordia dichotoma in alloxan induced diabetic rats. Int J Pharm Sci Res. 2011;2:2314–2319. [Google Scholar]
- 15.Rahman M.A., Akhtar J. Phytochemistry and pharmacology of traditionally used medicinal plant Cordia dichotoma Linn (Boraginaceae) Curr Trends Biotechnol Pharm. 2016;10:180–187. [Google Scholar]
- 16.Rahman M.A., Hussain A. Anticancer activity and apoptosis inducing effect of methanolic extract of Cordia dichotoma against human cancer cell line. Bangladesh J Pharmacol. 2015;10:27–34. [Google Scholar]
- 17.Nariya P.B., Bhalodia N.R., Shukla V.J., Acharya R., Nariya M.B. In vitro evaluation of antioxidant activity of Cordia dichotoma (Forst f.) bark. Ayu. 2013;34:124–128. doi: 10.4103/0974-8520.115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman M.A., Hussain A. Phytochemical and analytical evaluation of Cordia dichotoma Linn. leaves. Pharmacogn J. 2015;7:58–63. [Google Scholar]
- 19.Alam J., Mujahid M., Badruddeen, Jahan Y., Bagga P., Rahman M.A. Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J Tradit Complement Med. 2017;7(1):9–13. doi: 10.1016/j.jtcme.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 21.Chidambaram U., Pachamuthu V., Natarajan S., Elango B., Suriyanarayanan, Ramkumar K.M. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac J Trop Med. 2013;2013:188–194. doi: 10.1016/S1995-7645(13)60021-8. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini N., Re R., Yang M., Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2-azinobis (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 2002;299:379–389. [Google Scholar]
- 23.Sreejayan N., Rao M.N.A. Nitric oxide scavenging by curcuminoids. J Pharmacol Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitsuda H., Yasumoto K., Iwami K. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo. 1966;19:210–214. [Google Scholar]
- 25.Chen H., Yen G. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007;101:686–694. [Google Scholar]
- 26.Halliwell B., Gutteridge J.M.C., Aruoma O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 27.Berker K.I., Guclu K., Tor I., Apak R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta. 2007;72:1157–1165. doi: 10.1016/j.talanta.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Chowrasia D., Karthikeyan C., Choure L., Sahabjada, Gupta M., Arshad M. Synthesis, characterization and anticancer activity of some fluorinated 3,6-diaryl-[1,2,4] triazolo[3,4-b][1,3,4]thiadiazoles. Arab J Chem. 2013 (in press) [Google Scholar]
- 29.Lewandowska U., Szewczyk K., Owczarek K., Hrabec Z., Podsedek A., Koziolkiewicz M. Flavanols from Evening Primrose (Oenothera paradoxa) defatted seeds inhibit prostate cells invasiveness and cause changes in Bcl-2/Bax mRNA ratio. J Agric Food Chem. 2013;61:2987–2998. doi: 10.1021/jf304269x. [DOI] [PubMed] [Google Scholar]
- 30.Ahamed M., Ali D., Alhadlaq H.A., Akhtar M.J. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2) Chemosphere. 2013;93:2514–2522. doi: 10.1016/j.chemosphere.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 31.Wen J., Li R., Wen X. Dysregulation of cell cycle related genes and microRNAs distinguish the low- from high-risk of prostate cancer. Diagn Pathol. 2014;9:156. doi: 10.1186/s13000-014-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Cragg G.M., Newman D.J., Snader K.M. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 33.Ndhlala A.R., Moyo M., van Staden J. Natural antioxidants: fascinating or mythical biomolecules? Molecules. 2010;15(10):6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suffness M., Pezzuto J.M. Assays related to cancer drug discovery. In: Hostettmann K., editor. Methods in Plant Biochemistry: Assays for Bioactivity. Academic Press; London, UK: 1990. pp. 71–133. [Google Scholar]