Abstract
Background
There is a paucity of studies examining the safety of venom immunotherapy (VIT) in children. We aimed to assess the incidence of anaphylactic side effects during rush VIT in a cohort of pediatric patients and adult controls.
Methods
72 consecutive cycles of VIT-buildup in 71 children/adolescents aged 7–17 years were retrospectively evaluated and compared to an adult control group (n = 981) with regard to baseline parameters (sex, causative venom, severity of index sting reaction, results of allergy testing, comorbidities) and the incidence of anaphylactic adverse reactions.
Results
Compared to adults, severe index sting-induced anaphylaxis was significantly less common in children (P = .001). Children were more likely to suffer from bee venom allergy (P < .001) and showed higher levels of bee venom-specific IgE (P = .013), but lower serum tryptase concentrations (P = .014). The overall rate of VIT-induced anaphylactic reactions was higher in children than in adults (6.9% vs 2.5%, P = .046 by univariate analysis). In the final binary logistic regression model, however, only bee VIT (P = .039; odds ratio 2.25; confidence interval 1.04–4.87) and 5-day compared to 3-day buildup protocols (P = .011; odds ratio 2.64; confidence interval 1.25–5.57) were associated with an increased risk of treatment-induced anaphylaxis. All pediatric patients finally reached and tolerated the target maintenance dose of 100 µg.
Conclusions
The higher anaphylactic reaction rate observed in pediatric patients may be attributed to a greater prevalence of bee venom allergy. VIT-induced anaphylaxis in children is usually mild and does not affect further updosing and maintenance of VIT.
Keywords: Anaphylaxis, Bee, Buildup phase, Hymenoptera, Pediatric, Risk factor, Vespula
Background
As a result of age-specific outdoor activities, children are frequently exposed to stings by Vespula species or honey bees. Nonetheless, sting-induced anaphylaxis is considered to be infrequent. Epidemiologic studies dating from the early 70s and late 90s reported Hymenoptera sting-induced pediatric anaphylactic reaction rates to be as low as .3–.4% [1, 2]. A more recent questionnaire-based study in Irish school children, however, described a prevalence of 1.5%, which is closer to data derived from adult populations [3–5].
Sting-induced anaphylaxis commonly takes a benign course in pediatric patients, meaning that, first, reactions tend to be less severe than in adults [6–8] and, second, anaphylaxis is less likely to recur in the case of future re-stings [9]. Untreated children who have experienced moderate to severe index sting reactions, however, are at an increased risk of relapse compared to those with a history of urticaria or angioedema only, and the severity of recurring anaphylaxis was found to correspond to that of the initial reaction [10, 11]. Based on these observations, international guidelines state that venom immunotherapy (VIT) should be recommended for children with a history of moderate to severe sting-induced anaphylaxis, but not for those with only urticaria and/or angioedema [4, 5].
Several studies confirm the effectiveness of VIT in pediatric patients [9, 10, 12–14], and children are commonly considered to tolerate treatment at least as well or even better than adults [8]. There are, however, a surprisingly small number of studies addressing the safety of VIT in pediatric patients [12, 15–18], and even fewer including a direct comparison to adults [17, 18]. We aimed to assess the inpatient buildup phase of VIT using 3- or 5-day rush protocols in a cohort of pediatric patients, to examine the incidence of VIT-induced adverse reactions compared to a large and homogeneous adult control group and to work out age-specific characteristics.
Methods
Patients
Medical records of 1052 consecutive patients who had received at least one cycle of inpatient VIT buildup between January 2004 and May 2016 were available for retrospective evaluation. All patient-related procedures were part of routine practice; written informed consent was obtained from the patients and/or the patients’ caregivers for allergological work-up and initiation of VIT.
Collection of data
Clinical data and the results of allergy testing were retrieved from the medical records. Information on VIT-induced adverse reactions was gathered from standardized inpatient treatment protocols. In patients receiving more than one cycle of VIT buildup due to either double allergy to bee and Vespula venom, or following discontinuation of VIT for various reasons, each treatment cycle was evaluated separately. The local institutional review board consented to the retrospective review and publication of anonymized clinical data. Data from overlapping patient cohorts were part of previous publications [19, 20]; 309 patients (291 adults; 18 children/adolescents) were added for the current evaluation.
Allergy testing
Intradermal skin testing with Hymenoptera venoms (ALK-Abelló, Wedel, Germany) was carried out according to international guidelines [21]. Venom-specific serum IgE was measured using the ImmunoCAP™ method (Thermo Fisher Scientific, Freiburg, Germany). Baseline serum tryptase concentrations were determined using ImmunoCAP Tryptase™ (Thermo Fisher Scientific) in a subgroup of 628 patients including all patients with a history of severe sting-induced anaphylaxis.
Venom immunotherapy
VIT was indicated for adults with systemic sting reactions of any severity, though it was considered optional in the case of mild reactions only. Treatment was recommended for children with moderate to severe anaphylaxis, but not for those with urticaria/angioedema as the only symptom (exceptions were made for individual reasons such as high risk of exposure or impairment of quality of life). Aqueous allergen solutions (ALK-lyophilisiert SQ™, ALK-Abelló) were used for VIT buildup. The venom maintenance dose of 100 µg was achieved using standardized 3- and 5-day inpatient buildup protocols comprising 9 injections and a total dose of 301.1 µg, or 14 injections and a cumulative dose of 311.5 µg respectively (Table 1). 5-day protocols were used for all buildup-cycles before 2007, 3-day protocols were used afterwards. All injections were administered by trained physicians. Preventive medication with antihistamines was not routinely given.
Table 1.
Three and 5-day inpatient rush protocols for VIT buildup
| Protocol | 3 days | 5 days | ||
|---|---|---|---|---|
| Day | Dose (µg) | Day | Dose (µg) | |
| 1 | .1 | 1 | .02 | |
| 1 | .08 | |||
| 10 | .2 | |||
| .4 | ||||
| 2 | 20 | 2 | .8 | |
| 30 | 2 | |||
| 40 | 4 | |||
| 6 | ||||
| 3 | 50 | 3 | 8 | |
| 50 | 20 | |||
| 40 | ||||
| 4 or 5a | 100b | 4 | 50 | |
| 80 | ||||
| 5 | 100b | |||
| Cumulative dose (µg) | 301.1 | 311.5 | ||
| Number of injections (n) | 9 | 14 | ||
aOutpatient follow-up injection
bAluminium hydroxide adsorbed depot preparation
Classification of VIT-related adverse reactions and grading of anaphylaxis
VIT-related systemic reactions were classified as “objective” if any of the following symptoms occurred: urticaria with or without angioedema, respiratory compromise as a result of bronchospasm or laryngeal edema, arterial hypotension or collapse, distinct gastrointestinal involvement such as abdominal cramps, diarrhea, or vomiting [22]. Any undefined symptoms including sensations of warmth, pruritus, or dizziness were categorized as “subjective” as long as they were not accompanied or followed by the above defined objective signs. The severity of anaphylaxis was graded as mild (grade I), moderate (grade II), or severe (grade III) according to the classification system proposed by Muraro et al. [23].
Statistical analysis
Statistical analyses were performed with SPSS version 23 for Windows (SPSS Inc., Chicago, Ill). Interval-scaled data are reported as median and interquartile range (IQR). Ordinally and categorically scaled data are presented as absolute and relative frequencies. The Mann–Whitney U test was done to compare two groups for interval-scaled data. The Chi square or, if applicable, Fisher’s exact test were conducted for ordinal and categorical data. A binary logistic regression model was used to estimate the probability of a VIT-induced anaphylactic reaction in relation to several predictor variables (age group, venom, injection protocol). All tests were 2-tailed, and P values <.05 were considered statistically significant.
Results
Patient cohort
Figure 1 provides an overview on the total group’s age distribution. Of the total cohort (n = 1052), 71 (6.7%) were children or adolescents aged 7–17 years. Pediatric patients differed from adults with regard to a number of clinical baseline parameters (Table 2). A history of severe (grade III) index sting reactions was documented in only 9.9% of cases compared to 26.5% in adults (P = .001). There was a significantly higher proportion of bee venom allergic individuals among pediatric patients (32.4% vs 14.7%, P < .001). Bee venom allergic children had a significantly higher specific IgE than adults (median 15.3 kU/L vs 8.0 kU/L, P = .013), whereas the concentrations of specific IgE in children and adults sensitized to Vespula venom did not significantly differ (median 6.7 kU/L vs 4.8 kU/L, P = .37). Lower baseline serum tryptase concentrations were found in children (median 3.5 µg/L vs 4.4 µg/L, P = .014). No cardiovascular comorbidities were found in pediatric patients (0% vs 29.5% in adults, P < .001), and no children were on cardiovascular medication as opposed to 277 (28.2%) adults (P < .001). No significant differences were found with regard to the sex ratio (P = .17), the results of intradermal testing (P = .15), the latency time between the index sting and the initiation of VIT (P = .19), and/or the use of 3 or 5-day dose increase protocols (P = .53).
Fig. 1.
Age distribution of the total group
Table 2.
Clinical baseline parameters of patient cohort
| Total | Children | Adults | P valuea | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (years) at VIT-initiation, median (IQR) | 47 (20) | 14 (6) | 48 (19) | ||||
| Sex | |||||||
| Male | 580 | 55.1 | 45 | 63.4 | 535 | 54.5 | .17 |
| Female | 472 | 44.9 | 26 | 36.6 | 446 | 45.5 | |
| Diagnosis: IgE-mediated allergy to | |||||||
| Bee venom | 167 | 15.9 | 23 | 32.4 | 144 | 14.7 | <.001 |
| Vespula venom | 856 | 81.4 | 47 | 66.2 | 809 | 82.5 | |
| Bee and Vespula venom | 29 | 2.8 | 1 | 1.4 | 28 | 2.9 | |
| IgE to causative insect (kU/L), median (IQR) | |||||||
| Bee venom | 8.7 (22.2) | 15.3 (36.6) | 8.0 (21.0) | .013 | |||
| Vespula venom | 4.8 (11.4) | 6.7 (15.5) | 4.8 (11.2) | .37 | |||
| Baseline serum tryptase concentration | |||||||
| Availabilityb | 628 | 59.7 | 33 | 46.5 | 595 | 60.7 | |
| Median (µg/L) (IQR) | 4.4 (3.2) | 3.5 (1.9) | 4.4 (3.2) | .014 | |||
| Severity of index sting-induced anaphylaxis | |||||||
| Grade I (mild) | 138 | 13.1 | 5 | 7.0 | 133 | 13.6 | |
| Grade II (moderate) | 647 | 61.5 | 59 | 83.1 | 588 | 59.9 | |
| Grade III (severe) | 267 | 25.4 | 7 | 9.9 | 260 | 26.5 | .001 |
| Cardiovascular comorbidities | 289 | 27.5 | 0 | 0 | 289 | 29.5 | <.001 |
| Concurrent cardiovascular medication | |||||||
| Any | 277 | 26.3 | 0 | 0 | 277 | 28.2 | <.001 |
| ACE-inhibitor | 131 | 12.5 | 0 | 0 | 131 | 13.4 | <.001 |
| Beta-blocker | 59 | 5.6 | 0 | 0 | 59 | 6 | .028 |
| Latency (months) from index sting to VIT, median (IQR) | 7 (7) | 8 (6) | 7 (7.5) | .19 | |||
IQR interquartile range
aBased on univariate analysis comparing children and adults
bBaseline tryptase concentrations were determined in a subgroup of 628 patients (59.7%) including all patients with a history of severe index sting-induced anaphylaxis
Course of VIT
1101 consecutive cycles of VIT buildup were evaluated (children: 72 cycles; adults: 1029 cycles). 47 patients (1 child; 46 adults) underwent more than one cycle due to double allergy to honey bee and Vespula venom (1 child; 28 adults) or following discontinuation of VIT for various reasons (18 adults). In total, 728 injections were given to children, and 10,217 to adults.
Figure 2 provides information on the course of VIT in both age groups. 878 (79.7%) cycles of VIT buildup were administered without any complications (children: 79.2%; adults: 79.8%). In the total cohort, there were 77 (7.0%) reports of large local reactions (children: 9.7%; adults: 6.8%, P = .34). 104 (9.4%) patients (children: 4.2%; adults: 9.8%, P = .14) complained of subjective symptoms which did not fulfill the above defined criteria of objective anaphylaxis. There were 31 (children: 5; adults: 26) reports of VIT-induced anaphylactic reactions. Respective objective reaction rates were 2.8% (total), 6.9% (children), and 2.5% (adults). The higher objective reaction rate observed in children was statistically significant in the preliminary univariate analysis (P = .046). The highest reaction rate (8.3%) was observed in the subgroup of bee venom allergic children (Fig. 3).
Fig. 2.
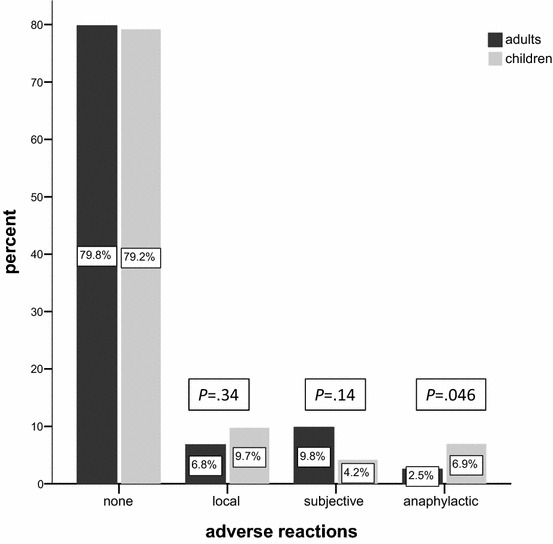
Large local reactions, subjective symptoms, and objective anaphylaxis during 1101 cycles of VIT buildup
Fig. 3.
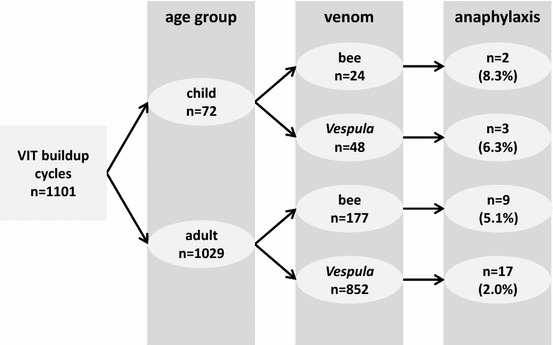
Incidence of VIT-induced anaphylactic reactions in venom-allergic children and adults
Anti-allergic treatment was given during 12 (16.7%) pediatric treatment cycles (oral antihistamine: 12.5%; intravenous steroid and/or antihistamine: 4.2%) and during 126 (12.2%) buildup cycles in adults (oral antihistamine: 10.2%; intravenous steroid and/or antihistamine: 2.0%). The difference was not statistically significant (P = .27).
Predictors of VIT-induced anaphylaxis
Of several parameters (causative venom, injection protocol, sex, severity of index sting reaction, tryptase elevation), two (venom, protocol) were likewise associated with the incidence of VIT-induced objective anaphylaxis in a preliminary univariate analysis (data not shown). Honey bee VIT (P = .039; odds ratio 2.25; confidence interval 1.04–4.87) and 5-day compared to 3-day injection protocols (P = .011; odds ratio 2.64; confidence interval 1.25–5.57) were found to be associated with an increased incidence of anaphylactic adverse reactions in a multivariate binary regression model whereas childhood/adolescence was not confirmed as an independent risk factor (P = .10; odds ratio 2.35; confidence interval .85–6.52).
Severity grading of VIT-induced anaphylaxis in pediatric patients
VIT-related anaphylaxis occurred in 5 children and adolescents aged 7–17 years (Table 3). 4 mild (grade I) reactions remained confined to the skin and responded well to treatment with antihistamines alone. In a 7-year old bee venom allergic girl, VIT-induced anaphylaxis was classified as moderate (grade II) due to additional bronchospasm. Her condition promptly improved upon treatment with inhalative salbutamol, intravenous antihistamines and corticosteroids. No epinephrine was given. The target venom maintenance dose of 100 µg was invariably reached and tolerated in all 5 reactors either within the regular inpatient dose increase schedule (n = 2) or following individually adjusted outpatient updosing protocols (n = 3).
Table 3.
Clinical details on VIT-induced anaphylactic reactions in children and adolescents
| Patient no. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| History | |||||
| Age (years) | 7 | 7 | 7 | 15 | 17 |
| Sex | F | M | M | F | M |
| Causative venom | Bee | Vespula | Vespula | Vespula | Bee |
| Severity of anaphylaxis at index sting (grade) | II | II | II | III | II |
| Relevant comorbidities | None | None | Asthma | None | None |
| Concurrent medication | None | None | Yesa | None | None |
| VIT-induced anaphylaxis | |||||
| Severity (grade) | II | I | I | I | I |
| Symptoms | |||||
| Urticaria/angioedema | + | + | + | + | + |
| Respiratory | + | − | − | − | − |
| Gastrointestinal | + | − | − | − | − |
| Cardiovascular | − | − | − | − | − |
| Injection protocol | 3 days | 3 days | 3 days | 3 days | 5 days |
| Venom dose prior to reaction (µg) | 50 | 40 | 50 | 40 | 8 |
| Time interval to injection (minutes) | 15 | 60 | 120 | 25 | 60 |
| Treatment | |||||
| Antihistamine | i.v. | Oral | i.v. | i.v. | Oral |
| Steroid | i.v. | − | − | − | − |
| Epinephrine | − | − | − | − | − |
| Inhalative beta2-adrenergic agonist | + | − | − | − | − |
aInhalative budesonide/formoterol
Discussion
VIT effectively protects venom allergic children and adults from future sting-induced anaphylaxis [9, 10, 12–14, 24]. VIT itself, however, is a potential elicitor of anaphylactic side effects, which are most likely to occur during the dose increase phase [24–27]. As opposed to ample data derived from adult populations, there is a paucity of studies examining the safety of VIT in children [12, 15–18]. Our present study including a total of 1052 individuals, 71 of whom were pediatric patients, was designed to compare VIT-related adverse reaction rates in children and adults and to assess age-specific clinical parameters.
Age-specific baseline characteristics of pediatric candidates for VIT
A preponderance of pediatric patients with a history of severe index sting-induced reactions might be expected to result from the fact that VIT was not recommended for children with mild (grade I) reactions only. Pediatric patients, however, reported a significantly lower rate of severe (grade III) index sting-related anaphylactic reactions than adults (9.9% vs 26.5%, P = .001). This is in accordance with previous publications stating that severe Hymenoptera sting-induced anaphylaxis is indeed very uncommon in children [6–9].
Our observation of a higher proportion of bee venom allergic subjects in the pediatric compared to the adult group likewise corroborates previously published data [7, 8, 18]. The majority of our young bee venom allergic patients (15 out of 23, i.e. 65.2%, data not shown) had a history of repetitive contact to bees due to beekeeping activities of family members or friends. We assume that high exposure is a risk factor for an early age onset of bee venom allergy. Our findings of higher bee venom-specific IgE levels in children (P = .013) and of an age-dependant increase of baseline serum tryptase concentrations (P = .014) are again in accordance with previous publications [18, 28]. It is tempting to speculate that lower serum tryptase concentrations during childhood might decrease the likelihood of severe anaphylactic sting reactions. Another important reason for the benign natural course of Hymenoptera venom allergy in children [6–9] might be the virtual absence of cardiovascular comorbidities which are considered an important risk factor for severe or even fatal anaphylaxis in adults [29].
Incidence of VIT-induced anaphylaxis in children versus adults
The VIT-related anaphylactic reaction rate (2.5% referring to the total number of treatment cycles) observed in our adult group is below that of previous studies which did not require documentation of objective symptoms to define an anaphylactic adverse reaction [25–27]. In this context, we would like to stress the importance of an accurate definition of anaphylaxis as misinterpretation of anxiety-related subjective symptoms may lead to an over-estimation of anaphylaxis rates, particularly in adults. Accordingly, we found a tendency towards a higher incidence of subjective symptoms in our adult group which, however, did not reach statistical significance (P = .14).
The pediatric VIT-related anaphylactic reaction rate of 6.9% in our series fits well within the—remarkably broad—range of previously published data. It slightly exceeds the values reported by Konstaninou et al. and Nittner-Marszalska et al. (3.7% respectively) [12, 18]. The higher rates described by Köhli-Wiesner et al. (16%), Birnbaum et al. (10.8%), and, most recently, by Confino-Cohen et al. (19%) might be attributed to high proportions of bee venom allergic children [15–17].
Whereas the tendency towards a higher objective reaction rate in our pediatric group in relation to the adult controls was statistically significant in the preliminary univariate analysis (P = .046), only bee VIT (P = .039; odds ratio 2.25; confidence interval 1.04–4.87) and the use of the 5-day injection protocol (P = .011; odds ratio 2.64; confidence interval 1.25–5.57) were found to be associated with an increased risk of VIT-induced anaphylaxis in the final multivariate model. Young age alone was not confirmed as an independent risk factor (P = .10). The use of bee venom has long been identified as a major predictor of VIT-induced anaphylactic side effects [14, 16–18, 24–26, 30]. Inpatient dose increase protocols including a higher number of injections and/or greater cumulative doses have likewise been shown to be associated with an increased incidence of treatment-related anaphylaxis [19, 30]. Children and adults in our series, however, did not differ with regard to the protocols used for VIT-buildup (P = .53). We conclude that the higher rate of VIT-induced anaphylactic reactions observed in our pediatric group might result from the greater proportion of bee venom allergic subjects.
Nittner-Marszalska et al. recently described a surprisingly high incidence of VIT-related anaphylactic reactions in bee venom allergic adults (21.4%) which significantly exceeded the pediatric reaction rate of 7.2% (P = .034) [18]. No significant differences between pediatric and adult reaction rates were found by Birnbaum et al. [17].
The target venom dose of 100 µg was reached and tolerated in all our pediatric patients. The authors of two recent studies demonstrate a reasonable efficacy of 50 µg maintenance doses in children and therefore advocate the use of reduced doses in order to improve the safety while decreasing the costs of treatment [12, 13]. Given the good tolerability of 100 µg doses in children and the clear dose-dependency of VIT [31], we and others opt for the use of standard dose increase protocols with a target dose of 100 µg in pediatric patients [15, 16, 18].
Conclusions
Our data demonstrate that, in contrast to previous assumptions [8], VIT-related pediatric anaphylactic reaction rates, are at least as high as those in adults. There is, however, need to clarify that VIT-induced anaphylactic reactions in children in both our series and previous studies [15–18] were invariably classified as mild to moderate and responded well to anti-allergic treatment, even without administration of epinephrine.
Authors’ contributions
JS contributed to the design of the study, collection and interpretation of data, and drafted the manuscript. CH and AK participated in the acquisition of data and assisted in drafting the manuscript. AT participated in the design of the study, collection and interpretation of data, and critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Petra Pfeuffer and Petra Raith for their unfailing technical assistance, and Carolin Malsch, Institute of Clinical Epidemiology and Biometry, University of Würzburg, for statistical advice.
Competing interests
AT serves on the scientific advisory board of ALK-Abelló.
Availability of data
The datasets analysed during the current study are not publicly available in order to protect the individual privacy of the participants. Anonymized data are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The local institutional review board consented to the retrospective review and publication of anonymized clinical data (Ethik-Kommission der Medizinischen Fakultät der Universität Würzburg).
Funding
This publication was funded by the German Research Foundation (DFG) and the University of Würzburg in the funding programme Open Access Publishing.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- IQR
interquartile range
- VIT
venom immunotherapy
Contributor Information
Johanna Stoevesandt, Phone: ++49 (0)931-201-26726, Email: Stoevesandt_J@ukw.de.
Christine Hosp, Email: Hosp_C@ukw.de.
Andreas Kerstan, Email: Kerstan_A@ukw.de.
Axel Trautmann, Email: Trautmann_A@ukw.de.
References
- 1.Settipane GA, Boyd GK. Prevalence of bee sting allergy in 4992 boy scouts. Acta Allergol. 1970;25:286–291. doi: 10.1111/j.1398-9995.1970.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 2.Novembre E, Cianferoni A, Bernardini R, Veltroni M, Ingargiola A, Lombardi E, Vierucci A. Epidemiology of insect venom sensitivity in children and its correlation to clinical and atopic features. Clin Exp Allergy. 1998;28:834–838. doi: 10.1046/j.1365-2222.1998.00313.x. [DOI] [PubMed] [Google Scholar]
- 3.Jennings A, Duggan E, Perry IJ, Hourihane JO. Epidemiology of allergic reactions to Hymenoptera stings in Irish school children. Pediatr Allergy Immunol. 2010;21:1166–1170. doi: 10.1111/j.1399-3038.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 4.Golden DB, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, Blessing-Moore J, Bernstein D, Dinakar C, Greenhawt M, Khan D, Lang D, Nicklas R, Oppenheimer J, Portnoy J, Randolph C, Schuller D, Wallace D. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Krishna MT, Ewan PW, Diwakar L, Durham SR, Frew AJ, Leech SC, Nasser SM. Diagnosis and management of Hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelines. Clin Exp Allergy. 2011;41:1201–1220. doi: 10.1111/j.1365-2222.2011.03788.x. [DOI] [PubMed] [Google Scholar]
- 6.Lockey RF, Turkeltaub PC, Baird-Warren IA, Olive CA, Olive ES, Peppe BC, Bukantz SC. The Hymenoptera venom study I, 1979–1982: demographics and history-sting data. J Allergy Clin Immunol. 1988;82:370–381. doi: 10.1016/0091-6749(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 7.Schuberth KC, Lichtenstein LM, Kagey-Sobotka A, Szklo M, Kwiterovich KA, Valentine MD. An epidemiologic study of insect allergy in children. I. Characteristics of the disease. J Pediatr. 1982;100:546–551. doi: 10.1016/S0022-3476(82)80750-6. [DOI] [PubMed] [Google Scholar]
- 8.Golden DB. Insect allergy in children. Curr Opin Allergy Clin Immunol. 2006;6:289–293. doi: 10.1097/01.all.0000235904.87676.40. [DOI] [PubMed] [Google Scholar]
- 9.Valentine MD, Schuberth KC, Kagey-Sobotka A, Graft DF, Kwiterovich KA, Szklo M, Lichtenstein LM. The value of immunotherapy with venom in children with allergy to insect stings. N Engl J Med. 1990;323:1601–1603. doi: 10.1056/NEJM199012063232305. [DOI] [PubMed] [Google Scholar]
- 10.Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med. 2004;351:668–674. doi: 10.1056/NEJMoa022952. [DOI] [PubMed] [Google Scholar]
- 11.Lange J, Cichocka-Jarosz E, Marczak H, Krauze A, Tarczoń I, Świebocka E, Lis G, Brzyski P, Nowak-Węgrzyn A. Natural history of Hymenoptera venom allergy in children not treated with immunotherapy. Ann Allergy Asthma Immunol. 2016;116:225–229. doi: 10.1016/j.anai.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinou GN, Manoussakis E, Douladiris N, Hatziioannou A, Giavi S, Saxoni-Papageorgiou P, Papadopoulos NG. A 5-year venom immunotherapy protocol with 50 µg maintenance dose: safety and efficacy in school children. Pediatr Allergy Immunol. 2011;22:393–397. doi: 10.1111/j.1399-3038.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 13.Houliston L, Nolan R, Noble V, Pascoe E, Hobday J, Loh R, Mallon D. Honeybee venom immunotherapy in children using a 50-µg maintenance dose. J Allergy Clin Immunol. 2011;127:98–99. doi: 10.1016/j.jaci.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Stritzke AI, Eng PA. Age-dependent sting recurrence and outcome in immunotherapy-treated children with anaphylaxis to Hymenoptera venom. Clin Exp Allergy. 2013;43:950–955. doi: 10.1111/cea.12144. [DOI] [PubMed] [Google Scholar]
- 15.Confino-Cohen R, Rosman Y, Goldberg A. Rush venom immunotherapy in children. J Allergy Clin Immunol Pract. 2017;5:799–803. doi: 10.1016/j.jaip.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Köhli-Wiesner A, Stahlberger L, Bieli C, Stricker T, Lauener R. Induction of specific immunotherapy with Hymenoptera venoms using ultrarush regimen in children: safety and tolerance. J Allergy (Cairo) 2012;2012:790910. doi: 10.1155/2012/790910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra-rush venom immunotherapy (210 min): a safety study and risk factors. Clin Exp Allergy. 2003;33:58–64. doi: 10.1046/j.1365-2222.2003.01564.x. [DOI] [PubMed] [Google Scholar]
- 18.Nittner-Marszalska M, Cichocka-Jarosz E, Małaczyńska T, Kraluk B, Rosiek-Biegus M, Kosińska M, Pawłowicz R, Lis G. Safety of ultrarush venom immunotherapy: comparison between children and adults. J Investig Allergol Clin Immunol. 2016;26:40–47. [PubMed] [Google Scholar]
- 19.Stoevesandt J, Hain J, Stolze I, Kerstan A, Trautmann A. Angiotensin-converting enzyme inhibitors do not impair the safety of Hymenoptera venom immunotherapy build-up phase. Clin Exp Allergy. 2014;44:747–755. doi: 10.1111/cea.12276. [DOI] [PubMed] [Google Scholar]
- 20.Stoevesandt J, Hosp C, Kerstan A, Trautmann A. Hymenoptera venom immunotherapy while maintaining cardiovascular medication: safe and effective. Ann Allergy Asthma Immunol. 2015;114:411–416. doi: 10.1016/j.anai.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Bilò BM, Ruëff F, Mosbech H, Bonifazi F, Oude-Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–1349. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 22.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569–574. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rance F. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62:857–871. doi: 10.1111/j.1398-9995.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyle RJ, Elremeli M, Hockenhull J, Cherry MG, Bulsara MK, Daniels M, Oude Elberink JN. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012;10:CD008838. doi: 10.1002/14651858.CD008838.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruëff F, Przybilla B, Bilò MB, Müller U, Scheipl F, Aberer W, Birnbaum J, Bodzenta-Lukaszyk A, Bonifazi F, Bucher C, Campi P, Darsow U, Egger C, Haeberli G, Hawranek T, Kucharewicz I, Kuchenhoff H, Lang R, Quercia O, Reider N, Severino M, Sticherling M, Sturm GJ, Wuthrich B. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126(105–11):e5. doi: 10.1016/j.jaci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Mosbech H, Müller U. Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:1005–1010. doi: 10.1034/j.1398-9995.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Lockey RF, Turkeltaub PC, Olive ES, Hubbard JM, Baird-Warren IA, Bukantz SC. The Hymenoptera venom study. III: safety of venom immunotherapy. J Allergy Clin Immunol. 1990;86:775–780. doi: 10.1016/S0091-6749(05)80182-4. [DOI] [PubMed] [Google Scholar]
- 28.Kucharewicz I, Bodzenta-Lukaszyk A, Szymanski W, Mroczko B, Szmitkowski M. Basal serum tryptase level correlates with severity of hymenoptera sting and age. J Investig Allergol Clin Immunol. 2007;17:65–69. [PubMed] [Google Scholar]
- 29.Müller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337–341. doi: 10.1097/ACI.0b013e328259c328. [DOI] [PubMed] [Google Scholar]
- 30.Brehler R, Wolf H, Kütting B, Schnitker J, Luger T. Safety of a 2-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. J Allergy Clin Immunol. 2000;105:1231–1235. doi: 10.1067/mai.2000.105708. [DOI] [PubMed] [Google Scholar]
- 31.Ruëff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001;108:1027–1032. doi: 10.1067/mai.2001.119154. [DOI] [PubMed] [Google Scholar]


