Abstract
The aim of the present investigation was to explore the constituents of the Arabian myrrh resin obtained from Commiphora myrrha. The organic and inorganic composition of the myrrh gum resin has been investigated using gas chromatography-mass spectrometry (GC–MS) and inductively coupled plasma-mass spectrometry (ICP-MS). Analysis executed by ICP-MS reveals the presence of various inorganic elements in significant amount in the myrrh resin. The elements that were found to be present in large amounts include calcium, magnesium, aluminum, phosphorus, chlorine, chromium, bromine and scandium. The important organic constituents identified in the myrrh ethanolic extract include limonene, curzerene, germacrene B, isocericenine, myrcenol, beta selinene, and spathulenol,. The present work complements other myrrh associated investigations done in the past and provides additional data for the future researches.
Keywords: Myrrh, Commiphora myrrha, GC–MS, ICP-MS, Metals, Inorganic constituents, Organic constituents
1. Introduction
Myrrh is a resinous exudate obtained from the tree Commiphora myrrha (family Burseraceae) and various other Commiphora species. The genus Commiphora includes over 150 species, and is mainly distributed in Eastern Africa, Arabia, and India (Vollesen, 1989). It is very well reported to possess medicinal properties and has been used in traditional medicines against a variety of diseases including ulcerative colitis, fever, ailments of gall bladder, skin infections, dysmenorrhea, amenorrhea, tumors, chest ailments and in burn treatment (Langhorst et al., 2013, El Ashry et al., 2003, Shen and Lou, 2008a, Su et al., 2008, Pećanac et al., 2013). Myrrh usually has been combined with frankincense during the treatment. Myrrh and frankincense resins were used for a long time in the traditional medicines of India, China, Rome, Greece, Babylon, and so on (Shen and Lou, 2008b).
A number of studies have been conducted for the evaluation of the biologically active constituents in the myrrh resin to deal with its medicinal properties reported since ages. The phytochemical investigations of myrrh started around a century ago, and hundreds of phytochemicals have been identified in this genus. El Ashry et al. (2003) and Hanus et al. (2005) reported isolation of secondary metabolites from myrrh. However, there is no data available regarding the mineral (inorganic) constituents of the myrrh resin.
Scientific publications in the past two decades have generated plenty of data to support the practices of the traditional medicines mainly encompassing plant based drugs. There are reports about hundreds of plant constituents, their identification, isolation and studies regarding the assessment of their pharmacological and toxicological properties. Most of the research investigations of plant based products are oriented toward the identification of organic constituents, which are mainly secondary metabolites. Indeed organic constituents are the ones known to possess pharmacological properties. On the other hand inorganic constituents are also there and they can play a significant influential role in the medicinal effects of the plant products. Inorganic elements can play a supplemental role in addition to the therapeutic effects of plant based medicines (Lozak et al., 2002, Pytlakowska et al., 2012).
A lot of research studies deal with the organic constituents of the myrrh; however, the inorganic mineral constituents are not yet reported. The present study focuses on the organic as well as inorganic constituents of the myrrh using GC–MS and ICP-MS techniques.
2. Materials and methods
All the solvents, nitric acid, perchloric acid, hydrogen peroxide and other reagents used were of one of the best quality grades available in the market. High quality Arabian myrrh resin was procured from local market in Riyadh, Saudi Arabia.
2.1. Preparation of myrrh resin extract for GC–MS analysis
In order to obtain myrrh resin extract the powdered myrrh resin was directly subjected to extraction with ethanol in a flask. The myrrh powder was soaked in ethanol at room temperature for a period of 48 h. Thus obtained extract was filtered and concentrated in a rotary evaporator under reduced pressure and controlled temperature, and then dried at room temperature. The extract was stored in airtight bottles at 4 °C until further investigations with GC–MS.
2.2. GC–MS analysis
Organic constituents were analyzed in Perkin-Elmer, Autosystem XL gas chromatograph linked to a mass spectrometer (Turbomass), available at Research center College of pharmacy King Saud University. 2 μl volume of myrrh resin extract was loaded into the glass capillary column (Elite-5MS column of 30 × 0.25 mm internal diameter). Oven temperature was set at 80 °C, 5 min, reaching up to 310 °C with an acceleration rate of 10 °C/min. The temperature of injector and interface was maintained at 260 and 250 °C respectively. The flow of mobile phase, helium, was set at a rate of 1.0 mL/min. Mass spectral scanning was done at 40–600 (m/z) in ionization mode. The source and inlet line temperature was set at 180 and 250 °C respectively. Spectra were compared with National Institute of Standard and Technology (NIST, 2005 v2.1) library to identify the unknown compounds. Blanks were also run after analysis of a set of five samples.
2.3. Sample preparation for ICP-MS analysis
One gram of dried sample and 50 ml of 20% Nitric acid (HNO3) were added to Erlenmeyer flask. The mixture was heated to 70–85 °C for 48 h. During heating period the volume of the flask was maintained at the same level by intermittently adding 20% nitric acid. After the completion of digestion the content of Erlenmeyer flask was filtered using Nalgene filter (Thermo scientific) unit. The filtrate was collected in 100 ml volumetric flask and allowed to cool. After cooling the volume was made up to 100 ml using deionized water (Milli Q) and analyzed with ICP-MS. For the sample preparation all the glassware was washed with deionized water and rinsed three times with 20% nitric acid.
2.4. ICP-MS analysis
For the elemental analysis of the myrrh resin the instrument ELAN-9000, Perkin Elmer, USA, was utilized. The Instrumental setting details for the analysis done are given in Table 1. Other details are given below.
Table 1.
Instrumental setting (ELAN-9000, Perkin Elmer) for the ICP/MS based elemental analysis of myrrh extract.
| ELAN-9000, Perkin Elmer |
|---|
| Parameters for the analysis of myrrh extract |
| RF power: 1500 W |
| Plasma gas flow: 15 L/min |
| Auxiliary gas flow: 1 L/min |
| Nebulizer gas flow: 0.83–0.88 L/min |
| Peristaltic pump speed: 0.5 mL/min |
| Nebulizer/spray chamber PFA-ST/Peltier-cooled cyclonic |
| Spray chamber temp: 2 °C |
| Detector mode dual lens/AutoLens enabled |
| Sampler/skimmer cones Nickel |
| Scanning mode: peak hopping |
| Number of points/peak: 1 |
| Number of sweeps/reading: 10 |
| Number of readings/replicate: 1 |
| Number of replicates: 3 |
2.4.1. Calibration of ICP/MS and internal standards
Instrument calibration was done using solution of Rh, Be, se, U, Co, Na, Mg, In, Fe, Pb, Cu, Ba 1 ppb in 1% HNO3. The same standard solution was used to optimize nebulizer gas flow, mass calibration, resolution and AutoLens calibration. A 20 ppb multi-element internal standard solution was used for all analyses. To prepare a 20 ppb internal standard solution, 1 ml of the 10 ppm stock (BDH Chemicals) was diluted into 500 ml 1% HNO3. LOD and LOQ values of the standard elements are given in Table 2.
Table 2.
LOD and LOQ values of the elements used for calibration of the ICP-MS instrument.
| S. no. | Element name | LOD (μg/g) | LOQ (μg/g) | S. no. | Element name | LOD (μg/g) | LOQ (μg/g) |
|---|---|---|---|---|---|---|---|
| 1. | Lithium (Li) | 0.0031 | 0.0093 | 32. | Rhodium (Rh) | 0.0002 | 0.0006 |
| 2. | Beryllium (Be) | 0.0025 | 0.0075 | 33. | Palladium (Pd) | 0.0001 | 0.0003 |
| 3. | Boron (B) | 0.0032 | 0.0096 | 34. | Silver (Ag) | 0.0009 | 0.0027 |
| 4. | Sodium (Na) | 0.0021 | 0.0063 | 35. | Cadmium (Cd) | 0.0001 | 0.0003 |
| 5. | Magnesium (Mg) | 0.0029 | 0.00087 | 36. | Tin (Sn) | 0.0008 | 0.0024 |
| 6. | Aluminum (Al) | 0.0016 | 0.0048 | 37. | Antimony (Sb) | 0.0009 | 0.0027 |
| 7. | Phosphorus (P) | 0.0095 | 0.0285 | 38. | Tellurium (Te) | 0.0006 | 0.0018 |
| 8. | Chlorine (Cl) | 0.0047 | 0.0141 | 39. | Iodine (I) | 0.0035 | 0.0105 |
| 9. | Potassium (K) | 0.0012 | 0.0036 | 40. | Cesium (Cs) | 0.0009 | 0.0027 |
| 10. | Calcium (Ca) | 0.0056 | 0.0168 | 41. | Barium (Ba) | 0.0006 | 0.0018 |
| 11. | Scandium (Sc) | 0.0016 | 0.0048 | 42. | Lanthanum (La) | 0.0001 | 0.0003 |
| 12. | Titanium (Ti) | 0.0003 | 0.0009 | 43. | Cerium (Ce) | 0.0002 | 0.0006 |
| 13. | Vanadium (V) | 0.0002 | 0.0006 | 44. | Praseodymium (Pr) | 0.0001 | 0.0003 |
| 14. | Chromium (Cr) | 0.0017 | 0.0051 | 45. | Neodymium (Nd) | 0.0005 | 0.0016 |
| 15. | Manganese (Mn) | 0.0005 | 0.0015 | 46. | Samarium (Sm) | 0.0002 | 0.0006 |
| 16. | Iron (Fe) | 0.0009 | 0.0027 | 47. | Europium (Eu) | 0.0008 | 0.0024 |
| 17. | Cobalt (Co) | 0.0008 | 0.0024 | 48. | Gadolinium (Gd) | 0.0007 | 0.0021 |
| 18. | Nickel (Ni) | 0.0016 | 0.0048 | 49. | Terbium (Tb) | 0.0001 | 0.0003 |
| 19. | Copper (Cu) | 0.0009 | 0.0027 | 50. | Dysprosium (Dy) | 0.0008 | 0.0024 |
| 20. | Zinc (Zn) | 0.0015 | 0.0045 | 51. | Holmium (Ho) | 0.0003 | 0.0009 |
| 21. | Gallium (Ga) | 0.0008 | 0.0024 | 52. | Erbium (Er) | 0.0009 | 0.0027 |
| 22. | Germanium (Ge) | 0.0016 | 0.0048 | 53. | Thulium (Tm) | 0.0001 | 0.0003 |
| 23. | Arsenic (As) | 0.0091 | 0.0273 | 54. | Ytterbium (Yb) | 0.0002 | 0.0006 |
| 24. | Selenium (Se) | 0.0001 | 0.0003 | 55. | Hafnium (Hf) | 0.0001 | 0.0003 |
| 25. | Rubidium (Rb) | 0.0007 | 0.0021 | 56. | Tantalum (Ta) | 0.0006 | 0.0018 |
| 26. | Strontium (Sr) | 0.0005 | 0.0015 | 57. | Rhenium (Re) | 0.0003 | 0.0009 |
| 27. | Yttrium (Y) | 0.0009 | 0.0027 | 58. | Iridium (Ir) | 0.0005 | 0.0015 |
| 28. | Zirconium (Zr) | 0.0007 | 0.0021 | 59. | Platinum (Pt) | 0.0006 | 0.0018 |
| 29. | Niobium (Nb) | 0.0002 | 0.0006 | 60. | Gold (Au) | 0.0001 | 0.0003 |
| 30. | Molybdenum (Mo) | 0.0007 | 0.0021 | 61. | Mercury (Hg) | 0.0035 | 0.0105 |
| 31. | Ruthenium (Ru) | 0.0006 | 0.0018 | 62. | Bismuth (Bi) | 0.0002 | 0.0006 |
3. Results and discussion
Medicinal properties of myrrh resin have been utilized in Chinese medicines and Indian traditional medicines since ages. It is mainly prescribed together with frankincense in case of traumatic pain and inflammation related diseases such as arthritis and fractures. Myrrh resin is widely used in dermatology for the treatment of skin ulcers and sores. Myrrh resin has been reported to be used as medicine in case of tumors, arthritis, trauma and fractures along with improvement in blood circulation (Shen et al., 2012).
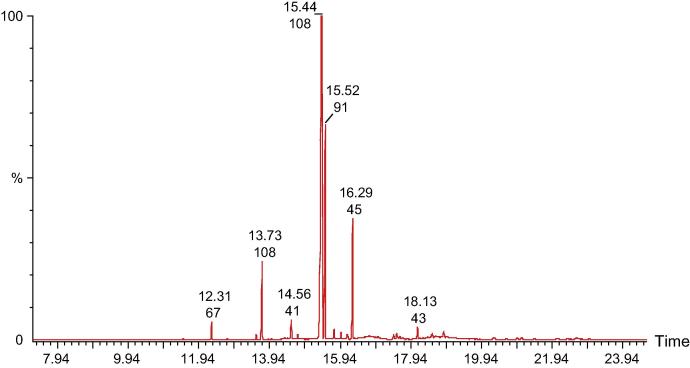
In the present phytochemical investigation the organic and inorganic constituents were identified and their amount was estimated in the Arabian myrrh resin. A significant amount of 27 organic compounds was estimated using GC/MS technique (Table 3). Fig. 1 shows the chromatogram of organic constituents taken by the GC–MS. In case of Inorganic constituents a total of 62 inorganic elements were identified using ICP/MS technique (Table 4). Results indicate significant amount of the important organic constituents identified in the myrrh resin including limonene, curzerene, germacrene B, isocericenine, myrcenol, beta selinene, and spathulenol.
Table 3.
Organic constituents in ethanolic extract of Arabian myrrh resin (from Commiphora myrrha) estimated by GC–MS.
| S. no. | Compound name | RT | Area | Height | N area% | Area% |
|---|---|---|---|---|---|---|
| 1 | R(+)-Limonene | 11.5 | 54,939 | 1,182,802 | 0.21 | 0.13 |
| 2 | (-)-Elema-1,3,11(13)-trien-12-ol | 12.3 | 386,465 | 8,658,952 | 1.47 | 0.95 |
| 3 | 2-Methyl-6-methylene-2,7-octadienal | 12.7 | 36,928 | 786,863 | 0.14 | 0.09 |
| 4 | Cis,cis,trans-3,3,6,6,9,9-hexamethyl-tetracyclo[6.1.0.0(2,4).0(5,7)]nonane | 13.5 | 191,332 | 4,192,220 | 0.73 | 0.47 |
| 5 | Curzerene | 13.7 | 2,872,761 | 63,333,412 | 10.90 | 7.05 |
| 6 | Germacrene B | 14.5 | 299,141 | 6,586,507 | 1.13 | 0.73 |
| 7 | Isosericenine | 14.7 | 337,138 | 7,574,696 | 1.28 | 0.83 |
| 8 | 3-[(E)-2-phenyl-1-propenyl]cyclohexanone | 15.4 | 26,362,042 | 419,107,232 | 100 | 64.66 |
| 9 | 2,5,8-trimethyl-1-nonen-3-YN-5-ol | 15.5 | 5,128,403 | 119,854,000 | 19.45 | 12.58 |
| 10 | Beta selinene | 15.7 | 478,044 | 6,710,925 | 1.81 | 1.17 |
| 11 | (-)-(1R,2S)-2,3-epoxy-2-(methoxymethyl)-6,6-dimethylbicyclo[3.1.1]heptane | 16.2 | 2,978,635 | 65,821,332 | 11.30 | 7.31 |
| 12 | Spathulenol | 16.7 | 79,499 | 1,672,474 | 0.30 | 0.19 |
| 13 | 1-deoxycapsidiol | 17.4 | 115,297 | 2,529,968 | 0.44 | 0.28 |
| 14 | Ethanone,1-(6,10,10-trimethylspiro[4.5]deca-6,8-dien-2-YL)-,(2R-trans)- | 17.5 | 134,501 | 2,814,635 | 0.51 | 0.33 |
| 15 | Bicyclo[3.1.1]hept-2-ene-2-carboxaldehyde,6,6-dimethyl-,(1S)- | 18.1 | 480,421 | 10,103,863 | 1.82 | 1.18 |
| 16 | 2-methylen-3-methyl-3-(4-hydroxymethyl-3-pentenyl)-bicyclo[2.2.2]oct-5-ene | 18.2 | 5153 | 133,641 | 0.02 | 0.01 |
| 17 | (-)-caryophyllene oxide | 18.6 | 23,502 | 437,846 | 0.09 | 0.06 |
| 18 | 4-[2′-methyl-5′-(2″-methyl-2″-propen-1″-YL)-2′-cyclopenten-1′-yliden]butan-2-one | 18.8 | 284,155 | 5,131,052 | 1.08 | 0.70 |
| 19 | Oxalic acid, hexyl 2-methylphenyl ester | 19.5 | 14,169 | 180,253 | 0.11 | 0.07 |
| 20 | 2-(2-hydroxy-2-methyl-2-phenylethyl)-3-methyl | 19.7 | 12,692 | 223,798 | 0.05 | 0.03 |
| 21 | 3-methyl-1-(4-methylphenyl)-1-phenyl-1,2-butanediol | 20.2 | 128,968 | 1,455,205 | 0.49 | 0.32 |
| 22 | 2,8-decadiene | 20.6 | 15,576 | 164,973 | 0.06 | 0.04 |
| 23 | 10-isopropyl-7-methyl-bicyclo(4.4.0)dec-1-en-3-one | 20.9 | 31,694 | 501,287 | 0.12 | 0.08 |
| 24 | (-)-(R)-ipsdienol | 21.4 | 37,608 | 452,066 | 0.14 | 0.09 |
| 25 | 2-(2-hydroxy-propyl)-3-one-[4,5]-spirodec-1-ene | 22.1 | 13,892 | 126,518 | 0.05 | 0.03 |
| 26 | 2,3,6,7-tetramethyl-1,4,4.alpha,5,8,8a beta,9 beta,9a alpha,10 beta,10a beta-decahydroanthracene | 22.3 | 10,021 | 153,213 | 0.01 | 0.01 |
| 27 | Myrcenol | 22.9 | 38,324 | 608,396 | 0.15 | 0.09 |
Figure 1.
Gas chromatogram of the ethanolic extract of the myrrh resin.
Table 4.
Inorganic constituents in myrrh resin (from Commiphora myrrha) estimated by ICP-MS.
| S. no. | Element name (symbol) | Level of element (ppm of myrrh resin) | S. no. | Element name (symbol) | Level of element (ppm of myrrh resin) |
|---|---|---|---|---|---|
| 1. | Lithium (Li) | 0.0173 | 32. | Rhodium (Rh) | 0.0159 |
| 2. | Beryllium (Be) | 0.0028 | 33. | Palladium (Pd) | 0.0285 |
| 3. | Boron (B) | 0.1758 | 34. | Silver (Ag) | 0.0243 |
| 4. | Sodium (Na) | 0.0943 | 35. | Cadmium (Cd) | 0.0092 |
| 5. | Magnesium (Mg) | 1.6269 | 36. | Tin (Sn) | 0.0118 |
| 6. | Aluminum (Al) | 11.5583 | 37. | Antimony (Sb) | 0.0213 |
| 7. | Phosphorus (P) | 99.8714 | 38. | Tellurium (Te) | 0.0215 |
| 8. | Chlorine (Cl) | 19.4685 | 39. | Iodine (I) | 0.0735 |
| 9. | Potassium (K) | 0.8781 | 40. | Cesium (Cs) | 0.0038 |
| 10. | Calcium (Ca) | 183.3582 | 41. | Barium (Ba) | 0.0032 |
| 11. | Scandium (Sc) | 17.3240 | 42. | Lanthanum (La) | 0.0020 |
| 12. | Titanium (Ti) | 1.2048 | 43. | Cerium (Ce) | 0.0014 |
| 13. | Vanadium (V) | 1.9019 | 44. | Praseodymium (Pr) | 0.0028 |
| 14. | Chromium (Cr) | 11.4872 | 45. | Neodymium (Nd) | 0.0048 |
| 15. | Manganese (Mn) | 0.5995 | 46. | Samarium (Sm) | 0.0094 |
| 16. | Iron (Fe) | 0.0407 | 47. | Europium (Eu) | 0.0032 |
| 17. | Cobalt (Co) | 0.0708 | 48. | Gadolinium (Gd) | 0.0022 |
| 18. | Nickel (Ni) | 0.3003 | 49. | Terbium (Tb) | 0.0008 |
| 19. | Copper (Cu) | 0.1118 | 50. | Dysprosium (Dy) | 0.0026 |
| 20. | Zinc (Zn) | 0.571 | 51. | Holmium (Ho) | 0.0026 |
| 21. | Gallium (Ga) | 0.2176 | 52. | Erbium (Er) | 0.0022 |
| 22. | Germanium (Ge) | 0.0058 | 53. | Thulium (Tm) | 0.0002 |
| 23. | Arsenic (As) | 0.8136 | 54. | Ytterbium (Yb) | 0.0018 |
| 24. | Selenium (Se) | 1.4127 | 55. | Hafnium (Hf) | 0.0004 |
| 25. | Rubidium (Rb) | 0.0157 | 56. | Tantalum (Ta) | 0.0002 |
| 26. | Strontium (Sr) | 0.0185 | 57. | Rhenium (Re) | 0.0012 |
| 27. | Yttrium (Y) | 0.0145 | 58. | Iridium (Ir) | 0.0018 |
| 28. | Zirconium (Zr) | 0.0185 | 59. | Platinum (Pt) | 0.003 |
| 29. | Niobium (Nb) | 0.0211 | 60. | Gold (Au) | 0.0064 |
| 30. | Molybdenum (Mo) | 0.0179 | 61. | Mercury (Hg) | 0.0225 |
| 31. | Ruthenium (Ru) | 0.0291 | 62. | Bismuth (Bi) | 0.0002 |
Curzerene is a sesquiterpenoid known to possess antioxidant and free radical neutralizing properties (Zhao et al., 2010). It plays a significant role against oxidative damage-associated diseases (Forman et al., 2013). Other diverse biological activities which sesquiterpenoids possess include antibacterial, antifungal, and anesthetic. Other sesquiterpenoids identified in the myrrh extract include germacrene B, Elema-1,3,11(13)-trien-12-ol.
GC/MS analysis identified and estimated the presence of (-)-Elema-1,3,11(13)-trien-12-ol in the myrrh resin (Table 3). (-)-Elema-1,3,11(13)-trien-12-ol is reported to exhibit immunomodulatory effects in in vitro studies. In a study (-)-Elema-1,3,11(13)-trien-12-ol inhibited the mast cell degranulation along with inhibition of IL-4 release, IL-4 mRNA expression and IL-4 protein expression in antigen-induced IgE-sensitized RBL-2H3 cells suggesting its candidature against IgE-mediated allergic disorders (Kim et al., 2013).
Anti-inflammatory activities of Commiphora myrrha extract has also been reported (Su et al., 2012) which indicate toward the probable role of compounds present in myrrh resin as well. In a study conducted with mice model the myrrh water extract at the 3.9 g/kg showed inhibition of formalin-induced paw edema in mice model (Su et al., 2012). In addition to its anti-inflammatory activities in this study the C. myrrha extract also indicates toward its analgesic activities.
Limonene, a monoterpene was also detected in myrrh resin, which is a very well-studied terpene. It is already in clinical practice for the treatment of cholesterol containing gall stones and gastroesophageal reflux disease (GERD). Limonene has very well reported chemopreventive properties against many types of cancer (Sun, 2007). In the past aqueous extract of myrrh resin inhibitory activities against various tumor cell lines have been reported by Shoemaker et al. (2005). However, it does not make a supporting base for the attribution of such kind of activities to any of the constituents, but it surly does indicate their part in the reported activities.
Reactive oxygen species (ROS) are very well known to cause oxidative damages to the cellular macromolecules including lipids, nucleic acids and proteins. These kinds of biological insults may result into cell death and disease progression. The essential oil of C. myrrha demonstrated potent superoxide anion radical scavenging activity. The study attributed this effect to the reaction between furan ring of C. myrrha constituents (particularly the furano-sesquiterpenoids) and superoxide anion radical (Racine and Auffray, 2005). Furanosesquiterpenoids from C. myrrha have also been reported to exhibit DPPH radical scavenging activity. A series of sesquiterpenoids from C. myrrha prevented neuronal cell death induced by MPP+ in H-SY5Y cells (Xu et al., 2011a, Xu et al., 2011b). Martins et al. (2010) in their in vitro study reported that the spathulenol inhibited human ABCB1 efflux pump and suggested its candidature to be used in combination chemotherapy of MDR cancer and recommended the spathulenol for further in vivo studies. All of these reported properties of the myrrh can be taken into consideration to support its therapeutic applications in traditional system of medicine. In the present study ample amount of sesquiterpenoids in the myrrh resin has been detected.
Inorganic elements play an indispensable role in the survival of the biological entities. In addition to the four basic elements carbon, hydrogen, nitrogen and oxygen (constituting formation of organic molecules) several inorganic elements are needed by the organisms for healthy survival. Various elements are known to be essentially needed for normal physiological functions in humans (Colotti et al., 2013). The present investigation found a total of 62 elements in the myrrh resin (Table 4), including those known to play their biological functions in humans.
Selenium is among the elements needed via food to assure the normal biological functions; it is known to play a role in antioxidant activities (Kieliszek and Błażejak, 2013) via certain enzymes. These enzymes include thioredoxin reductase (TRxR), glutathione peroxidase (GPx) and deiodinase iodothyronine (Kieliszek and Błażejak, 2013). Selenium also plays important role in reproductive health (Mistry et al., 2012). 1.41 ppm selenium was detected in the myrrh resin in the present investigation (Table 3). Higher selenium levels have been negatively correlated with risk of various cancers (Rayman, 2012).
Iron is an essential element which is required for the activities of hemoglobin for its oxygen carrying capacity. Iron is also an integral part of important enzymes like cytochrome p450. Results show very low amount of iron (0.04 ppm) in the myrrh resin (Table 4).
Chromium, which has been found in considerable amount in the myrrh (11.48 ppm, Table 4), is reported to exhibit significant role in biological system. Studies have reported to find positive correlation between chromium deficiency and the diabetes (Cefalu and Hu, 2004). Presence of the chromium in the myrrh resin can be viewed as a source of chromium supplement and be regarded as a medicinal asset along with its other therapeutic uses.
Calcium is essential for a number of physiological processes along with working as structural material for bone in combination with phosphorus. Supplementation of calcium can prevent the bone fracture (Bauer, 2013) and provide amelioration of calcium deficiency related disorders. Calcium is estimated to be present in the largest amount of all the inorganic elements identified in the myrrh extract (183.35 ppm) in the present study and phosphorus comes next to it (99.87 ppm) (Table 4). The significant amount of the calcium and the phosphorus found in the myrrh is unavoidable when its medicinal use is taken into consideration. Both the elements are essential for bone formation. Other significant nutrient elements are also identified in the myrrh resin, which includes magnesium, potassium, sodium, manganese and zinc (Table 4). Zinc is reported to play significant role in immune function and fights against infections (Shankar and Prasad, 1998, Rink and Gabriel, 2000). Zinc deficiency is correlated with increased incidences of infections (Keen and Gershwin, 1990). Hundreds of enzymes that maintain normal organ functions and immune system have zinc as a cofactor (Rink and Gabriel, 2000). In the present investigation zinc has been found to be present in myrrh resin in low amounts (0.57 ppm, Table 4). However, the amount of zinc is low in the myrrh resin but is not avoidable. Moreover, low amount simultaneously avoids the risk associated with high dose of zinc.
In the present investigation three elements, besides essential ones, draw the attention for their considerable amount present in the myrrh resin. These include aluminum (11.55 ppm), scandium (17.32 ppm), and chlorine (19.46 ppm). These elements may have toxic role if administered in higher doses. According to the Agency for Toxic Substances and Disease Registry (ATSDR, 2008, ATSDR, 2010) intermediate and chronic-duration minimal risk level (MRL) for the aluminum is 1 mg/kg/day which is much higher than the estimated amount of aluminum in the myrrh. ATSDR also indicates toward the safety of low level of chlorine. Moreover chlorine may be present as chloride of sodium and potassium. In case of scandium, no serious toxicities have been reported for this metal and it does not have any biological role either.
Arsenic which is a known toxic non-metal has been found in the myrrh extract (0.81 ppm). However, the level of arsenic in the myrrh, when compared with the reference dose of arsenic for humans (US EPA), was found to be much lower than the expected toxic level. Same is the case with other toxic metals (lead, mercury, etc.) found in the myrrh resin in the present study (Table 4). Table 4 also shows the other elements detected in trace amounts.
The present investigation gives an insight not only about the organic medicinal constituents of the myrrh resin but also about the inorganic beneficial elements. Probably, this is the first report regarding the inorganic constituents of the myrrh resin and supports the role of the resin as a medicine in the traditional system. As a whole myrrh resin contains essential elements required for biological functions and toxicologically insignificant amounts of arsenic, mercury and lead (and other probable toxic elements). The present investigation proposes the role of the inorganic elements in the medicinal effects along with its organic constituents. It is also suggested that the myrrh resin may be used as a source of supplement for several inorganic elements in case of deficiency.
Acknowledgment
The special thanks are due to Deanship of Scientific Research, King Saud University and Research Centre, College of Pharmacy, King Saud University, Riyadh, Kingdom of Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agency for Toxic Substances and Disease Registry (ATSDR), 2008. Toxicological profile for aluminum. ATSDR: United States. URL <http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=191&tid=34>. (Accessed November 2013). [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2010. Toxicological profile for chlorine. ATSDR: United States. URL <http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=1079&tid=36>. (Accessed November 2013).
- Bauer D.C. Clinical practice. Calcium supplements and fracture prevention. N. Engl. J. Med. 2013;369:1537–1543. doi: 10.1056/NEJMcp1210380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu W.T., Hu F.B. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- Colotti G., Ilari A., Boffi A., Morea V. Metals and metal derivatives in medicine. Mini Rev. Med. Chem. 2013;13:211–221. [PubMed] [Google Scholar]
- El Ashry E.S., Rashed N., Salama O.M., Saleh A. Components, therapeutic value and uses of myrrh. Pharmazie. 2003;58:163–168. [PubMed] [Google Scholar]
- Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radical Biol. Med. 2013 doi: 10.1016/j.freeradbiomed.2013.05.045. pii: S0891-5849(13)00273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L.O., Rezanka T., Dembitsky V.M., Moussaieff A. Myrrh-Commiphora chemistry. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2005;149:3–27. doi: 10.5507/bp.2005.001. [DOI] [PubMed] [Google Scholar]
- Keen C.L., Gershwin M.E. Zinc deficiency and immune function. Annu. Rev. Nutr. 1990;10:415–431. doi: 10.1146/annurev.nu.10.070190.002215. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S. Selenium: significance, and outlook for supplementation. Nutrition. 2013;29:713–718. doi: 10.1016/j.nut.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Lee T., Oh I. Mast cell stabilizing effect of (-)-Elema-1,3,11(13)-trien-12-ol and thujopsene from Thujopsis dolabrata is mediated by down-regulation of interleukin-4 secretion in antigen-induced RBL-2H3 cells. Biol. Pharm. Bull. 2013;36:339–345. doi: 10.1248/bpb.b12-00375. [DOI] [PubMed] [Google Scholar]
- Langhorst J., Varnhagen I., Schneider S.B. Randomised clinical trial: a herbal preparation of myrrh, chamomile and coffee charcoal compared with mesalazine in maintaining remission in ulcerative colitis - a double-blind, double-dummy study. Aliment Pharmacol. Ther. 2013;38:490–500. doi: 10.1111/apt.12397. [DOI] [PubMed] [Google Scholar]
- Lozak A., Sołtyk K., Ostapczuk P., Fijałek Z. Determination of selected trace elements in herbs and their infusions. Sci. Total Environ. 2002;289:33–40. doi: 10.1016/s0048-9697(01)01015-4. [DOI] [PubMed] [Google Scholar]
- Martins A., Hajdú Z., Vasas A. Spathulenol inhibit the human ABCB1 efflux pump. Planta Med. 2010;76:P608. [Google Scholar]
- Mistry H.D., Broughton Pipkin F., Redman C.W., Poston L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012;206:21–30. doi: 10.1016/j.ajog.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Pećanac M., Janjić Z., Komarcević A. Burns treatment in ancient times. Med. Pregl. 2013;66:263–267. [PubMed] [Google Scholar]
- Pytlakowska K., Kita A., Janoska P., Połowniak M., Kozik V. Multi-element analysis of mineral and trace elements in medicinal herbs and their infusions. Food Chem. 2012;135:494–501. doi: 10.1016/j.foodchem.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Racine P., Auffray B. Quenching of singlet molecular oxygen by Commiphora myrrha extracts and menthofuran. Fitoterapia. 2005;76:316–323. doi: 10.1016/j.fitote.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Rink L., Gabriel P. Zinc and the immune system. Proc. Nutr. Soc. 2000;59:541–552. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Shen T., Lou H.X. Chemical constituents from resin of Commiphora species and their biological activities. Nat. Prod. Res. Dev. 2008;20:360–366. [Google Scholar]
- Shen T., Lou H.X. Bioactive constituents of myrrh and frankincense, two simultaneously prescribed gum resins in Chinese traditional medicine. Chem. Biodivers. 2008;5:540–553. doi: 10.1002/cbdv.200890051. [DOI] [PubMed] [Google Scholar]
- Shen T., Li G.H., Wang X.N., Lou H.X. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012;142:319–330. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Shoemaker M., Hamilton B., Dairkee S.H. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother. Res. 2005;19:649–651. doi: 10.1002/ptr.1702. [DOI] [PubMed] [Google Scholar]
- Su S., Hua Y., Wang Y. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J. Ethnopharmacol. 2012;139:649–656. doi: 10.1016/j.jep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Su S.L., Bao X.J., Duan J.A. Evaluating bioactivity of myrrh oil from Commiphora myrrha and analyzing the volatile components by GC–MS. J. Nanjing Univ. Trad. Chin. Med. 2008;24:109–115. [Google Scholar]
- Sun J. D-Limonene: safety and clinical applications. Altern. Med. Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- United States Environmental Protection Agency (US EPA). Integrated Risk Information System. 1998. Arsenic, inorganic (CASRN 7440-38-2). US EPA. URL <http://www.epa.gov/iris/subst/0278.htm> (Accessed October 2013).
- Vollesen K. Burseraceae. In: Hedberg I., Edwards S., editors. vol. 3. Addis Ababa University Press; Addis Ababa: 1989. pp. 442–478. (Flora of Ethiopia). [Google Scholar]
- Xu J., Guo Y., Li Y. Sesquiterpenoids from the resinous exudates of Commiphora myrrha and their neuroprotective effects. Planta Med. 2011;77:2023–2028. doi: 10.1055/s-0031-1280087. [DOI] [PubMed] [Google Scholar]
- Xu J., Guo Y., Zhao P. Neuroprotective cadinane sesquiterpenes from the resinous exudates of Commiphora myrrha. Fitoterapia. 2011;82:1198–1201. doi: 10.1016/j.fitote.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang J.S., Yang B. Free radical scavenging activity and characterization of sesquiterpenoids in four species of Curcuma using a TLC bioautography assay and GC-MS analysis. Molecules. 2010;15:7547–7557. doi: 10.3390/molecules15117547. [DOI] [PMC free article] [PubMed] [Google Scholar]