Abstract
Purpose
Locally recurrent rectal cancer may cause significant morbidity. Prior reports of rectal cancer reirradiation following local recurrence suggest treatment efficacy, with variable rates of late toxicity. Modern techniques including intensity modulated radiation therapy (IMRT) may improve the therapeutic index. We report outcomes for pelvic reirradiation as treatment for rectal cancer using IMRT.
Methods and materials
The records of 31 patients undergoing reirradiation for rectal cancer between 2004 and 2013 were reviewed. All patients underwent IMRT using an accelerated hyperfractionation (39 Gy in 1.5-Gy fractions delivered twice daily, n=15) or once-daily fractionation technique (median dose, 30.4 Gy; range, 27-40 Gy in 15-22 fractions; n = 16). The median cumulative dose was 77 Gy (range, 59-113), and the median interval from prior pelvic radiation therapy was 39.8 months (range, 10.1-307.6). Treatment intent was palliative in 20 patients and neoadjuvant or adjuvant in 11 patients. Surgery was generally reserved for patients with an isolated local recurrence. Concurrent chemotherapy was administered for 25/31 patients, most frequently capecitabine (n=11) or continuous infusion 5-fluorouracil (n=10).
Results
Median follow-up was 11.3 months. The prescribed treatment was completed in 29/31 patients (93.5%). Among 18 patients with symptoms attributable to recurrent disease, successful palliation was achieved in 10/18 (55.6%). The rate of grade 2 and grade 3 acute toxicities was 32.3% and 3.2%, respectively. Local control rates at 1 and 2 years were 61.3% and 47.3%, respectively. Median overall survival was 21.9 months, and 1-year survival was 66.7% for patients who had surgical resection versus 58.7% for those who did not (P = .0802).
Conclusions
Rectal cancer reirradiation using IMRT is well-tolerated in the setting of prior pelvic radiation therapy. Given significant risk of local progression, further dose escalation may be warranted for patients with life expectancy exceeding 1 year.
Summary.
This manuscript reports on the treatment and oncologic outcomes of a patient series (n = 31) that received reirradiation for rectal cancer. One-half of the patients were treated with accelerated hyperfractionation and half with once-daily fractionation. Rectal cancer reirradiation using intensity modulate radiation therapy was well-tolerated; however, further dose escalation may be warranted for patients with life expectancy exceeding 1 year.
Introduction
Locally recurrent rectal cancer may cause significant morbidity, including debilitating pelvic pain, rectal bleeding, bowel obstruction, and sciatica, and has limited treatment options. Previous studies show local recurrence rates ranging from 4.4% to 11%.1, 2, 3 Prior reports of rectal cancer reirradiation suggest treatment efficacy, with variable rates of late toxicity. Das et al described the use of hyperfractionated accelerated radiation therapy (RT; 39 Gy in 1.5-Gy fractions twice daily) for palliative or neoadjuvant treatment of rectal cancer in patients with prior pelvic radiation therapy and demonstrated a 3-year rate of freedom from local progression of 33%, overall survival rate of 39%, and grade 3 to 4 late toxicity of 35%.4 Ng et al described a once-daily fractionation of 39.6 Gy in 22 fractions and demonstrated a median overall survival of 19 months, with 12.5% of patients experiencing grade 3 acute toxicity, no grade 4 acute toxicity, and 1 late toxicity in a patient irradiated palliatively.5
Modern treatment techniques including intensity modulated RT (IMRT) may improve the therapeutic index of reirradiation. This retrospective study was conducted to evaluate oncologic outcomes and toxicities for reirradiation of rectal cancer using IMRT in the setting of prior pelvic radiation therapy.
Methods and materials
Patient characteristics
An institutional review board approved review of the records of 31 patients with locally recurrent rectal cancer treated with reirradiation between 2004 and 2013 at our institution was performed. Prior extirpative resection of rectal cancer was performed in 24 of the 31 patients, with initial pathologic stage II (n=12), III (n=7), IV (n=2), or unknown (n=3). For 6 of the 31 patients, prior local treatment included chemo-RT alone (n=4) or chemo-RT with transanal excision (n=2). A single patient underwent prior neoadjuvant chemo-RT and definitive resection of a small bowel malignancy and was treated with reirradiation neoadjuvantly for a new primary rectal cancer. Prior pelvic RT was given as treatment for rectal or rectosigmoid cancer in 27 of the 31 patients and as treatment for other malignancies in 4 of the 31 patients including prostate (n = 2), endometrial (n=1), and small bowel (n=1) malignancies. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Median age (y) at retreatment (range): | 58.9 (31.0-85.6) |
| Gender | |
| Male | 18 (58.1%) |
| Female | 13 (41.9%) |
| Indication for prior radiation therapy | |
| Rectal/rectosigmoid cancer | 27 (87.1%) |
| Other cancers | 4 (12.9%) |
| Median prior radiation therapy dose (Gy) (range) | 45 (20-74) |
| Median retreatment interval (months) (range) | 39.8 (10.1-307.6) |
| Symptomatic at local recurrence | |
| Yes | 18 (58.1%) |
| No | 13 (41.9%) |
The median age at the time of retreatment was 58.9 years (range, 31.0-85.6). The median dose of previous RT was 45 Gy (range, 20-74), and 20 (64.5%) patients received conventionally fractionated treatment to a dose of 45 to 54 Gy. Short-course RT was previously used in 6 patients treated to a dose of 20 to 25 Gy in 5 fractions.6 All previous treatment was delivered with a conventional 4-field box technique. The median interval between the 2 courses of RT was 39.8 months (range, 10.1-307.6). Of the 31 patients, 22 (71.0%) received reirradiation after an interval of ≤5 years, and 18 (58.1%) were symptomatic at local recurrence, with 5 patients exhibiting more than 1 symptom. Symptoms were identified by retrospective review of chart records and most commonly included pelvic pain (n=14), rectal bleeding (n=3), increased bowel movement frequency (n=3), pelvic pressure/obstructive symptoms (n=2), or urinary symptoms (n=2). The symptomatic response rate was assessed within three months of completing reirradiation, and successful palliation was defined as a reduction or complete disappearance of symptoms. Retreatment intent was neoadjuvant in 9 patients, adjuvant (reirradiation given after surgery) in 2 patients, and for palliation in 20 patients. Surgery was generally reserved for patients with an isolated local recurrence.
Reirradiation treatment
All patients were treated with IMRT to a volume consisting of the gross tumor recurrence plus a planning target volume margin of 5 to 7 mm. Ninety-five percent of the prescriptive dose covered the whole target tumor volume. Generally, the conformality of treatment plans was optimized to keep cumulative dose to the femoral heads under 50 Gy. Because different areas of small bowel were likely in the pelvis at reirradiation compared with initial treatment, conformality of treatment was maximized, although specific small bowel objectives were not used.
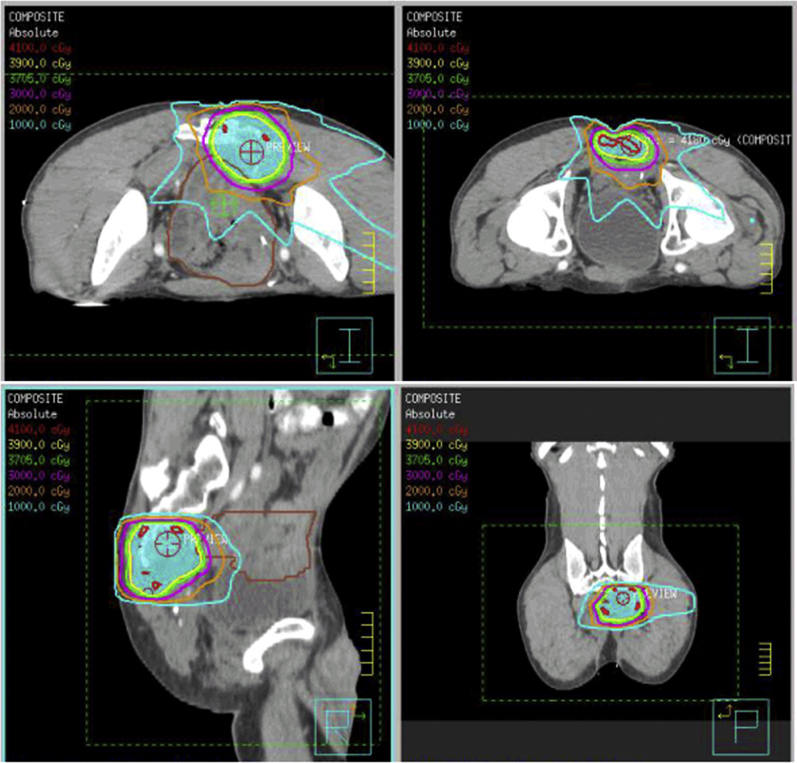
Over the time of the study, the prescribed dose was variable. Treatment characteristics are listed in Table 2. All patients underwent IMRT using either an accelerated hyperfractionation (39 Gy in 1.5-Gy fractions delivered twice daily, n=15) or once-daily fractionation technique (median dose, 30.4 Gy; range, 27-40 Gy in 15-22 fractions; n=16). All patients who underwent accelerated hyperfractionation RT were treated between 2010 and 2013. The median cumulative dose of RT (initial course plus reirradiation) was 77 Gy (range, 59-113). Radiation therapy was delivered using 6- to 18-MV photons. Examples of planning target volume with isodose distributions are shown in Fig 1. Concurrent chemotherapy was administered for 25 (80.6%) patients with capecitabine (n=11), continuous infusion 5-fluorouracil (5-FU) (n=10), bolus 5-FU (n=1), or other (n=3).
Table 2.
Treatment characteristics
| Fractionation schedule | |
| Accelerated twice-daily hyperfractionation | 15 (48.4%) |
| Once daily fractionation | 16 (51.6%) |
| Retreatment site | |
| Rectal/perirectal region | 15 (48.4%) |
| Presacral/pelvic sidewall region | 16 (51.6%) |
| Concurrent chemotherapy | |
| Yes | 25 (80.6%) |
| No | 6 (19.4%) |
Figure 1.
Planning target volume with isodose distributions for a typical case.
Follow-up
Follow-up was performed by colorectal surgery, radiation oncology, and medical oncology. Interval follow-up was generally completed every 3 months including physical examination, routine bloodwork, and cross-sectional imaging.
Statistical analysis
Statistical analysis was completed in StatView 5.0.1 (SAS Institute Inc.). Acute and late toxicity were graded using Common Terminology Criteria for Adverse Events, version 3.0.7 Local progression was defined as any recurrence in the pelvis or disease progression in the pelvis detected on radiographic studies or endoscopy. The rates of freedom from local progression and overall survival were estimated by Kaplan-Meier methods with log-rank test considered significant between 2 groups at 0.05 level.8 All time intervals were calculated from the date of completion of reirradiation.
Results
Treatment delivery
The prescribed treatment was completed in 29/31 (93.5%) patients. One patient had treatment discontinued at 16.5 Gy because of disease progression and 1 patient had the last treatment fraction omitted, reason not otherwise specified. Surgery was performed in 9 (29.0%) patients and was not performed in 2 patients with neoadjuvant treatment intent because of distant progression in 1 and local plus distant progression in the other. Among the 9 patients undergoing surgery, 2 had complete removal of all gross tumor with microscopically negative margins (R0 resection), 6 patients had complete removal of all gross tumor with microscopically positive margins (R1 resection), and 1 patient had unknown resection status. Among 18 patients with symptoms attributable to recurrent disease, successful palliation was achieved in 10/18 (55.6%).
Survival and recurrence outcomes
The median follow-up interval was 11.3 months for all patients and 20.7 months for patients alive at last follow-up.
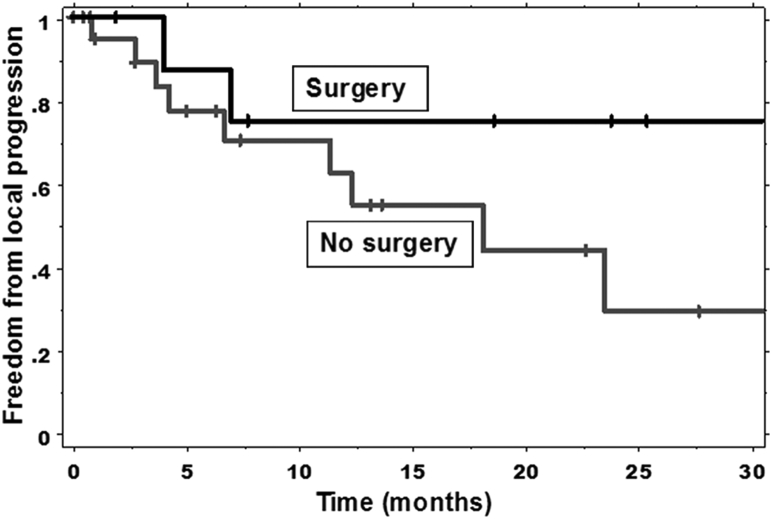
Twelve (38.7%) patients developed pelvic local progression. The 1- and 2-year actuarial rates of freedom from local progression were 61.3% and 47.3%, respectively (Table 3). The median duration of freedom from local progression was 23.6 months. There was no statistically significant difference in the rates of freedom from local progression in patients who had surgical resection compared with those who did not (P=.238) (Fig 2). The 1-year actuarial rate of freedom from local progression was 75.0% for patients who had surgical resection and 54.6% for patients who did not (Table 4). There was no significant association between the freedom from local progression and the retreatment interval (P=.846), the freedom from local progression and the retreatment dose (P=.440), or the freedom from local progression and fractionation technique (P=.286).
Table 3.
Local control and overall survival rates
| Parameter | 1-y rate (%) | 2-y rate (%) | Median (months) |
|---|---|---|---|
| Freedom from local progression | 61.3 | 47.3 | 23.6 |
| Overall survival | 60.8 | 45.4 | 21.9 |
Figure 2.
Kaplan-Meier estimates of freedom from local progression in patients who did and did not undergo surgery.
Table 4.
Pelvic control and overall survival rates by surgery, retreatment interval, and retreatment dose
| Parameter | 1-y rate (%) | P value |
|---|---|---|
| Freedom from local progression | ||
| Surgical resection | 75.0 | .238 |
| No surgical resection | 54.6 | |
| Freedom from local progression | ||
| Retreatment ≤3 y | 59.3 | .846 |
| Retreatment >3 y | 61.4 | |
| Freedom from local progression | ||
| High retreatment dose | 56.2 | .440 |
| Low retreatment dose | 71.6 | |
| Freedom from local progression | ||
| Accelerated hyperfractionation | 43.2 | .286 |
| Once- daily fractionation | 68.2 | |
| Overall survival | ||
| Surgical resection | 66.7 | .0802 |
| No surgical resection | 58.7 | |
| Overall survival | ||
| Retreatment ≤3 y | 59.8 | .811 |
| Retreatment >3 y | 61.1 | |
| Overall survival | ||
| High retreatment dose | 71.4 | .104 |
| Low retreatment dose | 38.5 | |
| Overall survival | ||
| Accelerated hyperfractionation | 65.5 | .379 |
| Once-daily fractionation | 50.0 | |
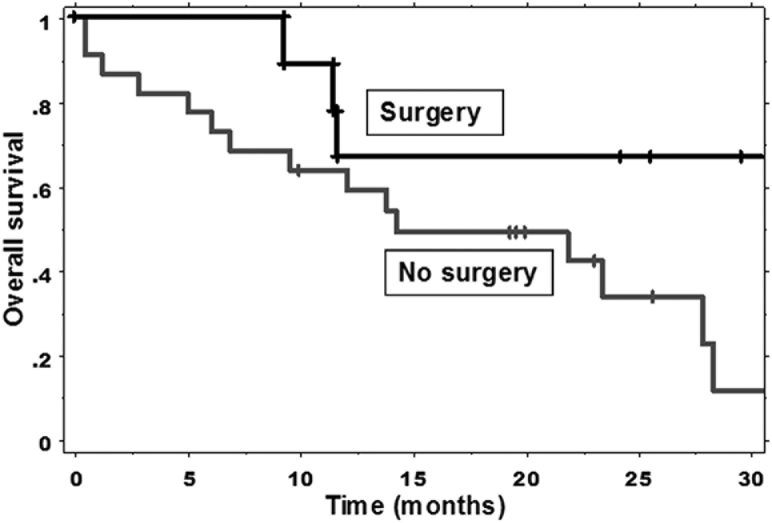
There were 19 (61.3%) deaths among the 31 patients. The 1- and 2-year actuarial overall survival rates were 60.8% and 45.4%, respectively. The median duration of overall survival was 21.9 months. There was no statistically significant difference in the rates of overall survival in patients who had surgical resection compared with those who did not (P=.0802) (Fig 3). The 1-year overall survival rate was 66.7% for patients who had surgical resection and 58.7% for patients who did not.
Figure 3.
Kaplan-Meier estimates of overall survival in patients who did and did not undergo surgery.
There was no significant association between retreatment interval and overall survival (P=.811). The 1-year actuarial overall survival rate was 61.1% for those patients with a retreatment interval >3 years and 59.8% for those with a retreatment interval ≤3 years.
Retreatment dose was not significantly associated with overall survival (P=.104). The 1-year actuarial overall survival rate was 71.4% for patients who were treated with high retreatment dose (median dose, 39 Gy; range, 39-40 Gy) and 38.5% for patients treated with low retreatment dose (median dose, 30 Gy; range, 27-36 Gy).
Fractionation technique was not significantly associated with overall survival (P=.379). The 1-year actuarial overall survival rate was 65.5% for patients treated with accelerated hyperfractionation and 50.0% for patients treated with once daily fractionation technique.
Toxicity
No patient developed grade 4 acute toxicity and only 1 (3.2%) patient developed grade 3 acute toxicity during chemoradiation. This patient had grade 3 diarrhea and required hospitalization. No other patient underwent hospitalization for acute toxicity. Ten (32.3%) patients developed grade 2 acute toxicity. The specific types of grade 2 acute toxicity included nausea/vomiting in 4 patients, skin toxicity in 4 patients, and diarrhea in 2 patients. No patient developed grade 4 late toxicity, and 1 (3.2%) patient developed grade 3 late toxicity. This patient experienced a sacral insufficiency fracture.
Discussion
Local recurrence in rectal cancer is a challenge, with salvage surgery preferred but not always possible. Reirradiation in recurrent pelvic disease may help downstage tumors to make surgical resection possible and improves palliative symptom control. In this study, reirradiation was well-tolerated, with a rate of a grade 2 acute toxicity of 32.3%, grade 3 acute toxicity of 3.2% (1 patient), grade 3 late toxicity of 3.2% (1 patient), and no grade 4 acute or late toxicities. Using a median retreatment dose of 39 Gy, the median duration of freedom from local progression was 23.6 months, the median duration of overall survival was 21.9 months, and the symptomatic response rate was 55.6%. Table 5 highlights the results of this study in the context of prior studies of rectal cancer reirradiation.
Table 5.
Comparison of prior studies of rectal cancer reirradiation
| Study | N | MRD (Gy) | MCD (Gy) | Acute toxicity |
Late toxicity |
Median OS (mo) | ||
|---|---|---|---|---|---|---|---|---|
| G3 (%) | G4 (%) | G3 (%) | G4 (%) | |||||
| Das et al | 50 | 39 | 89 | 4 | 0 | 24 | 2 | 26 |
| Lingareddy et al | 52 | 30.6 | 84.4 | 31 | 0 | 23 | 10 | 12 |
| Mohiuddien et al | 103 | 34.8 | 85.8 | 22 | 6 | 17 | 4 | 26 |
| Ng et al | 56 | 39.6 | 87.3 | 13 | 0 | 2 | 0 | 19 |
| Valentini et al | 59 | 40.8 | 90.8 | 5.1 | 0 | 12 | 0 | 42 |
| Youssef et al | 31 | 39 | 77 | 3 | 0 | 3 | 0 | 22 |
MCD, median cumulative dose; MRD, median retreatment dose.
Reirradiation up to 30 Gy, even with chemotherapy, has been shown to be safe for palliation and possible cure for resectable locally recurrent rectal cancer, and doses up to 40 Gy can be used for limited volumes.9 Mohiuddin et al published a study of 103 patients with recurrent rectal adenocarcinoma who underwent reirradiation (median dose, 34.8 Gy; range, 15-49.2 Gy) with concurrent continuous infusion 5-FU.10 The median cumulative dose was 85.8 Gy (range, 70.6-108), with 40 patients treated with 1.2-Gy fractions twice daily, 63 patients treated with 1.8-Gy fractions daily, and 34 patients undergoing surgery. The rates of grade 3 to 4 acute and late toxicities were 28.2% and 21.4%, respectively. The median overall survival was 26 months and the 5-year actuarial survival rate was 19%. For patients undergoing surgical resection, the median overall survival was 44 months and 5-year actuarial survival rate was 22%. The high rates of acute toxicity in this study may be due to larger field sizes or higher total doses. Mohiuddin et al also postulated that reirradiation dose may be titrated based on interval to reirradiation. A reirradiation dose of 35 Gy was recommended in their series if the interval to reirradiation is shorter than 12 months, and a reirradiation dose of 50 to 55 Gy if the interval to reirradiation is longer than 36 months.
Valentini et al reported a multicenter phase 2 study of preoperative hyperfractionated reirradiation in 59 patients for locally recurrent rectal cancer.11 Patients were treated with a dose of 40.8 Gy in 1.2-Gy fractions twice daily and with protracted venous infusion 5-FU. The median cumulative dose was 90.8 Gy (range, 70.8-95.8), and 39 patients underwent surgery. The rates of grade 3 to 4 acute and late toxicities were 5.1% and 11.9%, respectively. The 3- and 5-year actuarial rates of local control were 59% and 39%, respectively. The median overall survival was 42 months and the 5-year actuarial survival rate was 39%. For the 21 patients undergoing R0 (no residual tumor) resection, the 5-year actuarial survival rate was 66.8%.
Lingareddy et al reported a study of palliative reirradiation in 52 patients with recurrent rectal cancer.12 Twenty-two patients received 30 Gy in 1.2-Gy fractions twice daily and 30 patients received 30.6 Gy in 1.8- to 2.0-Gy fractions daily. The median reirradiation dose was 30.6 Gy and median cumulative dose was 84.4 Gy (range, 66.6-104.9). Ninety percent of patients received concurrent 5-FU. The median overall survival was 12 months and the 2- and 3-year overall actuarial rates were 25% and 14%, respectively. The rate of grade 3 acute toxicity was 30.7%, and no patient experienced grade 4 acute toxicity. The rate of grade 3 late toxicity was 23.1%, and 9.6% of patients experienced grade 4 late toxicity. Patients treated with hyperfractionated RT had a late toxicity rate of 18% compared with 47% with once-daily fractions (P < .05). On logistic regression analysis, hyperfractionated RT was significantly associated with reduced late toxicity (P < .04).
In Das et al, 94% of patients received accelerated hyperfractionation (39 Gy in 1.5-Gy fractions delivered twice daily). In Ng et al, 70% of patients received a reirradiation dose of 39.6 Gy in 1.8-Gy fractions daily. Although this dose fractionation regimen is higher than those previously described, it was deemed appropriate because of the median survival of 19 months, low rate of late toxicity, and practicality over hyperfractionated regimens.
In 2014, Guren et al reported a systematic review of reirradiation of locally recurrent rectal cancer.13 The median initial radiation dose was 50.4 Gy, with a median time of 8 to 30 months before reirradiation. The median reirradiation dose was 30 to 40 Gy to the gross tumor volume, which was mostly administered using hyperfractionation (1.2-1.5 Gy twice daily) or once-daily fractionation (1.8-Gy) technique. Median survival was 39 to 60 months in resected patients and 12 to 16 months in patients treated palliatively. Successful palliation was reported in 82% to 100% of patients, acute toxicities were reported in 9% to 20% of patients, and late toxicities were insufficiently reported.
In the current study, 15 patients received accelerated hyperfractionation (39 Gy in 1.5-Gy fractions delivered twice daily), whereas 16 patients received once-daily fractionation with a median dose of 30.4 Gy (range, 27-40) in 15 to 22 fractions. Overall treatment was well-tolerated in our group, and acute and late toxicities rates were favorable. However, 1- and 2-year local control was suboptimal. The low toxicity but suboptimal tumor control observed in the current series, which incorporated IMRT, suggests that greater reirradiation doses should be considered. Koom et al showed that in patients not undergoing surgery, reirradiation doses exceeding 50 Gyαβ10 (equivalent dose in 2 fractions, α/β=10) significantly increased the infield progression-free survival (P=.005).14
There are certain inherent limitations to this retrospective study, including limited sample size, although our study also has several strengths. Toxicity rates may have been underestimated because they were based on hospital records and physician rather than direct patient-reported outcomes. Although patient-reported quality of life outcomes would be preferable in this regard, based on physician reporting, the majority of patients that were symptomatic at presentation were palliated successfully by the described regimen. In addition to IMRT, the use of short-course RT as initial preoperative treatment may have affected the low rate of toxicity observed in our series. Finally, the date of retreatment was covariate to treatment dose and chemotherapy, which may confound the improved overall survival rates for patients treated with higher reirradiation doses, who also received more modern systemic therapy. This is likely mitigated by the relatively narrow and modern time window of patient treatments reviewed, from 2004 to 2013. In spite of these limitations, our results suggest that toxicity is acceptably low for rectal cancer reirradiation using IMRT, which allows for precise dose delivery to limited target volumes. In addition, hyperfractionation is used to reduce late effects of reirradiation, and although this is most important for patients with curative intent, hyperfractionated RT may still have a role in palliative treatment using dose-escalated regimens. However, for some patients treated with palliative intent, once-daily RT may be optimal depending on their performance status and extent of metastatic disease, along with practicality and convenience. Given successful palliation, low observed toxicity, but suboptimal tumor control rates in particular for inoperable patients, our current institutional practice is for reirradiation of 39 Gy (1.5 Gy twice-daily fractionation) in the preoperative setting and 45 Gy (1.5 Gy twice-daily fractionation) for patients not planned for salvage surgery.
Conclusion
In summary, rectal cancer reirradiation using IMRT is well tolerated in the setting of prior pelvic RT. Given significant risk of local progression for patients not undergoing surgery, further dose escalation may be warranted for such patients with life expectancy exceeding 1 year.
Footnotes
Conflicts of interest: P.J.P. reports grants from Philips Healthcare, grants and other from Varian Medical Systems, other from Holaira, Inc, and other from Medtronic/Covidien, outside the submitted work. J.R.O. reports grants, personal fees, and other from ViewRay, Inc, outside the submitted work. The other authors have nothing to declare.
References
- 1.Sauer R., Liersch T., Merkel S. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn W., Marijnen C.A., Nagtegaal I.D. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled tme trial. Lancet Oncol. 2011;12:575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 3.Sebag-Montefiore D., Stephens R.J., Steele R. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das P., Delclos M.E., Skibber J.M. Hyperfractionated accelerated radiotherapy for rectal cancer in patients with prior pelvic irradiation. Int J Radiat Oncol Biol Phys. 2010;77:60–65. doi: 10.1016/j.ijrobp.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Ng M.K., Leong T., Heriot A.G., Ngan S.Y. Once-daily reirradiation for rectal cancer in patients who have received previous pelvic radiotherapy. J Med Imaging Radiat Oncol. 2013;57:512–518. doi: 10.1111/1754-9485.12057. [DOI] [PubMed] [Google Scholar]
- 6.Myerson R.J., Genovesi D., Lockett M.A. Five fractions of preoperative radiotherapy for selected cases of rectal carcinoma: Long-term tumor control and tolerance to treatment. Int J Radiat Oncol Biol Phys. 1999;43:537–543. doi: 10.1016/s0360-3016(98)00435-0. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute . Department of Health and Human Servies, National Institutes of Health; Washington, DC: 2003. Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events, version 3.0. [Google Scholar]
- 8.Kaplan E., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 9.Glimelius B. Recurrent rectal cancer. The pre-irradiated primary tumour: Can more radiotherapy be given? Colorect Dis. 2003;5:501–503. doi: 10.1046/j.1463-1318.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 10.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144–1150. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 11.Valentini V., Morganti A.G., Gambacorta M.A. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Lingareddy V., Ahmad N.R., Mohiuddin M. Palliative reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1997;38:785–790. doi: 10.1016/s0360-3016(97)00058-8. [DOI] [PubMed] [Google Scholar]
- 13.Guren M.G., Undseth C., Rekstad B.L. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother Oncol. 2014;113:151–157. doi: 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Koom W.S., Choi Y., Shim S.J. Reirradiation to the pelvis for recurrent rectal cancer. J Surg Oncol. 2012;105:637–642. doi: 10.1002/jso.23023. [DOI] [PubMed] [Google Scholar]