Abstract
Background
There has been increased reporting of atypical meningioma (grade II) since the World Health Organization reclassification in 2000, and the use of postoperative radiation therapy (RT) in the treatment of these tumors is controversial. We evaluated patients treated at our institution to identify patient subgroups with increased risk of recurrence that may benefit from adjuvant RT.
Methods and materials
We retrospectively assessed 50 patients treated for World Health Organization grade II meningiomas between March 2000 and February 2013. Sex, race, age of diagnosis, tumor location, performance status, size of tumor, MIB-1 index, resection status, and RT were recorded. Patient follow-up, recurrence, and vital status were measured to assess 3-year overall survival (OS) and recurrence free survival (RFS).
Results
The median follow-up was 37 months (range, 1-148). Female sex was associated with decreased RFS compared with male sex (86.1% vs 100%, P = .047). Subtotal resection demonstrated both inferior RFS (67.5% vs 96.6%, P = .025) and OS compared with gross total resection (70.0% vs 100%, P < .001). Tumors >4.5 cm had worse RFS than tumors ≤4.5 cm (85.4% vs 100%, P = .025). Patient OS was lower in tumors with an MIB-1 index >5% than ≤5% (89.7% vs 100%, P = .008). Eastern Cooperative Oncology Group 2-4 negatively impacted OS relative to patients with an Eastern Cooperative Oncology Group 0-1 (66.7% vs 100%, P < .001).
Conclusions
Significantly higher rates of recurrence occurred in female sex, subtotal resection, and tumors larger than 4.5 cm. Further studies are needed to confirm these findings and determine whether patients without any of these risk factors can undergo surgical resection without adjuvant radiation therapy.
Summary.
We analyzed 50 patients treated for atypical meningioma at our institution to identify patient subgroups with an increased risk of recurrence. Female sex, subtotal resection, and large tumor size were found to be associated with a significantly higher risk of recurrence. Patients with these risk factors may warrant increased consideration of adjuvant radiation therapy to their treatment paradigms.
Introduction
Meningiomas represent 36% of all primary brain neoplasms in adults and are the most commonly reported central nervous system tumors.1 The incidence of meningiomas is approximately 6-7 in 100,000, and increases with age. They are often an incidental finding during autopsy or imaging.1 Pathogenesis is believed to be initiated by genetic loss of chromosome 22q12, with tumor aggressiveness corresponding to degree of genomic instability.2 Meningiomas are categorized histologically based on the World Health Organization (WHO) classification system as grade I (benign), grade II (atypical), and grade III (anaplastic), representing 80%, 15% to 20%, and 1% to 3% of all meningiomas, respectively.3
The WHO classification system underwent a significant revision in 2000 and an update in 2007.4, 5 These changes allowed clearer definitions of variants and resulted in a redistribution of many patients into different classes, with better correlation between grade and tumor behavior. Many originally “benign” or “malignant” meningiomas were reclassified as atypical, increasing the incidence of grade II from previously reported 5% to 7% to the current numbers of approximately 20% to 35%.6, 7 The sudden increase in reported grade II, and corresponding correction of reported grades I and III, meningiomas has obfuscated previous clinical data regarding outcomes and management.8
Given the increase of atypical meningiomas classified under the new guidelines, the need for reassessment of the current treatment approach is of growing importance.9, 10 Although gross total resection (GTR) is known to be critical, there is no consensus on the role of adjuvant radiation therapy (RT) in the treatment of atypical meningiomas, resulting in inconsistencies between institutions.11 Although prospective trials are in development (European Organization for Research and Treatment of Cancer 1308, Radiation Therapy Oncology Group 0539), the results of these studies will not be available for some time.12, 13
In this study, we analyzed outcomes of atypical meningiomas diagnosed in the modern treatment era at a single institution and assessed prognostic factors related to overall survival (OS) and recurrence free survival (RFS), with a focus on recurrence as these patients may warrant greater consideration for RT.
Methods
Patients
Fifty patients were treated for grade II meningiomas at the University of Illinois Hospital in Chicago, Illinois, between March 2000 and February 2013. This study was approved by the University of Illinois institutional review board. Electronic medical records (EMRs) were reviewed and clinical information recorded. Sex, race, age of diagnosis, tumor location, MIB-1 index, size of tumor, and RT status were documented. Preoperative performance status was retrospectively assessed using the Eastern Cooperative Oncology Group (ECOG) score. Meningioma grading via the 2007 WHO classification system was verified based on pathology reports along with MIB-1 status. Preoperative radiology reports were used to confirm tumor location and size based on largest single dimension.
Treatment
Extent of resection was based on surgical operative notes and post-operative imaging. GTR was defined as Simpson 1-2 and subtotal resection (STR) as Simpson 3-4. Pursuit of RT, dose, and modality were also recorded. Patient follow-up and vital status were based on medical record review, patient contact, and Social Security Death Index query.
Analysis
Statistical analysis was conducted using IBM SPSS Statistics for Windows, version 22. Date of diagnosis was defined as the date of surgical resection and was used as the starting date for OS, with survival measured until date of death or last follow-up. RFS was measured starting at date of resection until tumor growth was noted on routine follow-up imaging or until symptom development, verified by imaging. Kaplan-Meier curves and log-rank tests were used to compare groups for OS and RFS. Univariate analysis of each variable (sex, age, resection status, tumor size, MIB-1 status, ECOG status, tumor location, and RT) was conducted. Results from analysis were deemed significant at P <.05.
Results
Patient characteristics
A summary of patient characteristics is available in Table 1. Median age of diagnosis was 58 years (range, 26-82) and median follow-up was 37 months (range, 1-148). The cohort was predominately female (64%) with an ECOG of 0-1 (72%). Tumor locations were categorized as convexity (40%), parasagittal/falx (12%), skull base (30%), intraventricular (12%), and spinal (6%). Tumor size was not available in 4 patients because of incomplete medical records; these patients were excluded from analysis of size. A cutoff of 4.5 cm was used as this was the average greatest single dimension in our patient base. Tumor size was >4.5 cm in 52%, ≤4.5 cm in 40%, and unknown in 8% of patients. MIB-1 status was >5% in 64%, and GTR was obtained in 78% of patients. Of the cohort, 38% received postoperative RT and 73% of those who underwent STR received postoperative RT. A total of 63% of patients receiving postoperative RT received intensity modulated radiation therapy (IMRT), whereas 16% received stereotactic radiosurgery, and the remaining 21% were unknown. The specific dose used in cases using IMRT was available for 12 patients. The median postoperative radiation dose used in IMRT was 54 Gy (range, 50-60) and the median number of fractions delivered was 30 (range, 14-33). Specifics in regard to treatment approach, such as volume delineation, could not be obtained because of limitations of the EMR. Median time between surgery and RT was 2 months (range, 1-6).
Table 1.
Patient and treatment characteristics
| Characteristics | Total N (%) | Surgery only N (%) | Surgery + RT N (%) |
|---|---|---|---|
| Gender | |||
| Male | 18 (36) | 12 (39) | 6 (32) |
| Female | 32 (64) | 19 (61) | 13 (68) |
| Age of diagnosis | |||
| ≤60 y | 27 (54) | 15 (48) | 12 (63) |
| >60 y | 23 (46) | 16 (52) | 7 (37) |
| Race | |||
| Caucasian | 19 (38) | 11 (35) | 8 (42) |
| Hispanic | 12 (24) | 8 (26) | 4 (21) |
| African American | 17 (34) | 12 (39) | 5 (26) |
| Asian | 2 (4) | 0 (0) | 2 (11) |
| Resection | |||
| Subtotal (Simpson III-IV) | 11 (22) | 3 (10) | 8 (42) |
| Total (Simpson I-II) | 39 (78) | 28 (90) | 11 (58) |
| Size | |||
| ≤4.5 cm | 20 (40) | 16 (52) | 4 (21) |
| >4.5 cm | 26 (52) | 13 (42) | 13 (68) |
| Unknown | 4 (8) | 2 (6) | 2 (11) |
| MIB-1 | |||
| ≤5% | 18 (36) | 13 (42) | 5 (26) |
| >5% | 32 (64) | 18 (58) | 14 (74) |
| Location | |||
| Convexity | 20 (40) | 13 (42) | 7 (37) |
| Parasagittal/parafalcine | 6 (12) | 3 (10) | 3 (16) |
| Skull base | 15 (30) | 9 (29) | 6 (32) |
| Intraventricular | 6 (12) | 4 (13) | 2 (10) |
| Spinal | 3 (6) | 2 (6) | 1 (5) |
| Performance status | |||
| ECOG 0-1 | 36 (72) | 21 (68) | 15 (79) |
| ECOG 2-4 | 14 (28) | 10 (32) | 4 (21) |
| RT | |||
| Yes | 19 (38) | ||
| No | 31 (62) | ||
| Modality | |||
| SRS | 3 (16) | ||
| IMRT | 12 (63) | ||
| Unknown | 4 (21) | ||
| Dose | |||
| ≤54 Gy | 9 (75) | ||
| >54 Gy | 3 (25) | ||
| Recurrence | 7 (14) | 2 (6) | 5 (26) |
| Death | 10 (20) | 6 (19) | 4 (21) |
| Average follow-up (mo) | |||
| Mean | 41.76 | ||
| Median | 37 |
ECOG, Eastern Cooperative Oncology Group; IMRT, intensity modulated radiation therapy; RT, radiation therapy; SRS, stereotactic radiosurgery.
OS and RFS
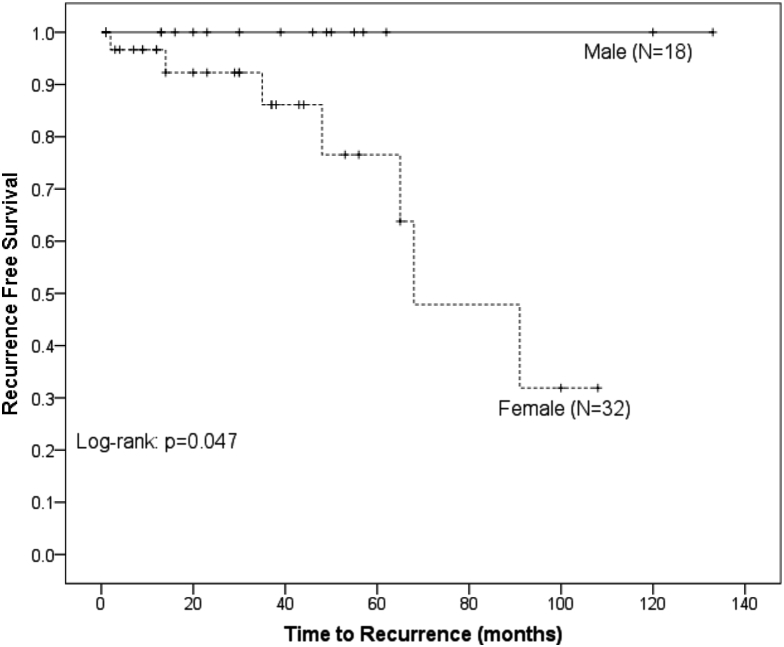
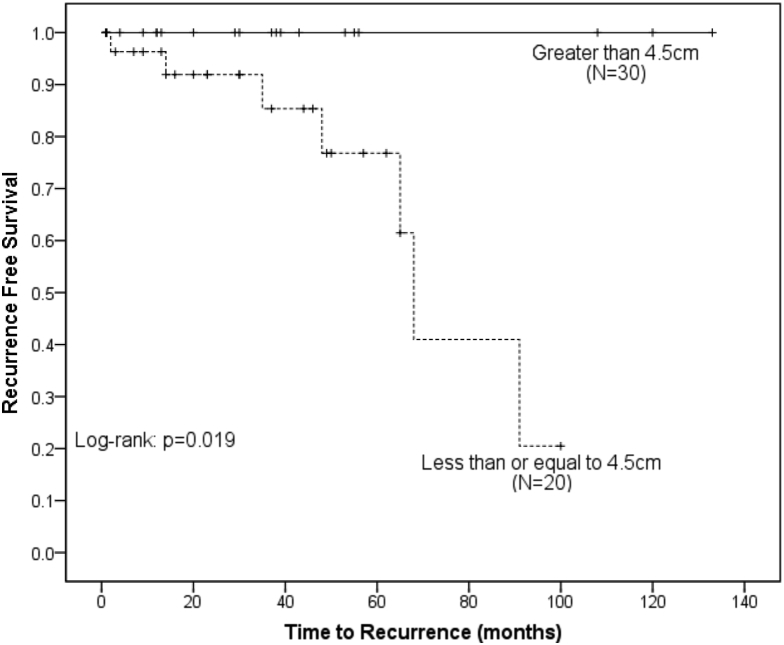
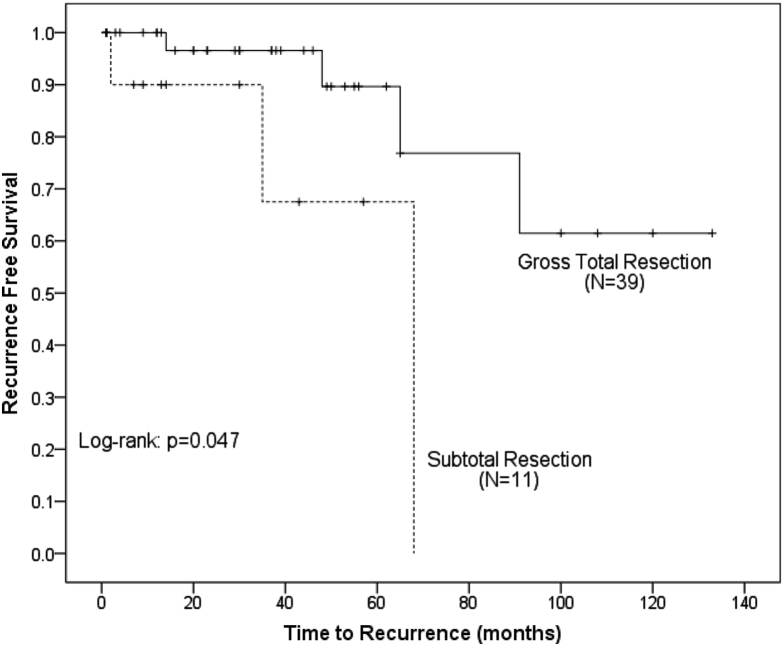
RFS was significantly higher in men compared with women (100% vs 88.1% at 3 years, P = .047) (Fig 1). Resection status had a significant impact on RFS, with GTR being superior to STR (96.6% vs 67.5% at 3 years, P = .025) (Fig 2). Tumor size had a significant impact on RFS (100% in ≤4.5 cm vs 85.4% in >4.5 cm, P = .019) (Fig 3). There was no benefit to RFS based on MIB-1 index, location, or performance status.
Figure 1.
Kaplan-Meier analysis of recurrence free survival comparing patients based on sex. Female patients had a statistically greater risk of recurrence.
Figure 2.
Kaplan-Meier analysis of recurrence free survival comparing patients based on tumor size. Patients with a tumor size >4.5 cm had a statistically greater risk of recurrence.
Figure 3.
Kaplan-Meier analysis of recurrence free survival comparing patients based on resection status. Patients with subtotal resections had a statistically greater risk of recurrence.
GTR had a significantly higher OS compared with STR (100% vs 70.0% at 3 years, P < .001). Tumors with an MIB-1 index ≤5% had a significantly greater OS compared with >5% (100% vs 89.7% at 3 years, P = .008). Patients with a better performance status had improved OS (100% in ECOG 0-1 vs 66.7% in ECOG 2-4, P < .001). Age and location trended towards significance for OS (P = .063 and P = .066, respectively). There was no difference in survival based on sex or tumor size.
Analysis of the effect of RT did not reveal a significant difference in OS (100% in no RT vs 100% in RT at 3 years, P > .1) or RFS (100% in no RT vs 90% in RT at 3 years, P > .1) in patients who had GTR. Patients who received RT after STR trended toward significance in OS (33.3% in no RT vs 85.7% in RT at 3 years, P = .054), but no significant benefit was observed for RFS (100% in no RT vs 64.3% in RT at 3 years, P > 1). IMRT dose did not appear to have significant benefit to OS or RFS (P > .1).
Discussion
The significant shift in rates of atypical meningiomas as a consequence of the WHO 2000/2007 classification revisions has made much of the prior research examining outcomes of atypical meningiomas inapplicable.14, 15 Therefore, this study has important clinical significance because it reports outcomes of patients diagnosed with atypical meningioma after the reclassification occurred in 2000. This study found that the most important prognostic factors of recurrence were size >4.5 cm, female sex, and subtotal resection.
The benefit to both OS and RFS with regard to the level of resection reaffirms the importance of GTR as the primary prognostic indicator in the treatment of atypical meningiomas. A recent review article demonstrated the impact of resection on improved outcomes and the importance of postoperative RT in those who have undergone a STR.16 This is consistent with our near significant findings for OS benefit from RT in STR, though we were perhaps not able to appreciate a significant effect because of our small sample size. Although no statistically significant difference in RFS was discovered from RT in STR, it was observed that STR patients that only received surgery appeared to have better RFS than those that received postoperative RT (100% vs 64.3%, respectively). This seemingly paradoxical effect of RT in the STR group is likely a manifestation of selection bias and differences in patient characteristics. As previously discussed, the benefit of RT to RFS has been described in a number of studies, and is generally recommended in the setting of STR. Kaur et al performed a systematic review of the literature specifically to evaluate the benefit of adjuvant RT in atypical and malignant meningioma.9 The authors also recommended RT in the setting of STR, citing benefit to both OS and RFS.
Tumor size was found in our study to be a significant contributing factor for recurrence. Detti et al found size to have a significant impact on recurrence at 5 cm, which is similar to the value used in our study.17 A study by Pizem et al provides a possible explanation for this association, by identifying both size and grade as predictors of brain invasion.18 Brain invasion has been demonstrated to have a powerful impact on recurrence and has been thoroughly reviewed by Modha et al.10 These studies together provide both support for our findings and rationale for the use of adjuvant RT in larger tumors, even when GTR is obtained.
Female sex was discovered to have an adverse effect on recurrence. To our knowledge, this study is only the second to indicate sex as a possible prognostic factor for recurrence.19 The incidence of meningiomas is well known to be higher in women.11 This is thought to correlate with the presence of androgen, estrogen, and progesterone receptors on meningiomas.2, 20 It is possible that differing hormone levels between men and women play a role in the risk of recurrence; further investigations are needed to confirm female sex as a negative prognostic factor.
This study has several limitations that are inherent in a single institution retrospective analysis. Using EMR for patient information allows for selection bias as the decision to refer or pursue for RT varies by provider. In addition, the availability and completeness of patient and treatment information is limited by the dependence on EMR. For example, information on specific dose for IMRT was only available for 12 patients. Although we found this to not be a significant contributor to RFS or OS, we lack power to thoroughly assess its contribution to these endpoints. Another major limitation was the small number of recurrences that occurred in the cohort, which precluded us from performing an accurate multivariate analysis examining prognostic factors. This in turn may increase the risk of finding incidentally significant P values, and restricts our ability to detect subtle differences. Last, retrospective studies result in the use of heterogeneous patient populations, using a range of patient demographics, tumor locations, and treatment techniques in a nonrandomized fashion. Considering these limitations, our results are interpreted with caution. We recommend considerations of the factors reported here on an individual basis until the prospective trials EORTC 1308 and RTOG 0539 are able to provide more specific guidelines.
Conclusion
In this retrospective review, we found that female sex, STR, and tumor size >4.5 cm negatively affect RFS. The identification of female sex as a risk factor for recurrence is a novel finding. Adjuvant RT should be considered in patients with these risk factors. Further studies are needed to confirm these findings and to elucidate if there is a subgroup of patients with atypical meningioma in which adjuvant RT can be safely avoided.
Acknowledgments
All procedures performed in studies involving human participants were done in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. For this type of study, formal consent is not required.
Footnotes
Conflicts of interest: None.
References
- 1.Ostrom Q.T., Gittleman H., Liao P. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the united states in 2007-2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choy W., Kim W., Nagasawa D. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 3.Riemenschneider M.J., Perry A., Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 4.Kleihues P., Cavenee W.K. 2nd ed. IARC; Lyon, France: 2000. WHO Classification of Tumours of the Central Nervous System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K. 4th ed. IARC; Lyon, France: 2007. WHO Classification of Tumours of the Central Nervous System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis J., Smith C., Ironside J., Erridge S., Whittle I., Everington D. The accuracy of meningioma grading: A 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–149. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 7.Pearson B., Markert J., Fisher W. Hitting a moving target: Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/5/E3. [DOI] [PubMed] [Google Scholar]
- 8.Smith S., Boddu S., Macarthur D. Atypical meningiomas: WHO moved the goalposts? Br J Neurosurg. 2007;21:588–592. doi: 10.1080/02688690701684246. [DOI] [PubMed] [Google Scholar]
- 9.Kaur G., Sayegh E., Larson A. Adjuvant radiotherapy for atypical and malignant meningiomas: A systematic review. Neuro Oncol. 2014;16:628–636. doi: 10.1093/neuonc/nou025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modha A., Gutin P. Diagnosis and treatment of atypical and anaplastic meningiomas: A review. Neurosurgery. 2005;57:538–550. doi: 10.1227/01.neu.0000170980.47582.a5. [DOI] [PubMed] [Google Scholar]
- 11.Simon M., Boström J., Koch P., Schramm J. Interinstitutional variance of postoperative radiotherapy and follow up for meningiomas in Germany: Impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry. 2006;77:767–773. doi: 10.1136/jnnp.2005.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson M., Weber D., Haylock B., Mallucci C., Zakaria R., Javadpour M. Radiotherapy versus observation following surgical resection of atypical meningioma (the ROAM trial) Neuro Oncol. 2014;16:1560–1561. doi: 10.1093/neuonc/nou149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radiation Therapy Oncology Group. RTOG 059 protocol information. Available at: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0539. Accessed March 9, 2016.
- 14.Combs S., Schulz-Ertner D., Debus J., von Deimling A., Hartmann C. Improved correlation of the neuropathologic classification according to adapted World Health Organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys. 2011;81:1415–1421. doi: 10.1016/j.ijrobp.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Yoon H., Mehta M., Perumal K. Atypical meningioma: Randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther. 2015;11:59–66. doi: 10.4103/0973-1482.148708. [DOI] [PubMed] [Google Scholar]
- 16.Sun S., Hawasli A., Huang J., Chicoine M., Kim A. An evidence-based treatment algorithm for the management of WHO grade II and III meningiomas. Neurosurg Focus. 2015;38:E3. doi: 10.3171/2015.1.FOCUS14757. [DOI] [PubMed] [Google Scholar]
- 17.Detti B., Scoccianti S., Di Cataldo V. Atypical and malignant meningioma: Outcome and prognostic factors in 68 irradiated patients. J Neurooncol. 2013;115:421–427. doi: 10.1007/s11060-013-1239-7. [DOI] [PubMed] [Google Scholar]
- 18.Pizem J., Velnar T., Prestor B., Mlakar J., Popovic M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin Neuropathol. 2014;33:354–363. doi: 10.5414/NP300750. [DOI] [PubMed] [Google Scholar]
- 19.Zhao P., Hu M., Zhao M., Ren X., Jiang Z. Prognostic factors for patients with atypical malignant meningiomas treated at a single center. Neurosurg Rev. 2015;38:101–107. doi: 10.1007/s10143-014-0558-2. [DOI] [PubMed] [Google Scholar]
- 20.Baxter D., Orrego A., Rosenfeld J., Mathiesen T. An audit of immunohistochemical marker patterns in meningioma. J Clin Neurosci. 2014;21:421–426. doi: 10.1016/j.jocn.2013.06.008. [DOI] [PubMed] [Google Scholar]