Abstract
Purpose
Accurate assessment of toxicity allows for timely delivery of supportive measures during radiation therapy for head and neck cancer. The current paradigm requires weekly evaluation of patients by a provider. The purpose of this study is to evaluate the feasibility of monitoring patient reported symptoms via mobile devices.
Methods and materials
We developed a mobile application for patients to report symptoms in 5 domains using validated questions. Patients were asked to report symptoms using a mobile device once daily during treatment or more often as needed. Clinicians reviewed patient-reported symptoms during weekly symptom management visits and patients completed surveys regarding perceptions of the utility of the mobile application. The primary outcome measure was patient compliance with mobile device reporting. Compliance is defined as number of days with a symptom report divided by number of days on study.
Results
There were 921 symptom reports collected from 22 patients during treatment. Median reporting compliance was 71% (interquartile range, 45%-80%). Median number of reports submitted per patient was 34 (interquartile range, 21-53). Median number of reports submitted by patients per week was similar throughout radiation therapy and there was significant reporting during nonclinic hours. Patients reported high satisfaction with the use of mobile devices to report symptoms.
Conclusions
A substantial percentage of patients used mobile devices to continuously report symptoms throughout a course of radiation therapy for head and neck cancer. Future studies should evaluate the impact of mobile device symptom reporting on improving patient outcomes.
Summary.
Data regarding feasibility of mobile device technology to collet patient-reported symptoms for patients receiving cancer treatment are sparse. The present study evaluates feasibility of mobile device symptom reporting for cancer patients receiving head and neck radiation therapy. Patients receiving radiation therapy for head and neck cancer were able to quickly and effectively use mobile devices to report symptoms during treatment.
Introduction
Head and neck cancer patients receiving radiation therapy often experience significant toxicity. Common side effects of head and neck radiation therapy include mucositis, pain, xerostomia, nausea, vomiting, weight loss, and fatigue. Monitoring patient symptoms during radiation therapy is important because accurate assessment of treatment toxicity allows for delivery of necessary supportive measures. Clinician assessments of toxicity are often discordant with patient-reported symptoms. One cross-sectional study examining toxicity reporting showed rates of discordance between clinician and patient reported toxicity ranging between 15% and 59% for various symptoms.1 Another study of patients enrolled in clinical trials found that patient-reported toxicity was higher than clinician-reported toxicity for a variety of endpoints.2 Some evidence suggests that patient-reported symptoms better correlate with underlying functional status and clinical outcomes than clinician-reported symptoms.3
Typically, patients receiving radiation therapy are evaluated once per week by a health care provider during a radiation treatment course. Although patients may report symptoms to a physician or health care provider at any time during treatment, it is common for symptoms to go unreported until the weekly visit. This delay in reporting of symptoms may lead to undertreatment of symptoms or delay in diagnosis and management of toxicity. Furthermore, evaluations of toxicity that are done periodically (ie, weekly, monthly, or with each cycle of chemotherapy) may be subject to recall bias and/or underreporting of toxicity related to treatment. Increased real-time reporting by patients may lead to improved symptom ascertainment by providers. If patients report symptoms immediately, this will provide the most accurate representation of how patients are tolerating treatment, which should allow providers to better manage toxicity.
Internet-based patient-reported symptom assessment has been reported for oncology and surgery patients, but these studies did not specifically evaluate the use of mobile device platforms.4, 5 Mobile device ownership is increasing, and reporting symptoms on a personal mobile device may be more convenient than using a computer to access an Internet-based reporting system. We developed a novel mobile application to allow patients to report symptoms related to their treatment in real time. The purpose of this study is to evaluate the feasibility of using mobile device technology to continuously collect patient-reported symptoms for head and neck cancer patients receiving curative radiation therapy.
Methods
Patient selection
We conducted a single-institution prospective study to evaluate the feasibility of using a mobile application to allow head and neck cancer patients receiving curative radiation therapy to report symptoms in real time.
This study was approved by the institutional review board, and all patients provided written informed consent. Enrollment was initiated in May 2014 and continued until completion of patient accrual (December 2014). Eligibility criteria included age ≥18 years with histologically confirmed T0-4 N0-3 M0 cancer of the head and neck. Disease sites allowed were nasal cavity and sinuses, nasopharynx, oropharynx, oral cavity, hypopharynx, larynx, salivary gland, and cutaneous malignancy. Patients with early-stage larynx cancer treated with definitive narrow-field radiation therapy were ineligible because of the low toxicity associated with this treatment.6 Planned radiation dose was required to be ≥60 Gy (1.2-2.25 Gy/fraction). All patients received either definitive (radiation therapy as primary treatment) or postoperative radiation therapy. Concurrent chemoradiation therapy was permitted but not required. Patient performance status was required to be ≤1 as measured by the Eastern Cooperative Oncology Group Performance Status scale.7
Intervention
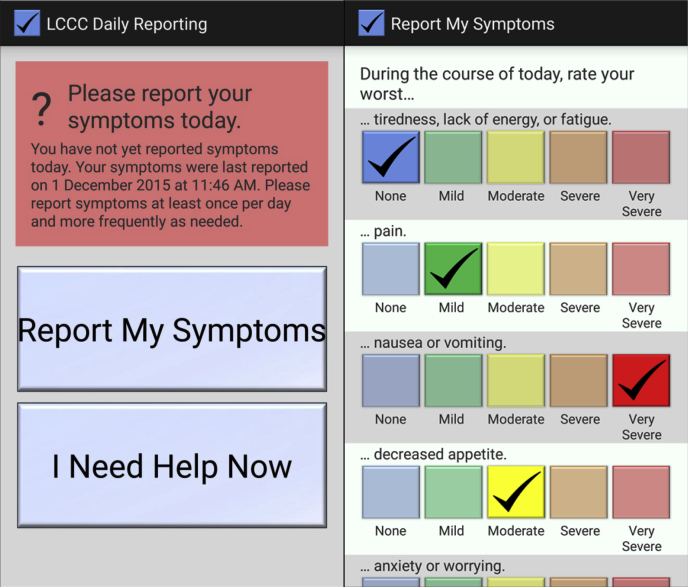
A Health Insurance Portability and Accountability Act–secure mobile application was developed for Android (Google Inc., Mountainview, CA) and iOS (Apple Inc., Cupertino, CA) mobile operating systems. The application requires a 1-time initialization with a unique patient identification number and password. No patient-identifying information or protected health information is transmitted by the application and therefore there is no risk of compromise of protected health information should an unauthorized person gain access to any data transmitted by the application. The format of the data transmitted to the server is a string consisting of 2 timestamps (indicating when patients opened the symptom-reporting screen and when the symptom report was submitted) as well as the numeric indices of patients' answers to the symptom survey questions. The mobile application was installed and initialized on patients' smartphones and/or tablet computers by the study coordinator. The study coordinator also provided instructions to patients regarding use of the application. If patients did not own a mobile device, a tablet computer was provided by the clinic and returned after completion of the study. The symptom-reporting application consists of a main screen that indicates to patients whether or not they have reported symptoms on the current date and displays the time and date of the most recent symptom report. A button entitled “Report My Symptoms” opens a screen, which allows patients to report symptom severity in 5 domains (fatigue, pain, nausea/vomiting, decreased appetite, anxiety) using questions adapted from the Patient Reported Symptom Monitoring System, a validated instrument to measure toxicity (other data; A. M. Stover, et al., unpublished data). For each domain, patients could report toxicity as follows: none, mild, moderate, severe, or very severe. Patients were asked to report symptoms using a smartphone and/or tablet at least once daily (including weekends) during treatment and encouraged to report multiple times daily as symptoms were occurring. The mobile application also provides a button to allow patients to call the clinic directly (not available for tablet computers). The application interface is shown in Fig 1. To ensure that the application was nonintrusive, there were no mobile-device notifications or other reminders to prompt patient reporting of symptoms. Patients were instructed that symptoms would not be immediately seen by the clinician in a timely manner, but would be reviewed retrospectively during the weekly clinical visit.
Figure 1.
Mobile application screenshots, showing the main screen (left) and survey screen (right) of the application.
The typical radiation therapy treatment course for head and neck cancer is once or twice daily, Monday through Friday for a total of 25 to 35 treatment days (approximately 5 to 7 weeks). As part of routine clinical practice, patients are seen by clinicians once weekly during treatment to manage treatment-related toxicity/symptoms. At the end of the visit, providers reviewed the patient-reported data with patients. Clinical impact of patient-reported symptoms on toxicity management and patient outcomes is not reported in this feasibility study, but data were collected for future analysis. Patient surveys regarding use of mobile devices were obtained before and after completion of radiation therapy. Patients also completed a survey to provide demographic information, such as race and income.
Outcome measures
The primary endpoint of this study was patient reporting compliance, which is defined as the number of days with any patient report submitted divided by the number of days between day 1 of treatment and the end of radiation therapy. A prespecified definition of feasibility is that at least 80% of patients use the application at least 80% of the days during radiation treatment. Patient survey responses regarding mobile device use were a secondary endpoint of this study. We also report total number of symptom reports submitted per patient, timing of report submissions, and time required for patients to complete and submit the symptom survey using mobile devices.
Statistical analysis
Descriptive statistics summarize patient demographics, treatment characteristics, patterns of use for the mobile application, and survey responses. A study sample size of 20 patients allows the proportion of patients who demonstrate reporting compliance of at least 80% to be estimated within 23% (using an exact 95% confidence interval). All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Fifty-five patients were eligible for enrollment, of which 22 (40%) were accrued and included in this analysis. Reasons for nonenrollment of eligible patients included: patient did not own a mobile device and no tablet was available to loan from clinic (20 patients), enrollment on a different clinical trial (6 patients), homelessness (2 patients), and unknown (5 patients). One patient withdrew consent during treatment for unknown reasons; data collected from this patient before withdrawal of consent were included for analysis. One patient was ineligible because of disease site (cervical esophagus) and dose (50.4 Gy); data collected from this patient were included in analysis of application feasibility. Patient demographics and treatment characteristics are shown in Table 1.
Table 1.
Patient demographics and treatment characteristics
| Characteristic | N | % | |
|---|---|---|---|
| Patient characteristics | Sex | ||
| Male | 15 | 68 | |
| Female | 7 | 32 | |
| Age (y) | |||
| <60 | 13 | 59 | |
| ≥60 | 9 | 41 | |
| Marital status | |||
| Married | 16 | 73 | |
| Not married | 6 | 27 | |
| Race | |||
| White | 18 | 82 | |
| Black | 4 | 18 | |
| College or advanced degree | |||
| No | 14 | 64 | |
| Yes | 8 | 36 | |
| Insurance coverage | |||
| No | 5 | 23 | |
| Yes | 17 | 77 | |
| Household income | |||
| <$70,000 | 11 | 50 | |
| ≥$70,000 | 11 | 50 | |
| Disease site | |||
| Oropharynx | 9 | 41 | |
| Sinonasal | 4 | 18 | |
| Oral cavity | 3 | 14 | |
| Unknown primary | 2 | 9 | |
| Othera | 4 | 18 | |
| Treatment characteristics | Concurrent chemotherapy | ||
| Yes | 18 | 82 | |
| No | 4 | 18 | |
| Radiation therapy course | |||
| Definitive | 13 | 59 | |
| Postoperative | 9 | 31 | |
| Radiation therapy technique | |||
| IMRT | 21 | 95 | |
| Conformal | 1 | 5 | |
| Median | IQR | ||
| Radiation therapy prescription dose (Gy) | 69.6 | 60-70 | |
| Radiation therapy treatment duration (d) | 45.5 | 42-49 |
IMRT, intensity modulated radiation therapy; IQR, interquartile range.
Other includes larynx, skin, nasopharynx, and cervical esophagus.
Patient symptom reporting
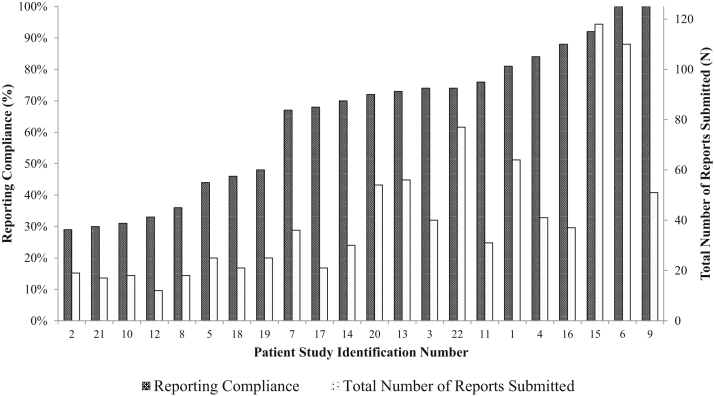
Patient compliance and the total number of reports submitted are shown in Fig 2. Median reporting compliance was 71% (interquartile range [IQR], 45%-80%). Six patients (27%) demonstrated reporting compliance ≥80% and 2 patients (9%) were 100% compliant. The median number of reports submitted per patient was 34 (IQR, 21-53).
Figure 2.
Reporting compliance and number of reports submitted, per patient. Reporting compliance is defined as the number of days with any patient reported symptom divided by the number of days between study enrollment and the end of radiation therapy.
Timing of symptom reporting via the mobile application is reported in Table 2. Mobile application use was consistent during all days of the week and symptom reporting did not decrease during the later weeks of treatment. Five hundred and sixteen (56%) of all patient reports were submitted during nonclinic hours (5 PM-7 AM or weekends). The median time between opening the symptom-reporting screen and pressing the submit button was 22 seconds (IQR, 15-35). Ninety-five percent of all symptom reports required 150 seconds or less to submit using the application. Subgroup analysis of reports in which any symptom was reported as severe or very severe (N = 209) demonstrated median time to report of 24 seconds (IQR, 17-43).
Table 2.
Timing of symptom reporting using the mobile application
| Reports per patient (median) | IQR | |
|---|---|---|
| Day of week | ||
| Sun | 5 | 3-7 |
| Mon | 5 | 3-8 |
| Tue | 5 | 4-8 |
| Wed | 5 | 3-8 |
| Thurs | 5.5 | 4-7 |
| Fri | 6 | 3-10 |
| Sat | 4 | 2-6 |
| Week of treatment | ||
| 1 | 7 | 3-10 |
| 2 | 6 | 5-7 |
| 3 | 6 | 5-9 |
| 4 | 6.5 | 4-9 |
| 5 | 6 | 4-8 |
| 6 | 5 | 3-7 |
| 7 | 6.5 | 5-12 |
| Report during clinic hours (7 AM-5 PM, weekdays) | ||
| Nonclinic hours | 17 | 10-30 |
| Clinic hours | 14.5 | 9.5-27.5 |
IQR, interquartile range.
Patient satisfaction with using mobile devices to report symptoms was high and overall pretreatment and posttreatment survey responses were positive (Table 3). Posttreatment survey data were available for 19 of the 21 patients who completed the study (90%). One hundred percent of respondents reported that participating in the trial was worthwhile and that they would recommend to others to participate in similar trials evaluating the use of mobile device technology.
Table 3.
Summary of pretreatment survey and posttreatment survey responses
| Response | N | % | |
|---|---|---|---|
| Pretreatment survey questions | |||
| Reporting treatment side effects daily will help my physician better manage my symptoms | Agree | 13 | 59 |
| Strongly agree | 9 | 41 | |
| I am confident that it will be easy to use the application to report symptoms daily | Agree | 13 | 59 |
| Strongly agree | 9 | 41 | |
| I am confident that I will report symptoms at least once per day, and more often as needed | Neutral | 1 | 5 |
| Agree | 12 | 55 | |
| Strongly agree | 9 | 41 | |
| I feel comfortable using my smartphone or tablet to report symptoms to my physician | Agree | 13 | 59 |
| Strongly agree | 9 | 41 | |
| The most accurate and convenient way to record symptoms on a daily basis is | Smartphone or tablet | 22 | 100 |
| Internet Web site | 0 | 0 | |
| Paper survey | 0 | 0 | |
| Posttreatment survey questions | |||
| Reporting treatment side effects on a daily basis helped my physician to better manage my symptoms | Agree | 11 | 58 |
| Strongly agree | 8 | 42 | |
| I found it convenient to use the application to report symptoms daily | Disagree | 1 | 5 |
| Neutral | 2 | 11 | |
| Agree | 8 | 42 | |
| Strongly agree | 8 | 42 | |
| I felt comfortable using a smartphone or tablet to report symptoms to my physician | Agree | 10 | 53 |
| Strongly agree | 9 | 47 | |
| What type of mobile device did you most frequently use to submit your daily symptoms | Smartphone | 7 | 37 |
| Tablet computer | 12 | 63 | |
| Who reported your symptoms most of the time? | Patient | 16 | 84 |
| Caregiver | 2 | 11 | |
| Both patient and caregiver | 1 | 5 | |
| The most accurate and convenient way to record symptoms on a daily basis is | Smartphone or tablet | 17 | 89 |
| Internet Web site | 1 | 5 | |
| Paper survey | 1 | 5 | |
| I felt that participating in this trial was worthwhile | True | 19 | 100 |
| False | 0 | 0 | |
| I would recommend to other people that they participate in this trial | True | 19 | 100 |
| False | 0 | 0 |
Discussion
This study demonstrates that substantial symptom reporting is possible using mobile device technology. The prespecified definition of feasibility, which required 80% of patients to report symptoms with 80% compliance, may have been an unrealistically high bar. Although this endpoint was not met, the significant levels of patient reporting observed in this study are encouraging and suggest that mobile devices can be a useful method to collect patient-reported outcomes. This study population was diverse with respect to race, age, gender, and other sociodemographic variables and is representative of the head and neck cancer population treated at our institution.
Previous studies evaluating mobile device symptom reporting for patients receiving chemotherapy experienced a steady decline in participants contributing data.8 Similarly, a study demonstrating feasibility of using tablet devices to collect quality of life assessment data from patients receiving radiation therapy for head and neck cancer showed a reduction in survey completion at later follow-up visits.9 In contrast, the present study demonstrated a high level of patient reporting throughout the completion of therapy, and we did not observe reductions in reporting compliance during later weeks of treatment.
Head and neck cancer patients are commonly treated with multimodality therapy that is associated with substantial toxicity and high rates of unplanned hospital admission.10 This patient population could therefore experience a significant benefit from aggressive symptom management facilitated by the use of mobile device technology.
Toxicity from radiation therapy increases throughout treatment, with minimal toxicity in the first 1-2 weeks and increasing toxicity in later weeks of treatment. This study demonstrated that mobile application symptom reporting can be used to collect data from patients even as they experience significant toxicity later in treatment. When analysis was restricted to only reports that contained at least 1 domain with severe or very severe symptoms, patients demonstrated an ability to quickly report symptoms. This may be due to the fact that mobile devices are easily accessible at short notice compared with other forms of symptom reporting. Furthermore, the design of this application did not require patients to log in with a username and password, which may have expedited patient reporting. The high level of patient reporting on nights and weekends is indicative that patients are willing to use mobile applications continuously to report symptoms as they occur, which can help eliminate issues related to recall when assessing toxicity. Given this high level of continuous reporting, data collected from mobile devices may provide a more complete picture of toxicity experienced by patients during treatment.
An important finding of this study is that even the least compliant patients provided a substantial number of symptom reports. The average number of total symptom reports submitted by the least compliant quartile of patients was 18 reports. In the absence of mobile device–based symptom reporting, these patients would typically have reported symptoms only once weekly (typically 6-7 times) during their course of radiation therapy. Therefore, the mobile application allowed for symptom reporting at least 3 times more often than would otherwise have been achieved.
A potential limitation of this study is that enrollment may have been higher among patients more likely to demonstrate willingness and ability to use mobile device technology to communicate symptoms. Approximately 60% of potentially eligible patients were not enrolled on this study. It is notable that, among these patients, 61% were not enrolled because of lack of a personal mobile device and lack of availability of a tablet to loan from the clinic. In the present study, no differences were seen in symptom reporting for patients who used their own device versus a clinic-provided tablet. Enrollment of patients was more likely limited by a lack of availability of tablets to loan rather than selection of only the most willing patients to participate. It is true that mobile-based symptom reporting will not be feasible for all patients, but this should not preclude us from offering this technology whenever possible to patients who could potentially benefit. Novel techniques and creative approaches are required to facilitate symptom reporting for patients who are less likely to use mobile device technology.
All patients in the present study were treated with curative intent. There is an opportunity to use mobile device technology to improve delivery of care for patients with metastatic cancer as well. Issues related to symptom management are different for patients receiving palliative radiation therapy. Doses of radiation prescribed for these patients are generally much lower than curative-intent cases and toxicity is typically more attributable to disease progression than treatment. Also, acuity of illness may be higher among patients with metastatic disease. Metastatic patients may benefit from rigorous symptom assessment to prevent unplanned admissions and maximize efforts to provide palliative care. Additional study is needed to determine if it is feasible to use mobile device technology to improve symptoms and minimize toxicity for patients with metastatic disease.
Future Directions/Conclusions
A substantial percentage of patients used mobile devices to continuously report symptoms throughout a course of radiation therapy for head and neck cancer. Electronic symptom reporting is emerging as a potentially powerful tool to improve the quality of health care.11 Pilot trials have demonstrated that patients are willing and able to report their own symptomatic toxicity in clinical trials.12 Patient-directed continuous self-reporting of symptoms is a method to improve accuracy of toxicity assessment in the setting of clinical trials.13
With continuous patient reporting of symptoms, the kinetics of patient reporting (by time, severity, frequency, etc.) can be studied and may be indicative or predictive of an impending clinical event (eg, hospital admission, urgent clinic visits). Earlier interventions may prevent escalation of symptoms and prevent hospital admission. The mobile application evaluated in this study is currently being used as part of a pilot study to reduce the rate of unplanned hospital admissions for high-risk patients receiving radiation therapy through intensive symptom management during treatment.
Footnotes
Conflicts of interest: None.
References
- 1.Basch E., Iasonos A., McDonough T. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 2.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basch E., Jia X.Y., Heller G. Adverse symptom event reporting by patients vs clinicians: Relationships with clinical outcomes. J Nat Cancer Inst. 2009;101:1624–1632. doi: 10.1093/jnci/djp386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basch E.M., Reeve B.B., Mitchell S.A. Electronic toxicity monitoring and patient-reported outcomes. Cancer J. 2011;17:231–234. doi: 10.1097/PPO.0b013e31822c28b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andikyan V., Rezk Y., Einstein M.H. A prospective study of the feasibility and acceptability of a Web-based, electronic patient-reported outcome system in assessing patient recovery after major gynecologic cancer surgery. Gynecol Oncol. 2012;127:273–277. doi: 10.1016/j.ygyno.2012.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki H., Nishiyama K., Tanaka E., Koizumi M., Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Oken M.M., Creech R.H., Tormey D.C. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 8.Kearney N., McCann L., Norrie J. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009;17:437–444. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 9.Pollom E.L., Wang E., Bui T.T. A prospective study of electronic quality of life assessment using tablet devices during and after treatment of head and neck cancers. Oral Oncol. 2015;51:1132–1137. doi: 10.1016/j.oraloncology.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Waddle M.R., Chen R.C., Arastu N.H. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol. 2015;5:e245–e253. doi: 10.1016/j.prro.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Johansen M.A., Henriksen E., Horsch A., Schuster T., Berntsen G.K. Electronic symptom reporting between patient and provider for improved health care service quality: A systematic review of randomized controlled trials. Part 1: State of the art. J Med Internet Res. 2012;14:e118. doi: 10.2196/jmir.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietanza M.C., Basch E.M., Lash A. Harnessing technology to improve clinical trials: Study of real-time informatics to collect data, toxicities, image response assessments, and patient-reported outcomes in a phase II clinical trial. J Clin Oncol. 2013;31:2004–2009. doi: 10.1200/JCO.2012.45.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed S., Berzon R.A., Revicki D.A. The use of patient-reported outcomes (PRO) within comparative effectiveness research implications for clinical practice and health care policy. Med Care. 2012;50:1060–1070. doi: 10.1097/MLR.0b013e318268aaff. [DOI] [PubMed] [Google Scholar]