Abstract
Background
Although cognitive deficits have consistently been characterized as core features of schizophrenia, they have not been incorporated into definitions of remission. Furthermore, just a few studies have examined the relationship between cognitive deficits and symptomatic remission. The main aim of the present study is to evaluate the executive functioning of nonremitted schizophrenia patients.
Methods
72 remitted and 42 nonremitted schizophrenia patients, and 119 healthy controls were examined. Subjects were tested with a comprehensive battery of cognitive tests, including a measure to assess the general components of executive functioning and individual tasks to tap the three specific executive dimensions assessed in the present study, namely updating, shifting and inhibition.
Results
Schizophrenia subjects performed poorly on general executive functioning and shifting tasks in comparison to healthy controls. Remitted subjects performed better than nonremitted on inhibition and updating tasks. Whereas being a male and showing decreases in updating increase the chances of being in the nonremitted schizophrenia subjects group, increases in shifting and updating enhance the odds of being in the healthy control group.
Conclusion
The present findings suggest that executive function deficits are present in chronic schizophrenic patients. In addition, specific executive processes might be associated to symptom remission. Future studies examining prospectively first-episode, drug naive patients diagnosed with schizophrenia may be especially elucidative.
Keywords: Schizophrenia, Remission, Neuropsychology, Executive functioning
Introduction
Schizophrenia (SCZ) is a chronic, disabling neuropsychiatric disease that affects 0.3% to 1.6% of the general population (Jablensky, 2000, Tandon et al., 2008). Notwithstanding the refinement of knowledge regarding its clinical course and advances in pharmacological and nonpharmacological interventions, the majority of individuals with schizophrenia still experience persistent incapacitating symptomatology and multiple relapses (Kane and Correll, 2010).
Different studies have found that remission, which is estimated to be achieved in only one-third of schizophrenia subjects (Lasser et al., 2007), is a good predictor of functional outcome (Andreasen et al., 2005, Emsley et al., 2013, Helldin et al., 2007). In order to provide a greater clarity about treatment goals and an improved framework for designing trials and test its effectiveness, the Remission in Schizophrenia Working Group (RSWG) has proposed consensual and operational criteria for remission in schizophrenia. According to RSWG, remission is defined as a state in which individuals with schizophrenia have experienced an improvement in core signs and symptoms considering that any remaining symptoms are of such low intensity that they no longer interfere significantly with behavior and are below the threshold typically used for establishing diagnose (Andreasen et al., 2005). The proposed symptom-based criteria include the seven diagnostically relevant items from the DSM-IV, which are cross-matched with eight items from the Positive and Negative Symptoms Scale (PANSS) (Kay et al., 1987). These eight items must score within a symptom level ≤ 3 points (mild or better severity) and include: delusions, unusual thought content, hallucinatory behavior, conceptual disorganization, mannerisms/posturing, blunted affect, passive/apathetic social withdrawal, lack of spontaneity and flow of conversation. There is also a minimum period of six months in which the symptoms severity must be maintained (Andreasen et al., 2005, Lambert et al., 2010).
Although clinical subtypes have been used in psychiatric nosology as a conceptual framework for understanding schizophrenia in past century, they have not proved useful for prognostic purposes (Braff et al., 2013). More recently, studies investigating different biomarkers have also reported disappointing findings (Asor and Ben-Shachar, 2012). Cognitive impairments have consistently been considered as core features of schizophrenia (Green and Harvey, 2014). They are present prior to onset of psychosis, are correlated with measurable brain dysfunction more than any clinical manifestation of the illness, are significantly associated with functioning in areas such as work performance, social relationships and independent living, and are increasingly considered as an important target for treatment (Green et al., 2000, Keef, 2008, Lewis, 2004, Palmer et al., 2009). Given the importance of reentering individuals with schizophrenia to community the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative of the U.S. National Institute of Mental Health (NIMH) set the development of a consensus cognitive battery (MCCB) for measuring cognition in schizophrenia, aiming to guide the design of clinical trials for cognition enhancing agents and encouraging new researches (Green and Nuechterlein, 2004).
It is noteworthy that cognitive impairments have not been incorporated into instrumental concepts of remission in schizophrenia, despite their clinical relevance (Andreasen et al., 2005, Helldin et al., 2006). Many studies have shown that schizophrenia subjects exhibit executive deficits, which are related to treatment refractoriness and poor functional outcomes and are likely to contribute to other cognitive deficits (Kerns et al., 2008). In addition, frontal lobe functioning measures have also been associated with recovery (Kopelowics et al., 2005). Executive functioning is a multidimensional process that covers a wide range of skills, which are used to guide behavior toward goals and to adapt to novel situations. It is considered the most sophisticated dimension of human behavior necessary for appropriate, socially responsible, independent and productive adult conduct. Executive processes are mediated by prefrontal regions and are associated with the ability to perform high-level tasks involving planning, organizing, initiating, monitoring and adapting behavior (Banish, 2009, Jurado and Rosselli, 2007, Lezak et al., 2004, Wilson et al., 2003).
The heterogeneity of the dysexecutive syndrome in schizophrenia, based on the theoretical model of Miyake et al. (2000), which postulates the notion, originally proposed by Teuber (1972), that different aspects of executive functioning correlate with one another, thus tapping some common underlying ability (unity), but also show some separability (diversity), has been emphasized in studies with chronic schizophrenia patients (Raffard and Bayard, 2012). Previous findings have suggested a specific impairment in the ability to update working memory in schizophrenia, and that this is associated with poor engagement with the environment (Galletly et al., 2006). Few studies have examined the relationship between cognitive deficits and symptomatic remission, and there were no clear evidence whether increased cognitive abilities, more specifically executive function, are a contributing factor for achieving remission. Some of these studies have found no significant differences in executive processes of fully remitted patients and healthy controls (Braw et al., 2012) or of remitted versus nonremitted schizophrenic patients (Brissos et al., 2011). On the other hand, some studies revealed marked differences in executive functioning between patients who have met the remission criteria and those who haven’t (Helldin et al., 2006, Hofer et al., 2011). At least part of this apparent inconsistency might be explained by methodological issues. Executive function tests differ in their complexity, specificity of the required specific executive abilities and even non-executive processes (Wood et al., 2009).
None of the previous studies systematically administered multiple executive tasks to understand the heterogeneity of the executive function impairments during symptomatic remission in schizophrenia patients. Therefore, the nature of how specific aspects of symptomatic remission affect, and are affected by, specific aspects of executive processes remains unclear.
The main aim of present study is to examine specific aspects of executive function of both remitted and nonremitted subjects, comparing with healthy controls. We hypothesized that both remitted and nonremitted subjects have executive function deficits in comparison to matched control group and that severity of deficits is greater in nonremitted subjects
Materials and methods
Subjects
A total of 114 subjects with DSM-IV schizophrenia were enrolled. Seventy-two (44 males; age = 35.98 ± 9.02 years and 28 females; age = 40.11 ± 11.57 years) patients were classified as remitted and 42 (36 males; age = 35.86 ± 9.39 years and 6 females; age = 32.33 ± 9.48 years) as nonremitted. 119 healthy controls (70 males; age = 33.56 ± 9.85 years and 49 females; age = 34.71 ± 11.28 years) were included in a cross-sectional study. Subjects who were not able to read and/or understand instructions of cognitive tests were excluded. Patients were recruited from an outpatient unit for treatment of schizophrenia, The Schizophrenia Program, Federal University of Sao Paulo (PROESQ). All participants provided written informed consent and the local ethics committee approved the study.
Diagnosis was assessed by using The Structured Clinical Interview for DSM-IV (SCID-I). Symptom severity was assessed by means of the Positive and Negative Symptoms Scale (PANSS) (Kay et al., 1987). Trained psychiatrists conducted all interviews.
To assess remission, the criteria proposed by Andreasen et al. (2005) were used, according to which 8 items of PANSS (delusions, conceptual disorganization, unusual thought content, hallucinatory behavior, mannerisms/posturing, blunted affect, social withdrawal and lack of spontaneity) should be scored ≤ 3 (mild). Yet, symptomatic remission criteria should be present for at least 6 months. Finally, the mean score on each group by gender was 69.72 ± 11.60 and 78.67 ± 2.25 for male and female individuals of nonremitted schizophrenia group and 49.8 ± 10.68 and 50.07 ± 11.18 for male and female individuals of remitted schizophrenia group, respectively.
Instruments
Nonverbal Intelligence Task (R-1)
This scale was created to allow measures of intelligence in low literacy populations. This test highly correlates with the Raven's Colored Progressive Matrices Test (r = 0.76, p = 0.001), and was chosen for the high frequency of low literacy found in the Brazilian schizophrenia population (Oliveira, 2002).
Computerized Stroop Test
The computerized Stroop Test (Capovilla et al., 2009) was used as a measure of inhibition. This test was composed of three parts of 24 stimuli each. In the first part, the subject was asked to read the word that appears in the computer display and the stimuli are names of four colors (yellow, blue, green and red) written in capital black font. The objective of this part was to evaluate the automation of reading, which was essential for the expected effect. The second part comprises 24 colored circles, six drawn in each of the four colors. Each circle was displayed for 40 ms and the participant had to name the color of the circle as fast as he/she can. The objective of this part was to provide a baseline measure for reaction time. In the third part of the test the subject had to read the color name but the stimuli were divergent; the word was displayed in a color different from the color it actually names. Each word was displayed for 40 ms and the participant had to name the color in which the word was written as fast as he/she can. In all the three parts, the reaction time and the number of correct answers were recorded using a microphone.
The performance in the Stroop Test depends on the interference of automatic processing of reading in the color identification. The scores both for reaction time and for the number of correct answers were based on the subtraction between the mean results in parts three and two.
Keep Track Task
The Keep Track Task was used to measure updating (Berberian et al., 2015). In this task, adapted from Yntema (1963), first several target categories were shown in the computer screen (animals, colors, countries, distances, metals, and relatives). Then, fifteen words were verbally presented in random order for 1500 ms apiece with the target categories remaining at the bottom of the computer screen. Each list had 2 to 3 exemplars from one of six possible categories. The task was to remember the last word presented in each of the target categories at the end of the trial. Thus, participants had to monitor the words presented and update their working memory representations for the appropriate categories when the presented word was a member of one of the target categories. The first three trials had four categories to be fulfilled, and the last three had five. The dependent measure was the proportion of words recalled correctly.
Letter Memory Task
Letter Memory Task was the second updating measure (Berberian et al., 2015). In this task, adapted from Morris and Jones (1990), several letters from a list were sequentially presented for 2000 ms per letter. The task was to recall the last two letters of each list. The instructions required the participants to rehearse out loud the last 2 letters by mentally adding the most recent letter and dropping the 3rd letter back and then saying the new string of 2 letters out loud. This instruction was given to ensure that participants were performing continuous updating. Although previous studies have used sequences ranging from 4 to 9 words, we had to adapt the task for a smaller sequence because most of the patients could not start performance with larger sequences.
Number–Letter Task
The Number–letter Task adapted from Rogers and Monsell (1995) was used to assess shifting. In this task a number–letter pair (e.g., 7G) was presented in one of four quadrants on the computer screen. In the first phase participants had to indicate whether the number was odd or even when the pair was presented in either of the top two quadrants. In the second phase, they had to indicate whether the letter was a consonant or a vowel when the pair was presented in the bottom two quadrants. Finally, in the last phase stimuli were presented in all the four quadrants, in a clockwise rotation and participants had to shift between these two types of categorization operations. The outcome variable was the mean of the total time taken to complete two first phases minus the total time taken to complete the last phase.
Plus–Minus Task
The Plus–Minus Task adapted from Jersild (1927) was used as a second measure of shifting. In this task, three lists of 30 two-digit numbers were presented in the top of the computer screen. On the first list, the participants were instructed to add 3 to each number and speak out loud their answers. On the second list, they had to subtract 3 from each number. Finally, on the third list, the participants were required to alternate between adding 3 to and subtracting 3 from the numbers (i.e., add 3 to the first number, subtract 3 from the second number, and so on). All the answers were recorded by using a microphone. The outcome variable was the mean of the total time taken to complete two first phases minus the total time taken to complete the third phase.
Tower of London Test
The Tower of London Test (TOL) was developed to identify impairments of supervening planning processes that monitor lower level structures, which are associated with frontal lobe dysfunction and are often incorporated in the executive function concept as a central cognitive component of many problem-solving activities (Krikorian et al., 1994). Although multiple strategies involving different sub-processes can be used in solving the TOL puzzle, the Tower of London is a neuropsychological instrument of choice to measure planning ability, the process of forming an effective task plan by activation of multiple goals or action constraints which sets the very general element (unity) of executive function (Duncan et al., 1997). The Tower of London, along with the similar Tower of Hanoi, was chosen mainly because it is frequently used as a measure of the integrity and complexity of executive function (Miyake et al., 2000). The Tower of London was used as a measure of general executive functioning. In Tower of London, participants were first shown three colored discs that are arranged in three pegs. The colored discs must be moved one-by-one from an initial state to matched goal states determined by the examiner. Subjects were instructed to use as few movements as possible to reach the goal and to never move more than one disc simultaneously or to place a disc out of the peg. The complete test comprises 12 items, with a rising complexity level. The dependent measure for this task was the total number of moves taken to complete the target problems.
Statistical analysis
All statistical analyses were performed with the SPSS for Windows software, version 21.0 (SPSS, Chicago, Illinois). Alpha was set at p < .05. Comparisons involving categorical variables such as gender and handedness were performed with a Chi-square test and comparisons involving age were assessed with Kruskal–Wallis non-parametric test, since age had a non-normal distribution as assessed by Shapiro–Wilk’s test.
Based on findings from previous studies (Miyake et al., 2000), tests were classified according to their corresponding construct: mental set shifting (“Shifting”, Plus–Minus and Number–Letter tests); information update and monitoring (“Updating”; Keep Track and Letter Memory tests); and inhibition of prepotent responses (“Inhibition”; Computerized Stroop). The equally weighted composite score of each construct was calculated. Comparisons involving those variables were assessed with Kruskal–Wallis non-parametric test, since they also had a non-normal distribution as assessed by Shapiro–Wilk’s test.
A multinomial logistic regression was performed to examine effects of gender, education level, intelligence, general executive measures, mental set shifting, information update and monitoring, and inhibition of prepotent responses on the likelihood that participants would belong to remitted, nonremitted or control group. In order to understand further ‘profiles’, a cross validated Exhaustive CHAID classification tree model was run using the same variables. Finally, a multiple regression was run to predict intelligence (R1percentile) and general executive functioning (performance on Tower of London) from shifting, updating, inhibition and group assignment (remitted, nonremitted and control).
Results
Groups were matched for age (Kruskal–Wallis; H(2) = 5.836; p = 0.54) and handedness (χ2(2) = 0.75; p = 0.963), but not for gender (χ2(2) = 10.274; p = 0.006), education level (χ2(8) = 89.250; p < 0.001), intelligence, general executive functioning, shifting, inhibition and updating. Please refer to Table 1 for group comparison results.
Table 1.
Mean and standard deviation of performance on R1 (IQ), Tower of London (general executive functioning), shifting, inhibition and updating for each group.
| NR-SCZ mean | R-SCZ mean | Controls mean | Three groups | NR-SCZ and R-SCZ | NR-SCZ and Controls | R-SCZ and Controls | |
|---|---|---|---|---|---|---|---|
| R1 | 22.60 ± 15.992 | 33.00 ± 27.591 | 38.91 ± 23.181 | H(2) = 15.448 p < .001⁎ |
U(112) = − 23.455 p = .214 |
U(159) = − 45.480 p < .001⁎ |
U(189) = − 22.025 p = .083 |
| TOL | 27.90 ± 3.512 | 29.31 ± 4.127 | 30.92 ± 2.970 | H(2) = 22.538 p < .001⁎ |
U(112) = − 31.029 p = .052 |
U(159) = − 55.700 p < .001⁎ |
U(189) = − 24.671 p = .041⁎ |
| “Shifting” | 1.366 ± .198 | 1.339 ± .199 | 16.333 ± 11.723 | H(2) = 162.699 p < .001⁎ |
U(112) = 6.805 p > .999 |
U(159) = − 115.091 p < .001⁎ |
U(189) = − 108.286 p < .001⁎ |
| “Inhibition” | .752 ± .432 | .614 ± .390 | .427 ± .182 | H(2) = 31.467 p < .001⁎ |
U(112) = 34.990 p = .002⁎ |
U(159) = 63.743 p < .001⁎ |
U(189) = 28.753 p = .084 |
| “Updating” | 58.059 ± 12.603 | 67.069 ± 13.476 | 73.882 ± 10.126 | H(2) = 45.142 p < .001⁎ |
U(112) = − 44.235 p = .002⁎ |
U(159) = − 79.223 p < .001⁎ |
U(189) = − 34.998 p = .002⁎ |
Nonremitted schizophrenia patients (NR-SCZ), remitted schizophrenia patients (R-SCZ) and healthy controls (controls). Results of Kruskal–Wallis and post hoc Mann–Whitney tests comparing the differences between groups for these variables.
Statistically significant (p < .05).
Between-group differences were found in all neuropsychological tasks, in which healthy control group had the highest performance means followed by the remitted group and the nonremitted group, which presented the lowest mean. Regarding pairwise comparisons, we observed differences between nonremitted and controls for intelligence; between both patients groups and controls for general executive functioning and shifting; and, finally, between nonremitted and the other two groups for inhibition and updating.
Gender differences on age, PANSS total score and duration of disease were assessed with Mann–Whitney nonparametric test. Only comparisons involving PANSS scores were significant (nonremitted group; males = 69.72 ± 11.607 vs. females = 78.67 ± 2.251; U = 359.500; p = 0.005).
The multinomial logistic regression model (χ2(16) = 325.528, p < 0.001) explained 86.7% (Nagelkerke R2) of the variance in remitted subjects and correctly classified 86.7% of cases. Three out of seven predictor variables were statistically significant: information update and monitoring, mental set shifting and gender (as shown in Table 2). Using the remitted group as a reference, we observed that decreasing performance on updating would slightly increase the odds of the individual to belong to the nonremitted group. In addition, male individuals had 7.14 more chance to belong to the nonremitted group than to the remitted group. Regarding the comparisons involving all groups, we observed that one unit increase in shifting and updating performance would increase by 164% and 16%, respectively, the chances of the individual to belong to healthy control group.
Table 2.
Summary of multinomial logistic regression analysis.
| Groupa | Variable | B | S.E. | Wald | df | Sig. | Odds |
95% C.I. for Odds Ratio |
|
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Lower | Upper | |||||||
| Nonremitted SCZ subjects | Intercept | − .358 | 1.776 | .041 | 1 | .840 | |||
| "Updating" | − .054 | .019 | 8.056 | 1 | .005⁎ | .947 | .912 | .983 | |
| "Shifting" | − .047 | .305 | .024 | 1 | .877 | .954 | .525 | 1.733 | |
| "Inhibition" | .711 | .598 | 1.411 | 1 | .235 | 2.036 | .630 | 6.576 | |
| Male | 1.966 | .609 | 10.411 | 1 | .001⁎ | 7.145 | 2.164 | 23.593 | |
| Femaleb | 0 | 0 | |||||||
| Finished Middle school or less | 2.052 | 1.186 | 2.996 | 1 | .083 | 7.784 | .762 | 79.506 | |
| High school incomplete | 1.899 | 1.228 | 2.391 | 1 | .122 | 6.680 | .602 | 74.157 | |
| Finished High School | .914 | 1.166 | .615 | 1 | .433 | 2.495 | .254 | 24.517 | |
| Undergraduate | 1.165 | 1.228 | .899 | 1 | .343 | 3.205 | .289 | 35.604 | |
| Graduateb | 0 | 0 | |||||||
| Healthy controls | Intercept | − 15.890 | 6.085 | 6.820 | 1 | .009 | |||
| "Updating" | .150 | .068 | 4.915 | 1 | .027⁎ | 1.162 | 1.018 | 1.328 | |
| "Shifting" | .973 | .268 | 13.222 | 1 | < .001⁎ | 2.646 | 1.566 | 4.471 | |
| "Inhibition" | − 3.947 | 2.575 | 2.349 | 1 | .125 | .019 | .000 | 3.005 | |
| Male | − 1.022 | 1.218 | .704 | 1 | .401 | .360 | .033 | 3.916 | |
| Femaleb | 0 | 0 | |||||||
| Finished Middle school or less | 6.068 | 3.415 | 3.157 | 1 | .076 | 431.719 | .535 | 348543.502 | |
| High school incomplete | 2.305 | 3.502 | .433 | 1 | .510 | 10.020 | .010 | 9584.593 | |
| Finished High School | 2.584 | 3.205 | .650 | 1 | .420 | 13.244 | .025 | 7087.350 | |
| Undergraduate | − 15.571 | .000 | 1 | 1.729x10− 7 | 1.729 x10− 7 | 1.729 x10− 7 | |||
| Graduateb | 0 | 0 | |||||||
Remitted SCZ subjects were used as reference for comparison with the other groups.
The reference category of the dependent variable for pairwise comparison is Remitted Schizophrenic Patients.
These are the reference categories for pairwise comparison of gender and years of education factors.
p-value below .050
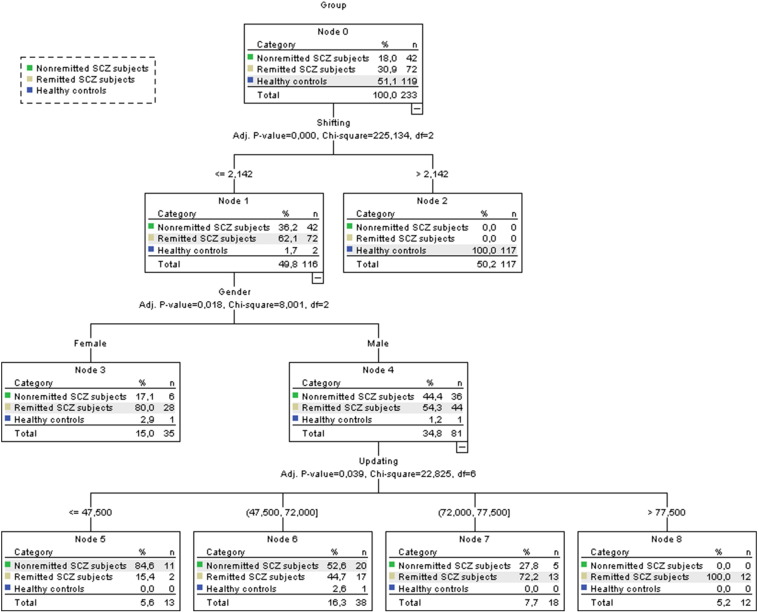
With the objective of further understanding the classification profiles of the individuals, a cross-validated Exhaustive CHAID classification tree model was run using the same variables described on the above table (Fig. 1).
Fig. 1.
Classification tree.
The first division shows that all schizophrenia subjects had scores below or equal to 2.142 on shifting, in contrast with individuals in healthy control group (χ2(2) = 225.134, p < 0.001). At the second division, an association between gender and symptom remission (χ2(2) = 8.001, p = 0.018) was observed, where 85.7% of the individuals on nonremitted group and 61.1% of the individuals on the remitted group were male. On the last level, an association between performance in updating and symptom remission (χ2(6) = 22.825, p = 0.039) was observed, where 83.3% of the individuals who scored higher than 72 on updating belonged to the remitted group.
The ranges for R1 percentile were 1 to 60 in nonremitted, 1 to 95 in remitted, and 5 to 95 in control group. A multiple regression was run to predict intelligence (R1percentile) and general executive functioning (performance on Tower of London) from shifting, updating, inhibition and group (remitted, nonremitted and healthy control groups). The assumptions of linearity, independence of errors, homoscedasticity, unusual points and normality of residuals were met. Those variables predicted intelligence and executive functioning (respectively, F(4,228) = 19.513, p < 0.001, adj R2 = 0.242; F(4,228) = 13.500, p < 0.001, adj R2 = 0.177).
Group also showed prediction of R1 score, although it did not reach the established level of significance (p < 0.05). The results are summarized in Table 3.
Table 3.
Summary of multiple regression analysis.
| Model |
Unstandardized Coefficients |
Standardized Coefficients |
t | Sig. |
95.0% Confidence Interval for B |
||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| Predicting TOL Score | |||||||
| Intercept | 23.173 | 1.423 | − 0.23 | 16.284 | < .001⁎ | 20.369 | 25.977 |
| "Shifiting" | − .008 | .025 | − .023 | − .301 | .764 | − .057 | − .042 |
| "Updating" | .087 | .020 | .312 | 4.400 | < .001⁎ | .48 | .126 |
| "Inhibition" | − .575 | .712 | − .053 | − .808 | .420 | − 1.978 | .827 |
| “Group” | .824 | .424 | .173 | 1.942 | .053 | .824 | .424 |
|
Predicting R1 Percentile |
|||||||
| Intercept | − 28.545 | 9.101 | − 3.136 | .002 | − 46.477 | − 10.612 | |
| "Shifiting" | − .204 | .161 | − .094 | − 1.268 | .206 | − .521 | .113 |
| "Updating" | 0.867 | 0.126 | 0.467 | 6.867 | < .001⁎ | .618 | 1.116 |
| "Inhibition" | 1.258 | 4.553 | .017 | .276 | .783 | − 7.713 | 10.228 |
| “Group” | 3.075 | 2.714 | .097 | 1.133 | .258 | − 2.274 | 8.424 |
p-value below .050.
Finally, analyses examining the relationship between sociodemographic and clinical variables with symptom remission showed an association between symptom remission and years of education, gender (female), occupation and schizophrenia clinical subtype. Results are presented in the Table 4.
Table 4.
Relationship between sociodemographic and clinical variables with symptom remission.
| Pearson Chi-squared | Asymp. Sig. (2-sided) | |
|---|---|---|
| Current antipsychotic in use | χ2(12) = 7.818 | p = .799 |
| Years of education | χ2(4) = 11.168 | p = .025⁎ |
| Ethnical group | χ2(4) = 2.147 | p = .709 |
| Family history of psychiatric diseases | χ2(4) = 4.489 | p = .344 |
| Gender | χ2(1) = 7.6720 | p = .006⁎ |
| Marital status | χ2(2) = 3.787 | p = .152 |
| Occupation | χ2(1) = 4.827 | p = .028⁎ |
| Religion | χ2(24) = 31.660 | p = .136 |
| Schizophrenia subtype | χ2(2) = 18.726 | p < .001⁎ |
| Socioeconomic status | χ2(6) = 8.922 | p = .178 |
| With whom the patient lives | χ2(3) = 3.924 | p = .349 |
p-value below .050.
Associations involving symptom remission and gender, schizophrenia subtype, occupation and years of education, were moderate (Cramer’s V = 0.254, p = 0.006 for gender; Cramer’s V = 0.415, p < 0.001 for schizophrenia subtype; Cramer’s V = 0.201, p = 0.028 for occupation and Cramer’s V = 0.313, p = 0.025 for years of education).
Discussion
The present study examined executive functioning in remitted and nonremitted schizophrenia subjects. In line with a priori hypothesis, schizophrenia subjects performed poorly on the general executive task (TOL) when compared to healthy controls. Remitted and nonremitted schizophrenia patients performed poorly on the tasks that tap the three specific executive dimensions likely implicated in the performance of general executive task: shifting, inhibition and updating. In addition, comparisons involving patient subgroups suggested that nonremitted subjects have impairments on tasks tapping updating and inhibition as compared with patients who attained remission.
Different studies have shown that intelligence measures are largely related to prognosis in schizophrenia. A robust body of evidence suggests that higher intelligence levels are associated with an increased likelihood of remission (Aylwand et al., 1984). Furthermore, longitudinal observational studies have suggested that the lower the performance on standardized measures of intelligence in childhood, adolescence and young adulthood, the higher is the risk for later development of schizophrenia (Maki et al., 2005). In addition, lower intelligence levels at first episode of psychosis predicted a schizophrenia diagnosis (Díaz-Caneja et al., 2015) and more severe symptoms (Leeson et al., 2011). Despite the observed association between intelligence and outcome in schizophrenia, general intellectual ability appears to remain stable after an initial decline associated with illness onset and concomitant, progressive loss of cortical matter (Rapoport et al., 2005, Goshman et al., 2005). In the present study, nonremitted, but not remitted schizophrenia patients, performed poorly on non-verbal intelligence tasks as compared to healthy controls, suggesting that higher level of intelligence is associated to remission.
Cognitive domains such as executive functioning are characteristically impaired in schizophrenia subjects irrespective of overall cognitive functioning, strengthening the argument that neurocognitive deficits are core deficits of illness (Weickert et al., 2000). Present findings indicate that, even remitted schizophrenia patients whose intelligence did not differ significantly from healthy controls performed poorly on general executive tasks. Nevertheless, no difference between remitted and nonremitted patients was found regarding those tasks. This finding suggests that the neuropsychological instrument to assess the general cognitive component of executive functioning process (TOL) used might be useful to differentiate healthy controls from chronic schizophrenia patients, but might not contribute to better understanding the nature of the executive function impairments in symptomatic remission.
Applying the framework of Miyake et al. (2000), the individual tasks (manifest variables) were chosen to tap the three specific executive dimensions (latent variables) examined in the present study, namely updating, shifting and inhibition. In general, increasing performance in all specific executive processes was associated with an increased chance of belonging to the healthy control group. The results showed that one unit increase in shifting and updating performance would increase by 164% and by 16%, respectively, the chances of belonging to the control group, suggesting that a poor performance on shifting and updating might be a good predictor of schizophrenia diagnosis. We also observed that increasing performance on updating and inhibition slightly affects the odds of belonging to remitted schizophrenia patients group, suggesting that a better performance on updating and inhibition might increase the likelihood of exhibiting symptom remission.
As mentioned earlier, only about a third of all patients reach remission (Lasser et al., 2007). The remission rates found in present study were significantly higher: of 80 males, 44 were in remission (55%) and of 34 females, 28 were in remission (82.4%). This finding might be explained at least in part by the fact that subjects were recruited from a university outpatient unit where subjects were seen in an interdisciplinary, intensive care program, which ensures medication adherence during treatment and clinical effectiveness, which improves treatment adherence (Tandon et al., 2006). All variables (outpatient population, treatment adherence and interdisciplinary, intensive care) have been associated to higher remission rates (Gasquet et al., 2008, Lambert et al., 2010, Malla et al., 2002). In line with the present findings, different studies have suggested that females, as compared to males, with schizophrenia show greater rates of symptomatic remission (Carpiniello et al., 2012, Lambert et al., 2010). However, a difference between male and female gender was found for PANSS total score in nonremitted group, suggesting a more severe expression of the illness in females. This finding contrasts with previous studies in which male gender was associated with more severe manifestations of the disease (Castle and Murray, 1991).
Analyses examining the effect of sociodemographic variables showed associations involving symptom remission and years of education and occupational adjustment. Those findings are in line with results of previous studies that have found an association between higher educational level (Geddes et al., 1994), better occupational adjustment (Spiker et al., 1987) and remission (McGlashan, 1988).
The present study has different strengths. First, sample size is considerably large as compared to most studies examining the relationship between cognitive functioning and remission in schizophrenia patients. Second, a validated model of executive function with multiple tasks providing purer measures of executive processes was applied. To our knowledge, this is the first study that systematically administered multiple executive tasks based on the theoretical model of Miyake et al. (2000) to understand the heterogeneity of executive function impairments in both remitted and nonremitted patients, compared to healthy controls. Finally, the present findings have demonstrated how specific aspects of executive function affect, or are affected by, symptomatic remission in schizophrenia patients.
Nevertheless, the present findings should be interpreted with caution, since different limitations were present. Firstly, groups were not matched for gender. Secondly, the present study design does not allow concluding about causal association involving cognitive functioning and symptomatic remission. Thirdly, the extent to which these specific aspects of executive functioning can be divided and treated as separate variables in a reliable manner should be considered. Finally, patients examined were chronic and influence of variables associated to disease course (e.g., treatment and social functioning) cannot be ruled out. However, the present findings are generally consistent with those reported by studies examining first episode subjects (Rund et al., 2007, Torgalsboen et al., 2014).
In conclusion, the present findings suggest that executive function deficits are present in chronic schizophrenic patients. In addition, specific executive processes might be associated to symptomatic remission, suggesting an important role of cognitive remediation in early interventions. Future studies examining prospectively first-episode, drug naive patients diagnosed with schizophrenia may be especially elucidative.
Role of funding source
The present study had financial support from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, grant numbers 2011/50740-5 and 2007/58630-9, Brazil).
Contributors
There are no other contributors other than the authors.
Conflict of interest
All authors declare that they have no conflicts of interest.
Acknowledgements
We acknowledge the contribution of the subjects who took part in this study, and we thank the Schizophrenia Program (PROESQ) for recruitment and clinical management of participants.
References
- Andreasen N.C., Carpenter W.T., Jr., Kane J.M., Lasser R.A., Mader S.R., Weinberger D.R. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatr. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Asor E., Ben-Shachar D. Platets: a possible glance into brain biological processes in schizophrenia. World J. Psychiatry. 2012;2(6):124–133. doi: 10.5498/wjp.v2.i6.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylwand E., Walker E., Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophr. Bull. 1984;10(3):430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- Banish M.T. Executive function: the search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18:89–94. [Google Scholar]
- Berberian A.A., Gadelha A., Dias N.M., Mecca T.P., Bressan R.A., Lacerda A.T. Investigation of cognition in schizophrenia: psychometric properties of instruments for assessing working memory updating. J. Bras. Psiquiatr. 2015;64(3):238–246. [Google Scholar]
- Braff D.L., Ryan J., Rissling A.J., Carpenter W.T. Lack of use in the literature from the last 20 years supports dropping traditional schizophrenia subtypes from DSM-5 and ICD-11. Schizophr. Bull. 2013;39(4):751–753. doi: 10.1093/schbul/sbt068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braw Y., Benozio A., Levkovitz Y. Executive functioning during full and partial remission (positive and negative symptomatic remission) of schizophrenia. Schizophr. Res. 2012;142:122–128. doi: 10.1016/j.schres.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Brissos S., Dias V.V., Balanzá-Martinez V., Carita A.I., Figueira M.L. Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr. Res. 2011;129:133–136. doi: 10.1016/j.schres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Capovilla A.G., Montiel J.M., Macedo E.C., Charin S. 2009. Teste de stroop computadorizado. Prelo. [Google Scholar]
- Carpiniello B., Pinna F., Tusconi M., Zaccheddu E., Fatteri F. Gender differences in remission and recovery of schizophrenic and schizoaffective patients: preliminary results of a prospective cohort study. Schizophr. Res. Treat. 2012 doi: 10.1155/2012/576369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle D.J., Murray R.M. The neurodevelopmental basis of sex differences in schizophrenia. Psychol. Med. 1991;21:565–575. doi: 10.1017/s0033291700022194. [DOI] [PubMed] [Google Scholar]
- Díaz-Caneja C.M., Pina-Camacho L., Rodríguez-Quiroga A., Fraguas D., Parellada M., Arango C. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr. 2015 doi: 10.1038/npjschz.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Johnson R., Swales M., Freer C. Frontal lobe deficits after head injury: unity and diversity of functions. Cogn. Neuropsychol. 1997;14(5):713–741. [Google Scholar]
- Emsley R., Chiliza B., Asmal L., Harvey B.H. The nature of relapse in schizophrenia. BMC Psychiatry. 2013;13:50. doi: 10.1186/1471-244X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly C.A., McFariane A.C., Clark C.R. Impaired updating working memory in schizophrenia. Int. J. Psychophysiol. 2006;63(3):265–274. doi: 10.1016/j.ijpsycho.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gasquet I., Haro J.M., Tcherny-Lessenot S., Chartier F., Lépine J.P. Remission in the outpatient care of schizophrenia: 3-year results from the Schizophrenia Outpatients Health Outcomes (SOHO) study in France. Eur. Psychiatry. 2008;23(7):491–496. doi: 10.1016/j.eurpsy.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Geddes J., Mercer G., Frith C.D., MacMillan F., Owens D.G., Johnstone E.C. Prediction of outcome following a first episode of schizophrenia. A follow-up study of Northwick Park first episode subjects. Br. J. Psychiatry. 1994;165(5):664–668. doi: 10.1192/bjp.165.5.664. [DOI] [PubMed] [Google Scholar]
- Goshman P.A., Greenstein D., Sporn A., Gogtay N., Keller B., Shaw P., Rapoport J.L. IQ stabilization in childhood-onset schizophrenia. Schizophr. Res. 2005;77(2-3):271–277. doi: 10.1016/j.schres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Green M.F., Harvey P.D. Cognition in schizophrenia: past, present and future. Schizophr. Res. Cogn. 2014;1:e1–e9. doi: 10.1016/j.scog.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Nuechterlein K.H. The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophr. Res. 2004;72(1):1–3. doi: 10.1016/j.schres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mint J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Helldin L., Kane J.M., Karilampi U., Norlander T., Archer T. Remission and cognitive ability in a cohort of patients with schizophrenia. J. Psychiatr. Res. 2006;40(8):738–745. doi: 10.1016/j.jpsychires.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Helldin L., Kane J.M., Karilampi U., Norlander T., Archer T. Remission in prognosis of functional outcome: a new dimension in the treatment of patients with psychotic disorders. Schizophr. Res. 2007;93(1-3):160–168. doi: 10.1016/j.schres.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Hofer A., Bodner T., Kaufmann A., Kemmler G., Mattarei U., Pfaffenberger N.M., Rettembacher M.A., Trebo E., Yalcin N., Fleischhacker W.W. Symptomatic remission and neurocognitive deficits in patients with schizophrenia. Psychol. Med. 2011;41(10):2131–2139. doi: 10.1017/S0033291711000353. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Epidemiology of schizophrenia: the global burden of disease and disability. Eur. Arch. Psychiatry Clin. Neurosci. 2000;250(6):274–285. doi: 10.1007/s004060070002. [DOI] [PubMed] [Google Scholar]
- Jersild A.T. Mental set and shift. Arch. Psychol. 1927;89:5–82. [Google Scholar]
- Jurado M.B., Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kane J.M., Correll C.U. Past and present progress in the pharmacologic treatment of schizophrenia. J. Clin. Psychiatry. 2010;71(9):1115–1124. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keef R.S.E. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry. 2008;7(1):22–28. doi: 10.1002/j.2051-5545.2008.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Nuechterlein K.H., Braver T.S., Barch D.M. Executive functioning component mechanisms and schizophrenia. Biol. Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kopelowics A., Liberman R.P., Ventura J., Zarate R., Mintz J. Neurocognitive correlates of recovery from schizophrenia. Psychol. Med. 2005;35(8):1165–1173. doi: 10.1017/s0033291705004575. [DOI] [PubMed] [Google Scholar]
- Krikorian R., Bartok J., Gay N. Tower of London procedure: a standard method and developmental data. J. Clin. Exp. Neuropsychol. 1994;16(6):840–850. doi: 10.1080/01688639408402697. [DOI] [PubMed] [Google Scholar]
- Lambert M., Karow A., Leucht S., Schimmelmann B.G., Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients perspective 5 years later. Dialogues Clin. Neurosci. 2010;13(3):393–407. doi: 10.31887/DCNS.2010.12.3/mlambert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser R.A., Nasrallah H., Helldin L., Peuskens J., Kane J., Docherty J., Tronco A.T. Remission in schizophrenia: applying recent consensus criteria to refine the concept. Schizophr. Res. 2007;96(1-3):223–231. doi: 10.1016/j.schres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Leeson V.C., Sharma P., Harrison M., Ron M.A., Barnes T.R.E., Joyce E.M. IQ trajetory, cognitive reserve, and clinical outcome following a first episode of psychosis: a 3-year longitudinal study. Schizophr. Bull. 2011;37(4):768–777. doi: 10.1093/schbul/sbp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. Should Cognitive deficit be a diagnostic criterion for schizophrenia? J. Psychiatry Neurosci. 2004;29(2):102–113. [PMC free article] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W. Oxford University Press; New York: 2004. Neuropsychological Assessment. [Google Scholar]
- Maki P., Veijola J., Jones P.B., Murray G.K., Koponen H., Tienari P., Miettunen J., Transkanen P., Wahlberg K.E., Koskinen J., Lauronen E., Isohanni M. Predictors of schizophrenia — a review. Br. Med. Bull. 2005;73–74(01):1–15. doi: 10.1093/bmb/ldh046. [DOI] [PubMed] [Google Scholar]
- Malla A.K., Norman R.M., Manchanda R., McLean T.S., Harricharan R., Cortese L., Townsend L.A., Scholten D.J. Status of patients with first-episode psychosis after one year of phase-specific community-oriented treatment. Psychiatr. Serv. 2002;53(4):458–463. doi: 10.1176/appi.ps.53.4.458. [DOI] [PubMed] [Google Scholar]
- McGlashan T.H. A selective review of recent North American long-term followup studies of schizophrenia. Schizophr. Bull. 1988;14(4):515–542. doi: 10.1093/schbul/14.4.515. [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morris N., Jones D.M. Memory updating in working memory: the role of the central executive. Br. J. Psychol. 1990;81:111–121. [Google Scholar]
- Oliveira R. Vetor Editora Psico-Pedagógica; São Paulo: 2002. Teste não verbal de inteligência. [Google Scholar]
- Palmer B.W., Dawes S.E., Heaton R.K. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol. Rev. 2009;19(3):365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffard S., Bayard S. Understanting the executive functioning heterogeneity in schizophrenia. Brain Cogn. 2012;79(1):60–69. doi: 10.1016/j.bandc.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Rapoport J.L., Addington A.M., Frangou S., Psych M.R.C. The neurodevelopmental model of schizophrenia. Mol. Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rogers R.D., Monsell S. Costs of a predictable switch between simple cognitive tasks. J. Exp. Psychol. Gen. 1995;124:207–231. [Google Scholar]
- Rund B.R., Melle I., Frils S., Johannessen J.O., Larsen T.K., Midboe L.J., Opjordsmoen S., Simonsen E., Vaglum P., McGlashan T. The course of neurocognitive functioning in first-episode psychosis and its relation to premorbid adjustment, duration of untreated psychosis, and relapse. Schizophr. Res. 2007;91(1-3):132–140. doi: 10.1016/j.schres.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Spiker H.T., Townsend B., Unterman D., Voisinet S., Welp R. Sustained remission in drug-free schizophrenic patients. Am. J. Psychiatry. 1987;144:1306–1309. doi: 10.1176/ajp.144.10.1306. [DOI] [PubMed] [Google Scholar]
- Tandon R., Targum S.D., Nasrallah H.A., Ross R. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. J. Psychiatr. Pract. 2006;12(6):348–363. doi: 10.1097/00131746-200611000-00003. [DOI] [PubMed] [Google Scholar]
- Tandon R., Keshavan M.S., Nasrallah H.A. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008;102(1–3):1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Teuber H.L. Unity and diversity of frontal lobe functions. Acta Neurobiol. Exp. (Wars.) 1972;32(2):615–656. [PubMed] [Google Scholar]
- Torgalsboen A.K., Mohn C., Rund B. Neurocognitive predictors of remission of symptoms and social and role functioning in the early course of first-episode schizophrenia. Psychiatry Res. 2014;216(1):1–5. doi: 10.1016/j.psychres.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Weickert T.W., Goldberg T.E., Gold J.M., Bigelow L.B., Egan M.F., Daniel R., Weinberger D.R. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch. Gen. Psychiatry. 2000;57(9):907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Wilson B.A., Alderman N., Burgess P.W., Emslie H., Evans J.J. Behavioural Assessment of Dysexecutive Syndrome (BADS) JOPED. 2003;5(2):33–37. [Google Scholar]
- Wood S.J., Allen N.B., Pantelis C. Cambridge University Press; New York: 2009. The Neuropsychology of Mental Illness. [Google Scholar]
- Yntema D.B. Keeping track of several things at once. Hum. Factors. 1963;5:7–17. doi: 10.1177/001872086300500102. [DOI] [PubMed] [Google Scholar]