Abstract
Background: Highly active antiretroviral therapy (HAART) is complex and many factors contribute to a patient’s response to initial therapy including adherence, drug effectiveness, and tolerance. Close HAART follow-up is needed, particularly when there are concurrent therapies such as prophylactic antibiotics and medications for the treatment of comorbidities. Objective: To assess the effectiveness of pharmacist intervention in reducing drug related problems in HIV/AIDS outpatients (intervention group) and in improving clinical parameters in the intervention group compared to the control group. Methods: We conducted a prospective controlled intervention study with patients paired by gender and initial T CD4+ lymphocyte (CD4) count. HIV-infected patients of a public outpatient service were enrolled for the study by consecutive and convenience sampling. Patients selected for the study were divided into a control group and an intervention group. Both groups were followed for one year; however, only the intervention group received pharmaceutical care. The primary outcome was the drug related problem (DRP) analysis for the intervention group. Secondary outcomes were CD4 count and viral load evaluation for both groups. Results: There was a total of 143 patients enrolled in this study, with 53 (37.06%) patients in the control group and 90 (62.94%) patients in the intervention group. A total of 202 pharmacist interventions with 193 pharmacist-patient and 9 pharmacist-physician interventions were proposed. After one year of pharmaceutical care, a reduction of 38.43% between the initial and final DRP was found (p = 0.0001). The most common DRPs found were related to medication safety. The intervention group showed a mean increase of 84% for the CD4 count in comparison with that observed in the control group. The viral load was not significantly different between the final and initial mean values for both groups. Conclusion: Pharmacist appointments enabled identification, prevention, and solving of drug related problems, especially those related to drug safety. Also, pharmacist interventions improved adherence and increased HAART effectiveness as suggested by the higher elevation in the CD4 count seen in the intervention group in comparison with the control group.
Keywords: HIV/AIDS, Brazil, CD4 lymphocyte count, Highly active antiretroviral therapy, Medication adherence, Pharmaceutical care
1. Introduction
In Brazil, from 1980 to June 2014, 757,042 cases of Acquired Immunodeficiency Syndrome (AIDS) were reported, of which 411,800 (54.4%) of the cases were reported in the southeast of Brazil (Brazil. Ministry of Health., 2014). Among those, 15,768 were new AIDS cases in 2014, with a national incidence rate of 20.4/100,000 individuals with AIDS reported in 2013 (Brazil. Ministry of Health., 2014). Although Brazil provides free therapy to treat against Human Immunodeficiency Virus (HIV), access to pharmaceutical care for people living with HIV/AIDS is a major challenge for public health systems, especially in developing countries (Oliveira et al., 2002, Remondi et al., 2014).
The goal of highly active antiretroviral therapy (HAART) is to achieve maximum suppression of HIV replication, resulting in an increased immune response. However, HAART is not effective unless patients are committed and able to adhere to the treatment regimen. In addition, health care professionals must be able to manage the therapy. Non-adherence or partial adherence to HAART can lead to virologic failure, development of viral resistance to antiretroviral drugs, and death (Bezabhe et al., 2016).
HAART is complex and many factors contribute to a patient’s response to initial therapy including adherence, drug effectiveness, and tolerance (Bolsewicz et al., 2015, Henderson et al., 2011, Verdugo et al., 2007). Furthermore, drug related problems (DRPs) are frequently found in HIV/AIDS patients. DPRs mainly occur due to drug-drug and drug-food interactions, adverse drug reactions, drug omission or unnecessary prescribed drug, no dosage adjustment in patients with renal or hepatic impairment, errors in drug indication, dosing, regimen and scheduling (Carcelero et al., 2011, Li and Foisy, 2014, Molino et al., 2014, Ojeh et al., 2015).
Drug interaction is commonly found between HAART, HAART and other drugs used to treat comorbidities, and HAART and food (Hughes et al., 2015, Ojeh et al., 2015, Rathbun and Liedtke, 2011). Antiretroviral drugs that function as protease inhibitors are extensively metabolized by the cytochrome P450. The concomitant use of these drugs and others also metabolized by CYP P450 can result in drug interaction (Hughes et al., 2015, Rathbun and Liedtke, 2011). Drugs with narrow therapeutic index and metabolized by CYP450, such as warfarin, require close monitoring when used with HAART (Esterly et al., 2013).
Besides some of the well-known mechanisms of drug interactions causing side effects, medications often present adverse effects leading to treatment discontinuation (Hughes et al., 2015, Rathbun and Liedtke, 2011, Verdugo et al., 2007, Verdugo et al., 2010). A multi-center study conducted in Spain found that a quarter of HIV patients had discontinued tenofovir treatment due to adverse drug effects (Verdugo et al., 2007). Also, the risk of virologic failure commonly associated with antiretroviral resistance increases when compliance decreases (Bezabhe et al., 2016, Pirkle et al., 2009). Close HAART follow-up is needed, particularly when there are concurrent therapies such as prophylactic antibiotics and medications for the treatment of comorbidities. Studies have shown a significant decrease in medication errors in institutions where pharmacists perform interventions with a team of clinicians. These studies support the fact that pharmacist intervention can reduce the number of adverse events, improve quality of health care, and minimize hospital costs (Carnevale et al., 2015, Chisholm-Burns et al., 2010, De Rijdt et al., 2008, Kaboli et al., 2006, Kopp et al., 2007, Stark et al., 2011).
1.1. Aim of the study
Pharmaceutical care encourages patients to be familiar with their own regimens, making it easier to understand the importance of drug adherence, as well as increasing therapy adherence, effectiveness, and tolerance. The aim of this study was to evaluate the effectiveness of pharmacist intervention in reducing drug related problems in the HIV/AIDS outpatients (intervention group) and in improving clinical parameters in the intervention group compared to the control group.
2. Methods
2.1. Design and setting
This study was performed at the Hospital Dia-University of Campinas Teaching Hospital, Campinas, Brazil, from January 2009 to June 2012.
We conducted a prospective controlled intervention study with patients paired by gender and initial T CD4+ lymphocyte (CD4) count. First, the clinical pharmacy staff analyzed all medical charts available during medical appointments in Hospital Dia to check whether patients fit the study criteria. Then, those who fit these criteria were assigned to Control/Intervention Group through a convenience sample. Both groups were followed for one year; however, only the intervention group received pharmaceutical care.
Each patient from the control group was matched with a patient from the intervention group according to their baseline CD4 count, considering a maximum variation range of 10% between their CD4 counts. Since the control group consisted of replaced patients, we could pair each intervention patient with a control patient and compare the outcomes.
Laboratory specimens were collected in the morning and subsequent analysis was carried out by a laboratory at the University of Campinas Teaching Hospital.
2.2. Subjects
HIV/AIDS outpatients from the Hospital Dia were eligible to participate in our study if they were between 18 and 65 years of age, with a body mass index of <30 kg/m2 and were undergoing HAART. Elderly patients (>65 years old) were excluded since it is known that elderly individuals present with more comorbidities; thus, their pharmacotherapy is more complex and associated with a greater number of drug interactions, adverse reactions, and toxicity (Hasse et al., 2011). Furthermore, previous data have shown that higher HIV infection progression and mortality rates have been identified among older patients compared with younger patients (Nguyen and Holodniy, 2008). Obese patients were also excluded since they present with higher incidences of hyperlipidemia and the fact that some HIV medications, such as protease inhibitors, are associated with weight gain and fat accumulation (Bavinger et al., 2013, Crum-Cianflone et al., 2010, Dube, 2000, Friis-Møller et al., 2003, Hasse et al., 2011).
Patients were excluded if they were homeless or prisoners, had severe psychiatric disease that limited their comprehension, did not follow up on previously scheduled appointments, refused to participate in the study, got pregnant, attended fewer than two pharmacist appointments, had pharmacist appointments at an interval greater than 6 months, did not complete one year of pharmaceutical care, were switched to another health service, or missed their appointments with their physician.
2.3. Pharmaceutical care service
A clinical pharmacy team analyzed patients’ medical charts to ensure they fit with the study criteria. Those who were eligible were assigned to an intervention or control group depending on the clinical pharmacist’s appointment schedule.
After undergoing their routine physician appointments, patients in the intervention group received pharmaceutical care appointments during the one-year follow-up. Control group patients continued with their usual care without pharmaceutical care service.
Patients in the intervention group had at least three pharmaceutical care appointments of 30–60 min each. The maximum interval between returns proposed to all patients from the Hospital Dia was four months.
The pharmaceutical care service provided for the intervention group was based on the pharmacotherapy workup. The definition of the pharmacotherapy workup employed was: a process in which pharmacists use rational decision-making to assess a patient’s drug-related needs, identify drug therapy problems, develop a care plan, conduct follow-up evaluations, and avoid safety and efficacy problems (Cipolle et al., 2004).
Pharmaceutical care activities comprised data collection through patient interview and medical history. The clinical pharmacist team used a standard form to collect data and guide appointments. The team consisted of two pharmacists trained in pharmaceutical care and four undergraduate pharmacist students.
The primary outcome was the drug related problem (DRP) analysis for the intervention group, considering all drugs in use by the patient: HAART, over the counter, and others to treat comorbidities. DRP analysis was not performed for the control group because these patients did not receive pharmaceutical care. Two pharmacists identified and classified individually the DRPs into four categories: (1) Indication: unnecessary drug therapy or additional drug therapy was required to treat or prevent a medical condition; (2) Efficacy: a drug was ineffective or the dosage was too low to produce the desired response; (3) Safety: the drug caused an adverse reaction or the dosage was too high which resulted in undesirable effects; (4) Adherence: the patient was not able or willing to take the drug regimen appropriately (Cipolle et al., 2004). Laboratory tests and patient’s symptoms were used to identify and monitor DRPs. Micromedex was used to check theoretical adverse drug reaction. Adverse drug reaction was considered when a patient reported a symptom or when there was an abnormality in laboratory tests which could not be explained by anything else other than adverse drug reaction.
Secondary outcomes were CD4 count and viral load evaluation for both groups. To analyze the influence of pharmaceutical care service on these parameters, we considered their results before and after one year of pharmaceutical care service in the intervention group. In the control group, initial CD4 count and viral load were compared to the final values.
After the clinical pharmacists identified the DRPs, they developed a care plan that comprised measures to be taken by the patient and the medical team. The care planning was checked by two pharmacists before initiation of the intervention. The pharmacist interventions were performed both orally and with standardized written form methods. Interventions were classified into 2 major categories: pharmacist-physician interventions or pharmacist-patient interventions. Then, interventions were divided into 4 categories: resolve DRP, prevent DRP, health promotion or quality of life. “Resolve DRP”: when there was a real problem, such as lack of adherence or presence of adverse drug reactions; “prevent DRP”: when there was a potential problem, such as a potential drug interaction; “health promotion”: patient education related to hygiene habits, disease, laboratory tests, drug storage orientation and condom use; “quality of life”: interventions related to lifestyle (proper eating and sedentarism), drug use (illicit drugs, tobacco and excessive intake of alcohol), and specialist referral, when necessary.
The clinical pharmacists were trained to properly classify the DRP and intervention categories. Once a week the clinical pharmacist team discussed all patient cases and solved issues regarding the DRP intervention classification. If no consensus was reached, a third pharmacist was consulted.
Antiretroviral drugs were provided to the patients by a specialized community pharmacy, also located at the University of Campinas.
2.4. Statistical analysis
Primary and secondary outcomes were analyzed using a paired t-test to compare the baseline values to the results found after the pharmacist interventions, considering a two-sided p < 0.05 as statistically significant. Graphics were made using Origin 6.0® and Microsoft Office Excel® software.
2.5. Ethical approval
This study was approved by the Research Ethics Committee at the School of Medical Sciences, University of Campinas, Brazil, (protocol number 727/2009). Each patient signed a consent form.
3. Results
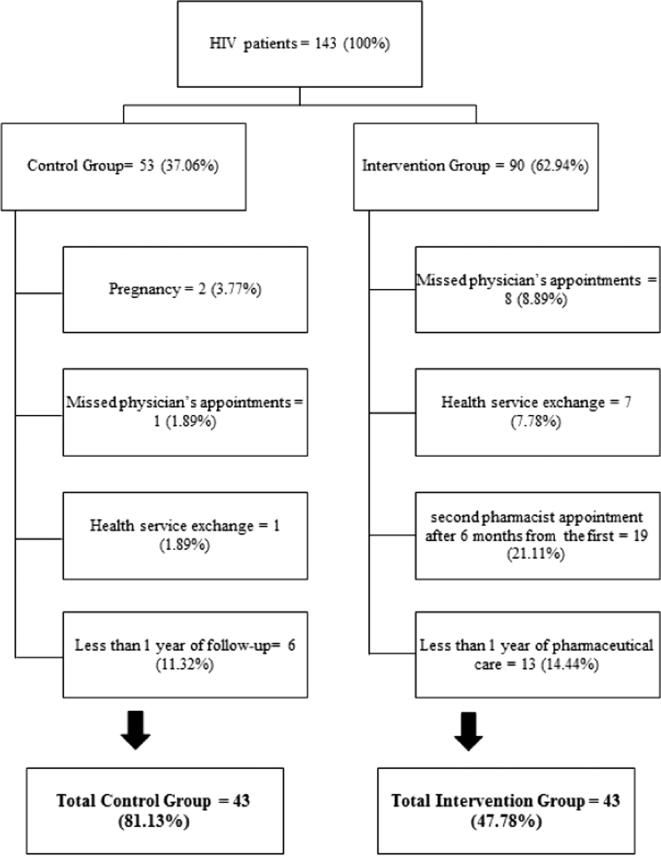
There was a total of 143 patients enrolled in this study, with 53 (37.06%) patients in the control group and 90 (62.94%) patients in the intervention group. Among them, 57 (39.86%) were excluded with 10 (17.54%) of the patients excluded from the control group and 47 (82.46%) from the intervention group. At the end, 43 patients were eligible in every arm (Fig. 1). Both groups were followed for 12 months.
Figure 1.
Total initially selected for the study and the reasons for discontinuing patients.
Each patient’s baseline characteristics are shown in Table 1. Since the patients were paired according to their gender and CD4 count, we found equal numbers of men (n = 28, 65.12%) and women (n = 15, 34.88%) between the groups and similar mean basal CD4 counts, where the control group presented with 291.09 [210.34–371.84] cells/mm3 and the intervention group presented with 297.72 [208.30–387.14] cells/mm3. The groups were similar in regard to their age, ethnicity, and time of HIV diagnosis and treatment. Most prescribed HAART was tenofovir, lamivudine and efavirenz in the control group (20%), and zidovudine, lamivudine and efavirenz in the intervention group (20%). Hepatitis C was the most frequent comorbidity in both groups with a 20% (n = 8.6) occurrence in each group.
Table 1.
Baseline characteristics of the control and intervention group.
| Characteristics | Control groupa | Intervention group |
|---|---|---|
| Age (Mean ± SD; years) | 41.49 ± 9.33 | 41.74 ± 7.93 |
| Gender | ||
| Men n (%) | 28 (65.12) | 28 (65.12) |
| Women n (%) | 15 (34.88%) | 15 (34.88%) |
| Ethnicity n (%) | ||
| Caucasian | 31 (72.09) | 26 (60.47) |
| Mulatto | 10 (23.26) | 13 (30.23) |
| Asian | – | 1 (2.33) |
| Black | 2 (4.65) | 3 (6.98) |
| HIV diagnosis (Mean [CI]; years) | 7.05 [5.32–8.78] | 8.02 [6.06–9.98] |
| HIV treatment duration (Mean [CI]; years) | 5.98 [4.41–7.55] | 6.35 [4.70–7.99] |
| Number of comorbidities (Mean ± SD) | 2.70 ± 1.70 | 2.80 ± 1.90 |
| Number of tablets per day (Mean ± SD) | 9.55 ± 4.70 | 10.25 ± 4.21 |
| Initial CD4 mean count ([CI]; cells/mm3) | 291.09 [210.34–371.84] | 297.72 [208.30–387.14] |
| Initial viral load mean count ([CI]; copies/ml) | 19 × 103 [50–42 × 103] | 40 × 103 [6 × 103–75 × 103] |
| Most prescribed HAART n (%)b | ||
| AZT + 3TC + EFV | 7 (17.50) | 8 (20.00) |
| AZT + 3TC + LPV/r + TDF | 2 (5.00) | 7 (17.50) |
| TDF + 3TC + EFV | 8 (20.00) | 6 (15.00) |
| TDF + 3TC + LPV/r | 5 (12.50) | 6 (15.00) |
| AZT + 3TC + LPV/r | 4 (10,00) | 4 (10.00) |
| ABC + 3TC + EFV | 0 | 3 (7.50) |
| Other | 14 (35.00) | 6 (15.00) |
Data from three patients in the control group were not considered to HIV diagnosis, treatment time, tablets per day and comorbidities (n = 40).
Most prescribed HAART was related to n = 40 in control and intervention group. 3TC: lamivudine. ABC: abacavir. AZT: zidovudine. EFV: efavirenz. LPV/r: lopinavir/ritonavir. TDF: tenofovir.
The clinical pharmacists team performed 194 appointments in the intervention group (mean of 4.51 [4.08–4.94] appointments per patient). A total of 202 pharmacist interventions with 193 (95.54%) pharmacist-patient and 9 (4.46%) pharmacist-physician interventions were proposed. A mean of 4.7 pharmacist interventions per patient and 1.04 pharmacist interventions per appointment was performed.
All pharmacist-physician interventions were accepted and aimed to resolve an identified DRP such as an additional drug therapy required (5/9, 55.56%), unnecessary drug therapy (2/9, 22.22%), and different drug required (2/9, 22.22%).
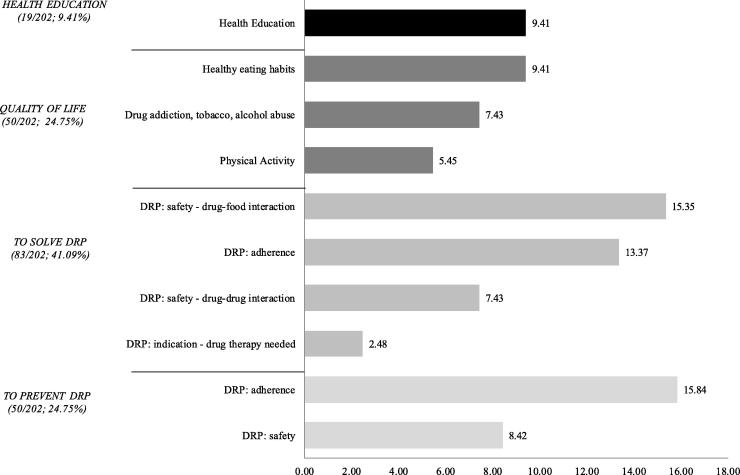
Pharmacist-patient interventions consisted of health education (19/193, 9.84%) and quality of life (50/193, 25.91%), to prevent (50/193, 25.91%) and resolve DRP (74/193, 38.34%). Pharmacist-patient interventions to prevent DRP were mostly related to a non-adherence problem (32/50, 64%). Most pharmacist-patient interventions aimed to resolve DRP adjusted dosing schedules in accordance with patient’s meal patterns (31/74, 41.89%). Fig. 2 shows how often pharmacist interventions were performed in regard to health education, quality of life, resolution, and prevention of DRPs.
Figure 2.
Percentage of pharmacist interventions commonly performed during the study. DRPs, Drug-related problems.
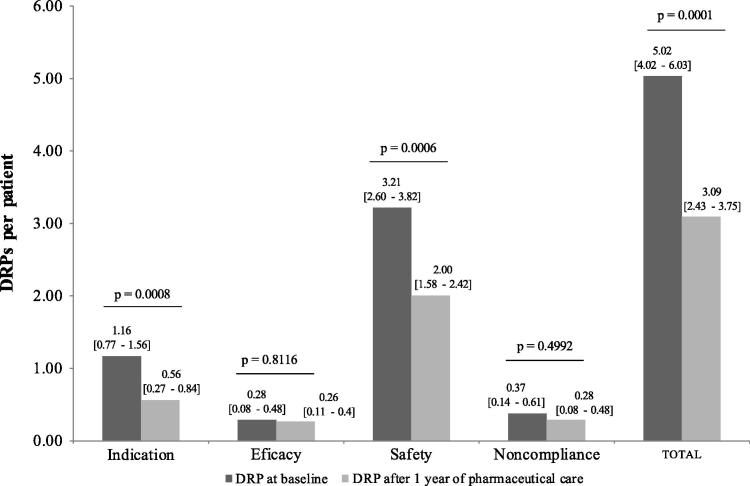
Fig. 3 shows DRPs from the first pharmacist appointment and after one year. Initially, 216 DRPs were identified with 5.02 [4.02–6.03] DRPs per patient. After one year of pharmaceutical care, 133 DRPs were found with 3.09 [2.43–3.75] DRPs per patient. A reduction of 38.43% between the initial and final DRPs was found and was statistically significant at p = 0.0001.
Figure 3.
Mean drug related problems per patient identified in first appointment and after 1 year of pharmaceutical care. DRPs, Drug-related problems.
The most common DRPs found were related to medication safety with 138 (3.21 [2.60–3.82] DRPs per patient) found initially and 86 (2.00 [1.58–2.42] DRP per patient) one year later. Indication DRP was the second most frequent with 50 (1.16 [0.77–1.56] DRP per patient) and 24 (0.56 [0.27–0.84] DRP per patient) initially and after one year, respectively (Fig. 3).
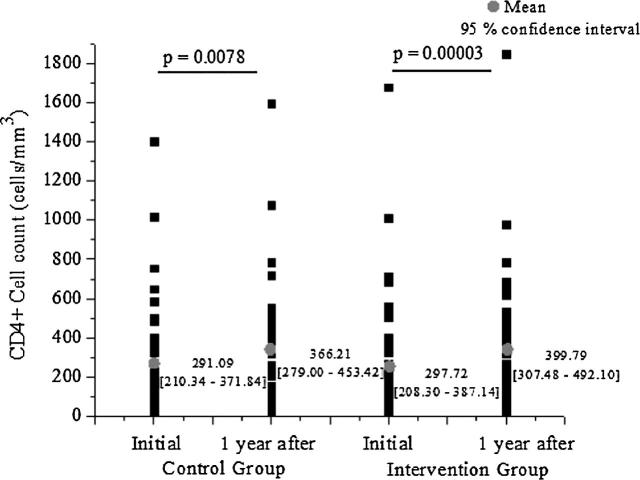
Fig. 4 shows CD4 count dispersion for both groups. The CD4 count mean presented with a significant increase in both the intervention (p = 0.00003) and the control group (p = 0.0078). However, the intervention group showed a mean increase of 84% for the CD4 count in comparison with that observed in the control group; the CD4 mean variation found was 154.66 and 83.80 in the intervention and control group, respectively (p = 0.4).
Figure 4.
CD4 count dispersion initially and after 1 year for intervention and control group.
At baseline, both groups had a statistically similar viral load (p = 0.10). The viral load was not significantly different between the final and initial mean values for both groups (p = 0.5 for the control group and p = 0.2 for the intervention group). Although the final control group viral load mean was lower than that presented by the intervention group (12 × 103 and 17 × 103 copies/mL, respectively), an approximate 4-fold viral load reduction was found in the intervention group when compared to the control group (23 × 103 for the intervention group and 6 × 103 for the control group).
Regarding the viral load limit of detection (viral load <50 copies/mL), 51.16% (n = 22) of individuals in the control group and 60.47% (n = 26) in the intervention group began and ended the study with a viral load <50. In addition, 9.3% (n = 4) of the patients in the control group and 2.33% (n = 1) of patients in the intervention group began the study with a viral load <50 and after one year they presented with a viral load >50. Finally, 3 (6.98%) patients in the intervention group presented with a clinically significant increase in their viral load compared with 11 (25.58%) patients in the control group.
4. Discussion
According to Brazilian epidemiological data, the prevalence of people living with HIV/AIDS in Brazil is 0.4% (Brazil. Ministry of Health., 2014). Of all cases from 1980 to June 2014, the highest concentration of those affected is among individuals aged 25–39, which comprises 54% and 50.3% of men and women, respectively (Brazil. Ministry of Health., 2014). Similarly, we found an age average of 41.63 [39.29–43.96] and 41.60 [38.41–44.79] years for all men and women enrolled in this study.
Viktil and Blix (2008) showed that a clinical pharmacist is able to identify, resolve, and prevent a clinically significant DRP (Viktil and Blix, 2008). Furthermore, they found evidence that pharmacist interventions positively influence clinical outcomes, such as improvement of drug and disease surrogate markers, reduction of hospitalization length of stay and re-admissions, and rates of disease events (Viktil and Blix, 2008). Likewise, studies have shown that clinical outcomes in HIV patients, as viral load reduction and CD4 increase, can be improved by pharmacist interventions, (Abah et al., 2014, Nevo et al., 2015, Reis et al., 2016, Saberi et al., 2012).
During the one-year of pharmaceutical care, most interventions (95.54%) were pharmacist-patient interventions, which resulted in a significant DRP reduction of 38.43% after one year of pharmaceutical care. Other studies had shown that pharmacist interventions are able to reduce DRPs, especially problems related to medication safety, such as the presence of adverse drug reactions for example (Blix et al., 2006, Chisholm-Burns et al., 2010, de Maat et al., 2004, Granas et al., 2010, Verdugo et al., 2010, Viktil and Blix, 2008). In our study, 46 interventions intended to solve an adverse drug reaction including a DRP drug-food interaction (n = 31) and/or drug-drug (n = 15) interaction. After those interventions, problems in regard to safety decreased from 138 to 86, a reduction of 37.68%, indicating that the pharmacist interventions influenced the reduction of adverse reactions.
A previous study showed that 50–80% of all DRPs are predictable (Viktil and Blix, 2008) and thus pharmacist interventions might resolve and prevent DRPs leading to a significant impact in DRP reduction; however, they were not enough to address all problems identified. Regarding other DRPs (indication, effectiveness, and adherence) no expressive reduction was observed as that presented for safety DRPs. These aspects might happen due to few pharmacist-physician interventions conducted (n = 9; 4.46%). Health team interventions were proposed to reduce indication and effectiveness DRPs, though these are the most complex interventions to be implemented for the pharmacy staff because many patients do not want a new drug added to their therapy regimen since they already take a large amount of tablets daily.
Although it is well known that a clinical pharmacist must be present at prescription time and actively participate in clinical case discussions in regard to the pharmacist-physician interventions (Blix et al., 2006, Kucukarslan et al., 2003, Viktil and Blix, 2008), our interventions were performed by medical record entries and a verbal approach. Nonetheless, our study had 100% of the pharmacist-physician interventions accepted suggesting that this combined method could also be effective and should be explored in the future.
As our data show, pharmacists should intercede with clinician teams in cases of HAART intolerance and with patients to instruct them on how to adequately take antiretroviral drugs, encouraging therapy adherence. Knowing that barriers to treatment adherence are complex and diverse but could also be related to patients’ cultural attitudes and beliefs (Bolsewicz et al., 2015) and it increases when patients have a greater understanding about their own health (Blix et al., 2004, de Lyra et al., 2007, Oliveira et al., 2002, de Oliveira, 2011, Strand et al., 2004), we performed interventions to enhance patient knowledge in relation to HIV/AIDS, HAART, and laboratory tests.
In the present study, we observed a significant increase in CD4 counts for both groups. However, the intervention group presented with a mean CD4 increase of 154.66 cells/mm3 while the control group had an increase of 83.8 cells/mm3 (p = 0.401) by the end of one year. Additionally, after one year of follow-up there were 9 (20.93%) patients in the intervention group and 6 (13.95%) in the control group with CD4 counts >500 cells/mm3 and an undetectable viral load. These data demonstrate the clinical relevance of the pharmacist interventions in the intervention group.
The viral load parameter did not demonstrate a statistical difference; however, the viral load reduction was greater in the intervention group, resulting in a mean reduction of 23.52 × 103 RNA copies/mL, while in the control group there was 6.23 × 103 RNA copies/mL. Viral load quantification is an important marker of HAART adherence (Bonner et al., 2013). Previous studies had shown that the pharmacist, through pharmaceutical care and interventions, could improve adherence which would help with reducing the viral load (Henderson et al., 2011, Nevo et al., 2015, Reis et al., 2016, Saberi et al., 2012).
4.1. Strength and limitations
This study had some limitations. For instance, the pharmacist appointments had to follow the physician’s schedule, and therefore some data could not be analyzed due to a lack of information in the medical charts, such as laboratory tests, medications dose, and over-the-counter DRPs. Thus, certain cases were not considered for analyses such as DRPs. Furthermore, due to the lack of information we could not find out the control DRP rate. Although there was no randomization due to the healthcare facility routine, pharmacists selected any patients that accepted to participate in this study. After that, the pharmacist checked whether the patient satisfied the inclusion/exclusion criteria. Thus, future studies should assess whether pharmacist interventions would have an influence on adverse drug reaction outcomes and confirm and quantify them by the use of specific laboratory tests.
5. Conclusion
The results of this study indicate that pharmacist interventions positively influenced the patients’ clinical outcomes. Pharmacist appointments enabled identification, prevention, and solving of drug related problems, especially those related to drug safety. Lastly, pharmacist interventions improved adherence and increased HAART effectiveness as suggested by the higher elevation in the CD4 count and the viral load reduction seen in the intervention group in comparison with the control group.
We suggest governments and healthcare managers should encourage the use of pharmaceutical care as a public health service, particularly by HIV patient assistance programs.
Funding
Coordination for the Improvement of Higher Level Personnel (CAPES) and State of São Paulo Research Foundation (FAPESP).
Acknowledgments
We gratefully acknowledge the following pharmacist graduate students: Cristiane Zanin, Luana da Silva Baleeiro, Natalia Cavalheiro Braz, and Valéria de Souza Santos Holsback. We would like to thank the Hospital Dia staff for their support, collaboration and care dedicated during this research. We are also grateful for the patients for participating in this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abah I.O., Ojeh V.B., Falang K.D., Darin K.M., Olaitan O.O., Agbaji O.O. Pharmaceutical care outcomes in an outpatient human immunodeficiency virus treatment center in Jos, Nigeria. J. Basic Clin. Pharm. 2014;5:57–61. doi: 10.4103/0976-0105.139727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavinger C., Bendavid E., Niehaus K., Olshen R.A., Olkin I., Sundaram V., Wein N., Holodniy M., Hou N., Owens D.K., Desai M. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS ONE. 2013;8:e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezabhe W.M., Chalmers L., Bereznicki L.R., Peterson G.M. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine (Baltimore) 2016;95:e3361. doi: 10.1097/MD.0000000000003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix H.S., Viktil K.K., Moger T.A., Reikvam A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm. World Sci. 2006;28:152–158. doi: 10.1007/s11096-006-9020-z. [DOI] [PubMed] [Google Scholar]
- Blix H.S., Viktil K.K., Reikvam A., Moger T.A., Hjemaas B.J., Pretsch P., Vraalsen T.F., Walseth E.K. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur. J. Clin. Pharmacol. 2004;60:651–658. doi: 10.1007/s00228-004-0830-4. [DOI] [PubMed] [Google Scholar]
- Bolsewicz K., Debattista J., Vallely A., Whittaker A., Fitzgerald L. Factors associated with antiretroviral treatment uptake and adherence: a review. Perspectives from Australia, Canada, and the United Kingdom. AIDS Care. 2015;27:1429–1438. doi: 10.1080/09540121.2015.1114992. [DOI] [PubMed] [Google Scholar]
- Bonner K., Mezochow A., Roberts T., Ford N., Cohn J. Viral load monitoring as a tool to reinforce adherence: a systematic review. J. Acquir. Immune Defic. Syndr. 2013;64:74–78. doi: 10.1097/QAI.0b013e31829f05ac. [DOI] [PubMed] [Google Scholar]
- Brazil. Ministry of Health., 2014. Boletim Epidemiologico - Aids e DST 2014. Brasília - DF.
- Carcelero E., Tuset M., Martin M., De Lazzari E., Codina C., Miró J., Gatell J. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med. 2011;12:494–499. doi: 10.1111/j.1468-1293.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- Carnevale R.C., Molino C.de G.R.C., Visacri M.B., Mazzola P.G., Moriel P. Cost analysis of pharmaceutical care provided to HIV-infected patients: an ambispective controlled study. Daru. 2015;23:1–9. doi: 10.1186/s40199-014-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm-Burns M.A., Kim Lee J., Spivey C.A., Slack M., Herrier R.N., Hall-Lipsy E., Graff Zivin J., Abraham I., Palmer J., Martin J.R., Kramer S.S., Wunz T. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med. Care. 2010;48:923–933. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- Cipolle R.J., Strand L.M., Morley P.C. second ed. McGraw-Hill; United States of America: 2004. Pharmaceutical Care Practice. [Google Scholar]
- Crum-Cianflone N., Roediger M.P., Eberly L., Headd M., Marconi V., Ganesan A., Weintrob A., Barthel R.V., Fraser S., Agan B.K. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS ONE. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lyra D., Kheir N., Abriata J., da Rocha C., dos Santos C., Pelá I. Impact of Pharmaceutical Care interventions in the identifi cation and resolution of drug-related problems and on quality of life in a group of elderly outpatients in Ribeirão Preto (SP), Brazil. Ther. Clin. Risk Manag. 2007;3:989–998. [PMC free article] [PubMed] [Google Scholar]
- de Maat M.M.R., de Boer A., Koks C.H.W., Mulder J.W., Meenhorst P.L., van Gorp E.C.M., Mairuhu a.T.a., Huitema a.D.R., Beijnen J.H. Evaluation of clinical pharmacist interventions on drug interactions in outpatient pharmaceutical HIV-care. J. Clin. Pharm. Ther. 2004;29:121–130. doi: 10.1111/j.1365-2710.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- De Rijdt T., Willems L., Simoens S. Economic effects of clinical pharmacy interventions: a literature review. Am. J. Health Pharm. 2008;65:1161–1172. doi: 10.2146/ajhp070506. [DOI] [PubMed] [Google Scholar]
- Dube M.P. Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 2000;31:1467–1475. doi: 10.1086/317491. [DOI] [PubMed] [Google Scholar]
- Esterly J.S., Darin K.M., Gerzenshtein L., Othman F., Postelnick M.J., Scarsi K.K. Clinical implications of antiretroviral drug interactions with warfarin: a case-control study. J. Antimicrob. Chemother. 2013;68:1360–1363. doi: 10.1093/jac/dkt043. [DOI] [PubMed] [Google Scholar]
- Friis-Møller N., Sabin C.A., Weber R., Monforte A.d’Arminio, El-Sadr W.M., Reiss P., Thiébaut R., Morfeldt L., Wit S.De, Pradier C., Calvo G., Law M.G., Kirk O., Phillips A.N., Lundgren J.D., Group, D.C. on A.E. of A.-H.D. (DAD) S. Combination Antiretroviral Therapy and the Risk of Myocardial Infarction. N. Engl. J. Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- Granas A.G., Berg C., Hjellvik V., Haukereid C., Kronstad A., Blix H.S., Kilhovd B., Viktil K.K., Horn A.M. Evaluating categorisation and clinical relevance of drug-related problems in medication reviews. Pharm. World Sci. 2010;32:394–403. doi: 10.1007/s11096-010-9385-x. [DOI] [PubMed] [Google Scholar]
- Hasse B., Ledergerber B., Furrer H., Battegay M., Hirschel B., Cavassini M., Bertisch B., Bernasconi E., Weber R. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin. Infect. Dis. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- Henderson K.C., Hindman J., Johnson S.C., Valuck R.J., Kiser J.J. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDS. 2011;25:221–228. doi: 10.1089/apc.2010.0324. [DOI] [PubMed] [Google Scholar]
- Hughes C.A., Tseng A., Cooper R. Managing drug interactions in HIV-infected adults with comorbid illness. CMAJ. 2015;187:36–43. doi: 10.1503/cmaj.131626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboli P.J., Hoth A.B., McClimon B.J., Schnipper J.L. Clinical pharmacists and inpatient medical care: a systematic review. Arch. Intern. Med. 2006;166:955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- Kopp B.B.J., Mrsan M., Erstad B.B.L., Duby J.J.J. Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am. J. Health Pharm. 2007;64:2483–2487. doi: 10.2146/ajhp060674. [DOI] [PubMed] [Google Scholar]
- Kucukarslan S.N., Peters M., Mlynarek M., Nafziger D.A. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch. Intern. Med. 2003;163:2014–2018. doi: 10.1001/archinte.163.17.2014. [DOI] [PubMed] [Google Scholar]
- Li E.H., Foisy M.M. Antiretroviral and medication errors in hospitalized HIV-positive patients. Ann. Pharmacother. 2014;48:998–1010. doi: 10.1177/1060028014534195. [DOI] [PubMed] [Google Scholar]
- Molino C.de G.R.C., Carnevale R.C., Rodrigues A.T., Visacri M.B., Moriel P., Mazzola P.G. Impact of pharmacist interventions on drug-related problems and laboratory markers in outpatients with human immunodeficiency virus infection. Ther. Clin. Risk Manag. 2014;10:631–639. doi: 10.2147/TCRM.S61821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo O.N., Lesko C.R., Colwell B., Ballard C., Cole S.R., Mathews W.C. Outcomes of pharmacist-assisted management of antiretroviral therapy in patients with HIV infection: a risk-adjusted analysis. Am. J. Health Pharm. 2015;72:1463–1470. doi: 10.2146/ajhp140727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N., Holodniy M. HIV infection in the elderly. Clin. Interv. Aging. 2008;3:453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh V.B., Naima N., Abah I.O., Falang K.D., Lucy O., London I., Dady C., Agaba P., Agbaji O. Pattern of drug therapy problems and interventions in ambulatory patients receiving antiretroviral therapy in Nigeria. Pharm. Pr. 2015;13(566):2015. doi: 10.18549/pharmpract.2015.02.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M.A., Esher Â.F.S.do C., Santos E.M.dos, Cosendey M.A.E., Luiza V.L., Bermudez J.A.Z. Evaluating pharmaceutical services for people living with HIV/AIDS in the city of Rio de Janeiro. Cad Saúde Pública. 2002;18:1429–1439. doi: 10.1590/s0102-311x2002000500036. [DOI] [PubMed] [Google Scholar]
- de Oliveira D.R. first ed. RCN Editora; São Paulo: 2011. Atenção Farmacêutica: da filosofia ao gerenciamento da terapia medicamentosa. [Google Scholar]
- Pirkle C.M., Boileau C., Nguyen V.-K., Machouf N., Ag-Aboubacrine S., Niamba P., Drabo J., Koala S., Tremblay C., Rashed S. Impact of a modified directly administered antiretroviral treatment intervention on virological outcome in HIV-infected patients treated in Burkina Faso and Mali. HIV Med. 2009;10:152–156. doi: 10.1111/j.1468-1293.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- Rathbun R.C., Liedtke M.D. Antiretroviral drug interactions: overview of interactions involving new and investigational agents and the role of therapeutic drug monitoring for management. Pharmaceutics. 2011;3:745–781. doi: 10.3390/pharmaceutics3040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis H.P.L.C., Darlan daS.C.acirc ndido, Catarine V.L., Juliana de O.C., Ana M.S.de A.uacutejo, Maria J.B., Marta M.de F.F. Evaluation of clinical parameters in people living with HIV undergoing pharmacotherapeutic monitoring: Viral load, CD4+ T lymphocytes and adherence to antiretrovirals. Afr. J. Pharm. Pharmacol. 2016;10:236–243. [Google Scholar]
- Remondi F.A., Cabrera M.A.S., de Souza R.K.T. Non-adherence to continuous treatment and associated factors: prevalence and determinants in adults 40 years and older. Cad Saúde Pública. 2014;30:126–136. doi: 10.1590/0102-311x00092613. [DOI] [PubMed] [Google Scholar]
- Saberi P., Dong B.J., Johnson M.O., Greenblatt R.M., Cocohoba J.M. The impact of HIV clinical pharmacists on HIV treatment outcomes: a systematic review. Patient Prefer. Adherence. 2012;6:297–322. doi: 10.2147/PPA.S30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R.G., John J., Leidl R. Health care use and costs of adverse drug events emerging from outpatient treatment in Germany: a modelling approach. BMC Health Serv. Res. 2011;11:9. doi: 10.1186/1472-6963-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand L.M., Cipolle R.J., Morley P.C., Frakes M.J. The impact of pharmaceutical care practice on the practitioner and the patient in the ambulatory practice setting: twenty-five years of experience. Curr. Pharm. Des. 2004;10:3987–4001. doi: 10.2174/1381612043382576. [DOI] [PubMed] [Google Scholar]
- Verdugo R., Lisón L., Fernández M., Conde M., Morales J., Camps R., Hazas J., Barrueta O., Uranga A. The role of the hospital pharmacist in the prevention, treatment and management of the side effects associated with antiretroviral treatment. Farm Hosp. 2010;34:237–250. doi: 10.1016/j.farma.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Verdugo R.M., Navarro M.V.G., Martín L.A.-K., Muñoz A.C., Roldán U.B., Criado S.A. Analysis of the causes and predictive factors for discontinuing treatment with tenofovir in pretreated HIV patients. Farm Hosp. 2007;31:200–205. doi: 10.1016/s1130-6343(07)75374-6. [DOI] [PubMed] [Google Scholar]
- Viktil K.K., Blix H.S. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin. Pharmacol. Toxicol. 2008;102:275–280. doi: 10.1111/j.1742-7843.2007.00206.x. [DOI] [PubMed] [Google Scholar]