Abstract
The present study assessed the comparative antioxidant potential of the ethanol extract (EE) of leaves of four Acacia species (Acacia salicina, AS; Acacia laeta, AL; Acacia hamulosa AH; and Acacia tortilis, AT) grown in Saudi Arabia, including RP-HPTLC quantification of antioxidant biomarker rutin. In vitro DPPH radical scavenging and β-carotene-linoleic acid bleaching assays showed the promising antioxidant activities of Acacia extracts: ASEE (IC50: 60.39 and 324.65 μg/ml) >ALEE (IC50: 217.06 and 423.36 μg/ml) >ATEE (IC50: 250.13 and 747.50 μg/ml) >AHEE (IC50: 255.83 and 417.28 μg/ml). This was comparable to rutin tested at 500 μg/ml. Further, a RP- HPTLC densitometric method was developed (acetonitrile:water; 6:4; v/v) using glass-backed RP-18 silica gel F254 plate, and scanned at UV max 254 nm. The method was validated as per the ICH guidelines. Analysis of the validated RP-HPTLC displayed an intense peak (Rf = 0.65 ± 0.004) of rutin that was estimated (μg/mg dry weight) to be highest in ASEE (10.42), followed by ALEE (2.67), AHEE (1.36) and ATEE (0.31). Taken together, presence of rutin strongly supported the high antioxidant property of the tested Acacia species, especially Acacia salicina. The developed RP-HPTLC method therefore, affirms its application in the quality control of commercialized herbal drugs or formulation containing rutin.
Keywords: Acacia species, Fabaceae, Antioxidant activity, Rutin, RPHPTLC
1. Introduction
The genus Acacia (family: Fabaceae) is widely distributed in the arid zones, rainforests and in the drier parts of the world, including Arabian and African countries (Hall and Johnson, 1993, Ibrahim and Aref, 2000). Acacia contains 1350 species, found as shrubs of height 1–3 m (Acacia iteaphylla), small trees of 8–15 m (Acacia excels and Acacia cambagei) and tall trees growing to 20–30 m (Acacia bakeri and Acacia melanoxylon) (Hall et al., 1972, Muhaisen Hasan et al., 2002). Several Acacia species have been proved to be useful in the treatment of various diseases. Different extracts of Acacia etbaica, Acacia laeta, Acacia origena, Acacia pycnantha and Acacia salicina showed high antimicrobial activity against isolated pathogenic bacteria (Mahmoud et al., 2016, Chatti et al., 2011). Acacia nilotica lignin wood extracts were found to be very impressive in abating the overproduction of free radicals, including its high cytotoxic potential against breast cancer cell line, MCF-7 (Barapatre et al., 2016). Moreover, the aqueous and methanol extracts of Acacia karroo bark had been demonstrated for their antiviral property against HIV-1 (Mulaudzi et al., 2011). An antioxidant active compound isolated from Acacia mearnsii, showed neuroprotection against acrolein-induced oxidative damage by the attenuation of reactive oxygen species (Huang et al., 2010). The chloroform extract of leaves of Acacia salicina was found to possess significant antimutagenic and antioxidant activities against superoxide radicals. The antioxidant property of medicinal plants is associated with the presence of several phytoconstituents such as flavonoids (Hamouz et al., 2011), anthocyanins and phenolics (Basar et al., 2013). A flavonol 3-O-glycosides (Isorhamnetin 3-O-neohesperidoside) isolated from A. salicina leaves protected the cells against oxidative stress by inhibiting xanthine oxidase and superoxide anion scavengers (Bouhlel et al., 2010). Phytochemical investigation of Acacia tortilis revealed the presence of several flavonoids such as vicenin and rutin (Seigler, 2003) and also found to possess a strong anticonvulsant property (Mohammad Alharbi and Azmat, 2015). A. tortilis was also found to be very effective in the treatment of skin allergy as well as inflammatory reactions (Derbel et al., 2007) and Diabetes (Kumar and Singh, 2014).
Rutin (quercetin-3-rutinoside) (Fig. 1) is a flavonol glycoside (Calabro et al., 2005) widely available in fruits, especially in citrus fruits such as orange, grapefruit and lemon. It was found to possess several pharmacological actions such as antitumor (Deschner et al., 1991), antidiabetic, hypolipidemic and antioxidant (Ahmed et al., 2010) due to its potent scavenging property of oxidizing free radicals (hydroxide, peroxide and superoxide) (Mauludin et al., 2009). Rutin being a nontoxic and nonoxidizable molecule has advantages over other flavonoids such as myricetin, and quercetagenin that catalyzes the production of oxygen radical (Hodnick et al., 1986). Rutin has been quantitatively estimated by HPTLC method in different extracts of many plants such as Ficus species (Alajmi et al., 2015) and Ocimum sanctum Linn (Ilyas et al., 2015) as well as in pharmaceutical preparations (Soponar et al., 2010). HPLC has been also used for the estimation of rutin in the extracts of Althea rosea (Muhetaer et al., 2015), Syringa vulgaris (Toth et al., 2016) and tea (Porto-Figueira et al., 2015). Till date not any HPTLC method has been reported for comparative analysis of antioxidant biomarker rutin in different Acacia species (Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis).
Figure 1.
Chemical structure of biomarker rutin.
With this background information indicating promising antioxidative potential of Acacia extracts with few applied examples, we argue that this genus may offer a promising natural source of commercial antioxidants. In this study, we therefore, planned to perform a comparative antioxidant assay on 95% ethanol extracts of leaves of Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis grown in Saudi Arabia, including quantification of antioxidant biomarker rutin by validated RP-HPTLC method.
2. Materials and methods
2.1. Plant material collection and authentication
The leaves of four different Acacia species i.e. Acacia salicina (Voucher No. 15007; collected in March, 2008), Acacia laeta (Voucher No. 15081; collected in March, 2008), Acacia hamulosa (Voucher No. 16221; collected in March, 2014) and Acacia tortilis (Voucher No. 14977; collected in March, 2007) were collected from eastern part of Saudi Arabia. These samples were authenticated by Dr. Mohammed Yusuf (Field taxonomist, Department of Pharmacognosy, College of Pharmacy, KSU) and specimens were deposited at the college herbarium.
2.2. Plant material extraction by ultrasonic method
The leaves of Acacia salicina (AS), Acacia laeta (AL), Acacia hamulosa (AH) and Acacia tortilis (AT) were dried in air, pulverized and passed through a 0.75-mm sieve. The extraction process was carried out in an ultrasonic cleaner Transsonic-460/H (ELMA, Germany). The powdered materials (5.0 g) of AS, AL, AH and AT were mixed with 95% ethanol in a conical flask and sonicated (frequency 20 kHz, power 100 W) for 30 min at room temperature. The AS, AL, AH and AT ethanol extracts (EE) were centrifuged at 5000 rpm for 20 min and finally filtered through Whatman filter paper No. 1. The obtained extracts were concentrated and dried under reduced pressure using rotary evaporator (R-210, BUCHI). The estimated yields (w/w) of ASEE, ALEE, AHEE and ATEE were found to be 7.2%, 6.3%, 5.6% and 5.9% w/w for samples, respectively.
2.3. Apparatus and reagents
Rutin (standard), Tween-40, 1,1-diphenyl-2-picrylhydrazyl (DPPH), β-carotene, and linoleic acid were procured from Sigma Aldrich (USA). The AR grade ethanol and Chloroform were procured from BDH (UK), and HPLC grade acetonitrile and glass-backed silica gel 60F254 RP-HPTLC plate were procured from Merck (Germany). Automatic TLC Sampler-4 (CAMAG, Switzerland) was used to apply the rutin and different Acacia species extracts (ASEE, ALEE, AHEE and ATEE) bandwise to RP-HPTLC plates and plate development was carried out in ADC2 (automatic development chamber) (CAMAG, Switzerland). The scanning of developed RP-HPTLC plate was done by CATS 4 (CAMAG, Switzerland) and documented by TLC Reprostar 3 (CAMAG, Switzerland).
2.4. In vitro antioxidant assays
2.4.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
Antioxidant activities of ASEE, ALEE, AHEE and ATEE were evaluated quantitatively by free-radical scavenging ability against DPPH as per the previously described method (Lee et al., 2013) with minor alteration to suite 96-well microtitre plates format. In brief, 100 μL of different concentrations (31.25, 62.5, 125, 250 and 500 μg/ml) of each extract was mixed with 40 μL of DPPH (0.2 mM in methanol) in wells of a 96-well microtitre plate. Appropriate control was prepared using the solvent only in addition to the same amount of DPPH reagent to get rid of any inherent solvent effect. Rutin (31.25, 62.5, 125, 250 and 500 μg/ml) was used as standard. After 30 min incubation in dark at 25 °C, the decrease in absorbance (Abs) was measured at λ = 517 nm using microtitre plates reader. The test was carried out in triplicate. The radical scavenging activity was calculated from the following equation:
2.4.2. β-Carotene-linoleic acid bleaching assay
The antioxidant activities of ASEE, ALEE, AHEE and ATEE were evaluated by using the β carotene bleaching method (Miller, 1971) with minor modifications for working with 96 well plate. Briefly, 0.25 mg β-carotene was dissolved in 0.5 ml of chloroform and added to flasks containing 12.5 μg of linoleic acid and 100 mg of Tween-40. The chloroform was evaporated at 43 °C using speed vacuum concentrator (Savant, Thermo Electron Co.). The resultant mixture was immediately diluted to 25 ml with distilled water and shaken vigorously for 2–3 min to form an emulsion. A 150 μl aliquot of the emulsion was added to wells of a 96-well plate containing 50 μl of each plant extract or rutin at 500 μg/ml. A control containing solvent instead of extract was also prepared. The plate was incubated at 50 °C for 2 h. Absorbance was taken at 470 nm at 30 min intervals using microtitre plate spectrophotometer (BioRad). The test was carried out in triplicate. Then, antioxidant activity was expressed as % inhibition of lipid peroxidation using the following formula:
where Abssam120 and Abscont120 are the absorbance of the sample and control, respectively at time 120 min, and Abscon0 is the absorbance of the control at time 0 min.
2.5. HPTLC instrumentation and conditions
The estimation of rutin in ASEE, ALEE, AHEE and ATEE was accomplished on RP-HPTLC plate (20 × 10 cm) where the band size of each track was 6 mm wide and 9.4 mm apart. Rutin, ASEE, ALEE, AHEE and ATEE were applied on the RP-HPTLC plate with an application speed of 160 nl/s, and development of the applied plate was carried out in pre-saturated twin-trough glass chamber (20 × 10 cm) at 25 ± 2 °C under 60 ± 5% humidity. The developed RP-HPTLC plate was dried and analyzed quantitatively at λ = 254 nm in absorbance mode.
2.6. Preparation of standard stock solutions
A standard stock solution of rutin (1 mg/ml) was prepared in HPLC grade methanol, following further dilution of the standard stock solution with methanol to get ten different dilutions of standard rutin ranging from 10 to 180 μg/ml. All the ten dilutions of rutin were applied (10 μl, each) through microliter syringe attached with the applicator on the RP-HPTLC plate to furnish the linearity range of 100–1800 ng/band.
2.7. Validation of method
Validation of the proposed RP-HPTLC method was performed according to the International Conference on Harmonization guidelines (2005), for the determination of linearity range, limit of detection (LOD), limit of quantification (LOQ), precision, recovery as accuracy and robustness.
2.8. Statistical analysis
The statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Dunnet’s test for the estimation of total variation in a set of data. Results were expressed as mean ± SD. P < 0.01 was considered significant.
3. Results and discussion
3.1. Antioxidant activity of tested Acacia species
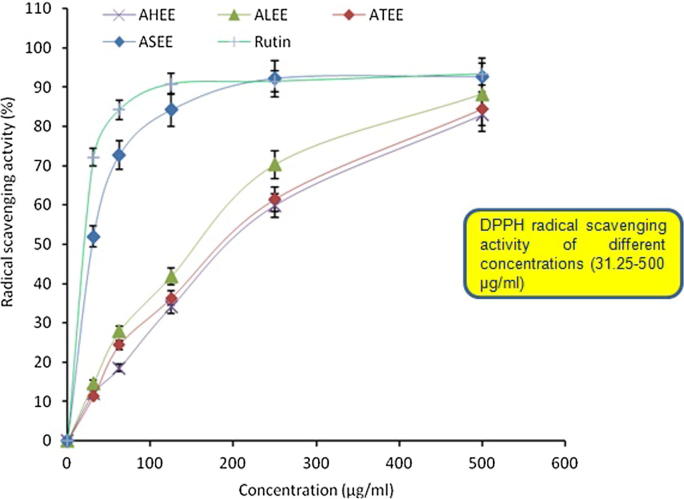
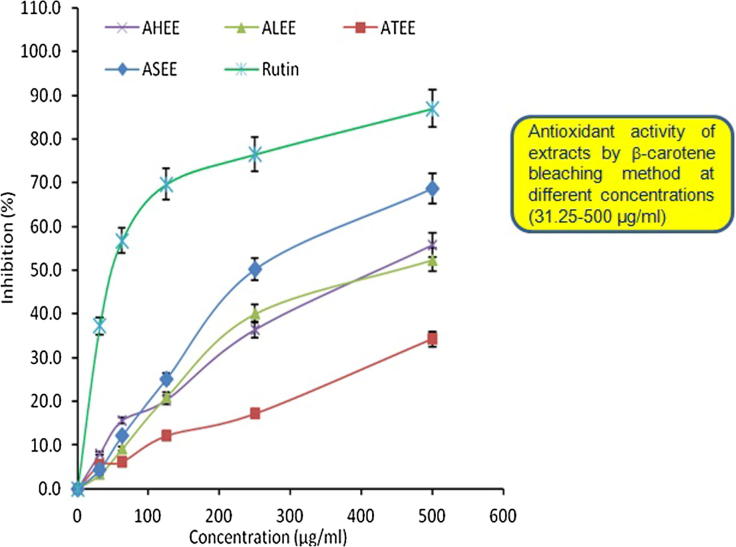
Free radical scavenging activities (DPPH) of ASEE, ALEE, AHEE and ATEE were tested for the first time and the results were represented in Fig. 2. The IC50 values for ASEE, ALEE, AHEE and ATEE in DPPH assay were found to be 60.39, 217.06, 255.83 and 250.13 μg/ml, respectively whereas in case of β-carotene-linoleic acid bleaching assay those were 324.65, 423.36, 417.28 and 747.50 μg/ml, respectively. At 500 μg/ml, the antioxidant activity in DPPH assay was in the following order: ASEE > ALEE > ATEE > AHEE. Interestingly at concentrations higher than 250 μg/ml, the radical scavenging activity of ASEE was approximately similar to the antioxidant standard Rutin. In accordance with the DPPH radical scavenging assay results, the different Acacia extracts showed concentration dependent antioxidant activity. Similarly, the β-carotene-linoleic acid bleaching assay also showed the antioxidant property of all extracts (500 μg/ml) that was however, in the order of ASEE > AHEE > ALEE > ATEE (Fig. 3).
Figure 2.
DPPH radical scavenging activity of different concentrations (31.25–500 μg/ml) of ASEE, ALEE, AHEE and ATEE. Values are means of three experiments.
Figure 3.
Antioxidant activity of ASEE, ALEE, AHEE and ATEE in comparison with the standard antioxidant (Rutin) assayed by the β-carotene bleaching method showing percentage of inhibition of lipid peroxidation by different concentrations (31.25–500 μg/ml) of the extracts. Values are means of three experiments.
Both the analyses demonstrated that the ethanol extract of A. salicina had highest antioxidant potential compared to the other alcoholic extracts of A. laeta, A. hamulosa and A. tortilis.
3.2. Development of RP-HPTLC method and validation
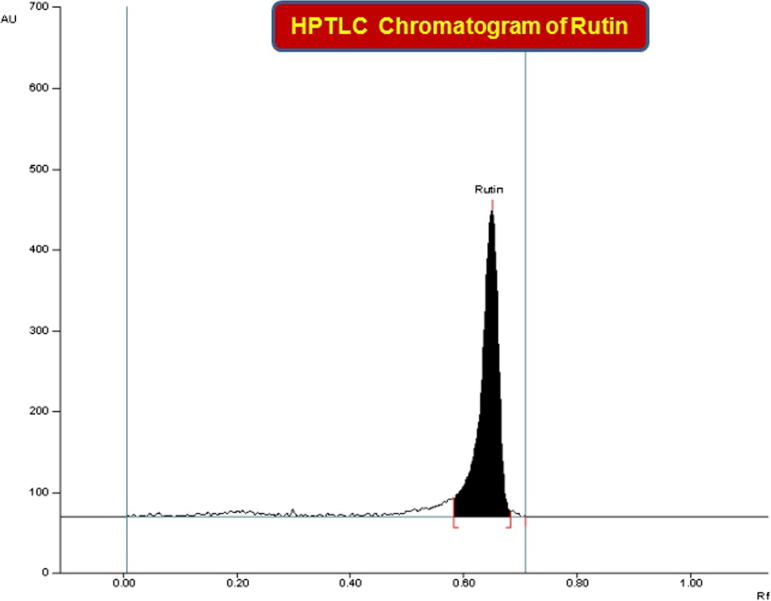
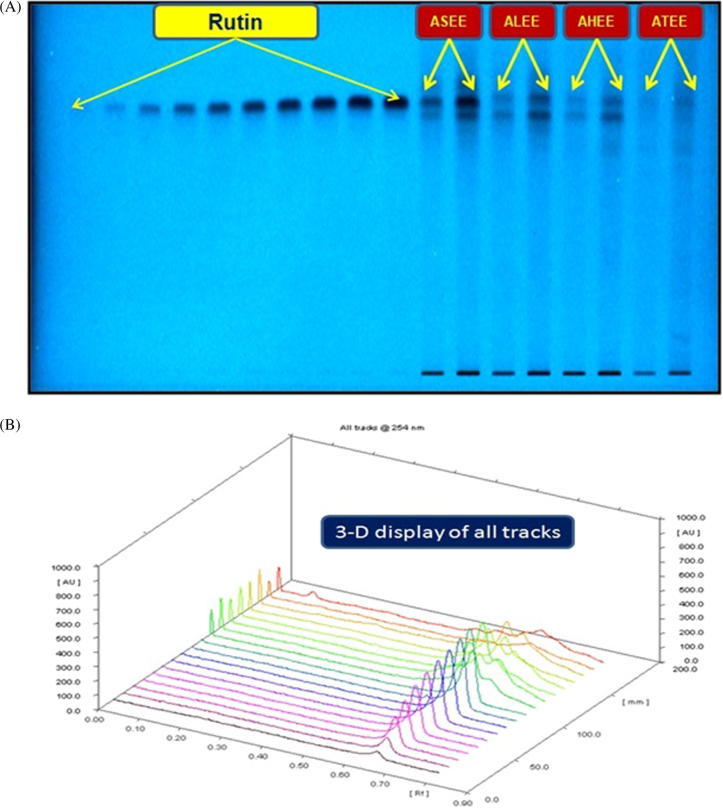
The mobile phase used in RP-HPTLC analysis was selected by analyzing various compositions of different solvents. Of these, a mixture of acetonitrile and water in the ratio of 4:6 (V/V) was found to be the best mobile phase for development and analysis of rutin in ASEE, ALEE, AHEE and ATEE. The developed RP-HPTLC method gave an intense, compact and sharp peak of rutin at Rf = 0.65 ± 0.004 (Fig. 4). This method was found to be very efficient in clearly separating the biomarker rutin and various constituents of ASEE, ALEE, AHEE and ATEE (Fig.5a and b). During the development of the RP-HPTLC plate the optimized mobile phase volume for saturation was found to be 20 ml and saturation time was found to be 20 min. The developed RP-HPTLC method was found to be selective with high resolution baseline. The regression equation and square of correlation coefficient (r2) for rutin were found to be Y = 6.34x + 787.32 and 0.9985 ± 0.0002 in the linearity range 100–1800 ng/spot. The Limit of detection (LOD) and limit of quantification (LOQ) for rutin were found to be 29.77 and 90.22 ng/band, respectively (Table 1). The recoveries as accuracy study for the proposed method were recorded (Table 2). The recovery (%) and RSD (%) for rutin were found to be 98.25–99.52% and 0.827–1.151%. The intra-day and inter-day precision for the proposed method was recorded (Table 3). The %RSD for intra-day and inter-day precisions (n = 6) were found to be 0.528–0.651% and 0.509–0.552%, respectively, which showed the good precision of the proposed method. The robustness study was performed by introducing small deliberate changes in the mobile phase composition, duration of saturation and mobile phase volume used in the saturation at the 300 ng/band concentration of rutin. The obtained data of this study in the form of SD and % RSD are reported in Table 4. The SD and % RSD values were found to be very low which indicated that the method was robust.
Figure 4.
Chromatogram of standard rutin (Rf = 0.65; 800 ng/spot) at 254 nm.
Figure 5.
Quantification of rutin in ASEE, ALEE, AHEE and ATEE by RP-HPTLC. (A) Pictogram of developed RP-HPTLC plate at short UV length (254 nm) [mobile phase: acetonitrile: water, (4:6, v/v)]; (B) 3-D display of all tracks at 254 nm.
Table 1.
Rf, Linear regression data for the calibration curve of Rutin (n = 6).
| Parameters | Rutin |
|---|---|
| Linearity range (ng/spot) | 100–1800 |
| Regression equation | Y = 6.34x + 787.32 |
| Correlation (r2) coefficient | 0.9985 ± 0.0002 |
| Slope ± SD | 6.34 ± 0.057 |
| Intercept ± SD | 787.32 ± 13.723 |
| Standard error of slope | 0.023 |
| Standard error of intercept | 5.601 |
| Rf | 0.65 ± 0.004 |
| LOD | 29.77 ng/band |
| LOQ | 90.22 ng/band |
Table 2.
Recovery as accuracy studies of the proposed RP-HPTLC Method (n = 6).
| Percent (%) of rutin added to analyte | Theoretical concentration of rutin (ng/ml) | Concentration of rutin found (ng/mL) ± SD | %RSD | SEM | % Recovery |
|---|---|---|---|---|---|
| 0 | 200 | 198.93 ± 2.29 | 1.151 | 0.934 | 99.46 |
| 50 | 300 | 295.97 ± 2.91 | 0.983 | 1.187 | 98.65 |
| 100 | 400 | 393.04 ± 3.67 | 0.933 | 1.497 | 98.25 |
| 150 | 500 | 494.64 ± 4.12 | 0.827 | 1.681 | 99.52 |
Table 3.
Precision of the proposed RP-HPTLC Method (n = 6).
| Conc. of Rutin (ng/band) | Intra-day Precision |
Inter-day Precision |
||||
|---|---|---|---|---|---|---|
| Average Conc. found ± SD | %RSD | SEM | Average Conc. found ± SD | %RSD | SEM | |
| 400 | 397.26 ± 2.59 | 0.651 | 1.057 | 394.58 ± 2.18 | 0.552 | 0.889 |
| 600 | 595.98 ± 3.65 | 0.612 | 1.489 | 592.03 ± 3.41 | 0.575 | 1.391 |
| 800 | 793.03 ± 4.13 | 0.528 | 1.710 | 790.51 ± 4.03 | 0.509 | 1.644 |
Table 4.
Robustness of the proposed RP-HPTLC Method (n = 6).
| Optimization condition | Rutin (300 ng/band) |
||
|---|---|---|---|
| SD | %RSD | SEM | |
| Mobile phase composition (Acetonitrile: Water) | |||
| (4:6) | 2.41 | 0.814 | 0.983 |
| (3.9:6.1) | 2.33 | 0.793 | 0.951 |
| (4.2:5.8) | 2.21 | 0.737 | 0.902 |
| Mobile phase volume (for saturation) | |||
| 18 ml | 2.41 | 0.814 | 0.983 |
| 20 ml | 2.39 | 0.809 | 0.975 |
| 22 ml | 2.36 | 0.798 | 0.963 |
| Duration of saturation | |||
| 10 min | 2.35 | 0.798 | 0.959 |
| 20 min | 2.29 | 0.783 | 0.934 |
| 30 min | 2.23 | 0.759 | 0.910 |
3.3. Estimation of rutin in ASEE, ALEE, AHEE and ATEE by applying developed RP-HPTLC method
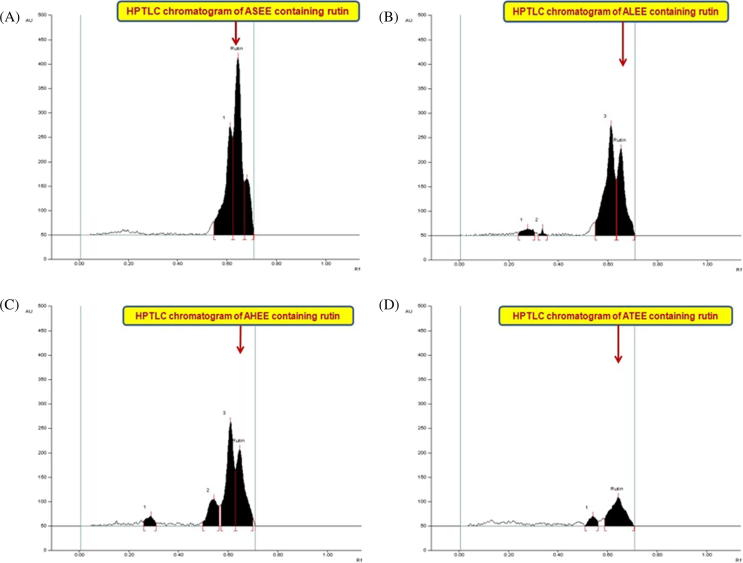
The application of developed RP-HPTLC method was exercised in the estimation of rutin in ASEE, ALEE, AHEE and ATEE (Fig. 6). The content of rutin in different extracts ASEE, ALEE, AHEE and ATEE by employing the above developed RP-HPTLC method was found to be: 10.42 μg/mg (ASEE) > 2.67 μg/mg (ALEE) > 1.36 μg/mg (AHEE) > 0.31 μg/mg (ATEE) of the dried weight of extracts. This is a maiden report, which demonstrated the development of an economical, precise, accurate and simple RP-HPTLC method for estimation of rutin in different Acacia species (Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis).
Figure 6.
Chromatogram of rutin estimation in the extracts of Acacia species at 254 nm [mobile phase: acetonitrile: water, (4:6, v/v)]. (A) Acacia salicina ethanol extract (ASEE; spot 2, Rf = 0.65); (B) Acacia laeta ethanol extract (ALEE; spot 4, Rf = 0.65); (C) Acacia hamulosa ethanol extract (AHEE; spot 4, Rf = 0.65); (D) Acacia tortilis ethanol extract (ATEE; spot 2, Rf = 0.65).
Several biochemical reactions in our body are responsible for the formation of large number of free radicals. These free radicals intervene the normal metabolic processes inside and outside of our body cells which leads to the occurrence of many pathological changes (Pandey and Rizvi, 2009). Exhaustive research has been done on the useful effects of several antioxidant phenolic and flavonoidal molecules which help in neutralizing the deleterious effects produced by free radicals (Benavente-Garia et al., 1997). The Antioxidant property of A. nilotica, Acacia seyal and A. laeta has been compared by DPPH free radical scavenging method and it was found that A. nilotica and A. seyal showed highest antioxidant property while A. laeta showed less antioxidant property. The phytochemical investigation of these three species indicated that both A. nilotica and A. seyal were enriched in phenolics and flavonoidal compounds in comparison with A. laeta which might be the reason of their high antioxidant potential (Abdel-Farid et al., 2014). To prove the antioxidant property of plant materials several antioxidant assays have been recommended (Alam et al., 2013).
In our study we used DPPH radical scavenging assay and β-carotene-linoleic acid bleaching methods for the comparative analysis between four species of genus Acacia (A. salicina, A. laeta, A. hamulosa and A. tortilis). DPPH is a molecule containing a stable free radical, in DPPH assay, in the presence of an antioxidant which can donate an electron to DPPH, the purple color of free DPPH radical decays, and the extent of decrease in the absorbance correlates with the free-radical scavenging potential of the test sample. This is one of the most common in vitro methods used for assessing plant extracts ability to scavenge free radical, owing to its simplicity and compatibility with both hydrophilic and lipophilic antioxidant samples regardless of pH, temperature and light (Kedare and Singh, 2011). On the other hand, in β-carotene bleaching method, the linoleic acid radical formed by the loss of a hydrogen atom from one of its diallylic methylene groups attacks unsaturated β-carotene molecules. As a result, β-carotene is oxidized and subsequently the system loses its chromospheres and characteristic orange color. In our in vitro assays, the antioxidant property of all the extracts was found to be in the order of A. salicina > A. laeta > A. tortilis > A. hamulosa in both the antioxidant assays.
The available literature also revealed the presence of flavonoids and phenolic compounds in Acacia species which supports our finding of good antioxidant property of all the Acacia species. Rutin being a flavonoidal compound may possess the ability to reduce or inhibit the hazardous actions of free radicals produced in our body. According to Yang et al. (2008), rutin exhibited a strong DPPH free radical scavenging property along with the inhibition of lipid peroxidation. Rutin was also found to be very effective in the treatment of diabetes by reducing thiobarbituric acid reactive species (TBARS), lipid hydroperoxides (HP) and increasing superoxide dismutase (SOD) and catalase antioxidant enzymes activities in liver, kidney and brain (Kamalakkannan and Stanely, 2006). The antioxidant effect of rutin was also studied by Bhandary et al., 2012, against Ischemia/reperfusion-associated hemodynamic alterations in which ROS produces toxic effects on various injured organs. In this case rutin showed very promising regulatory effects against Cardiac I/R and also showed significant DPPH and SOD activity. The finding of rutin in large quantity in A. salicina by RP-HPTLC supported its highest antioxidant potential which also indicated that the presence of rutin in all these Acacia species along with other flavonoids and phenolic compounds might be responsible for their antioxidant potential.
Recently, HPTLC has been extensively employed in quality control of herbal drugs because of its various unique features such as inexpensive, high sample throughput as well as requirement of very less solvent for cleaning (Alam et al., 2014). These days it is also used for HPTLC has been extensively employed in the identification of herbs and their constituents: for purity testing of herbals and stability testing of their preparations, and analysis of uniformity of herbal extracts, animal extracts, drugs as well as excipients. It has been widely employed recently in the standardization and quality control of formulated products, viz. pharmaceuticals, cosmetics, and herbal nutritional supplements (Alajmi et al., 2013). The above developed validated RP-HPTLC method for the estimation of rutin in different Acacia species will prove beneficial in helping and facilitating the quality control of herbal drugs.
4. Conclusion
Our finding of promising antioxidant property of alcoholic extracts of four Acacia species especially A. salicina will provide an opportunity for further evaluation of these and other species in the various diseases caused by free radicals such as cancer, hepatic disorder, and aging. The RP-HPTLC method developed in this work for the comparative analysis of antioxidant biomarker rutin in Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis is a maiden method and it may be employed further for the study of degradation kinetics and quality control of herbal drugs as well as herbal formulations containing rutin.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. (RGP-150).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Perwez Alam, Email: alamperwez007@gmail.com, aperwez@ksu.edu.sa.
Mohamed F. Alajmi, Email: alajmister@gmail.com.
Ahmed H. Arbab, Email: arbabssn@gmail.com.
Mohammad K. Parvez, Email: khalid_parvez@yahoo.com.
Nasir A. Siddiqui, Email: nasiratksu@gmail.com.
Saleh I. Alqasoumi, Email: sqasoumi@ksu.edu.sa.
Adnan J. Al-Rehaily, Email: ajalreha@ksu.edu.sa.
Mohammed S. Al-Dosari, Email: msdosari@yahoo.com.
Omer A. Basudan, Email: omer_basodan@yahoo.com.
References
- Abdel-Farid I.B., Sheded M.G., Mohamed E.A. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J Biol Sci. 2014;21:400–408. doi: 10.1016/j.sjbs.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed O.M., Moneim A.A., Yazid I.A., Mahmoud A.M. Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide- Streptozotocin-induced diabetic rats. Diabetol. Croat. 2010;39:15–35. [Google Scholar]
- Alajmi M.F., Alam P., Shakeel F. Quantification of bioactive marker β-amyrin by validated high-performance thin-layer chromatographic-densitometric method in different species of Maytenus grown in Saudi Arabia. J Planar Chromatogr. 2013;26:475–479. [Google Scholar]
- Alajmi M.F., Alam P., Siddiqui N.A., Basudan O.A., Hussain A. Quantitative analysis of biomarker rutin in different species of genus Ficus by validated NP and RP-HPTLC methods. Pak. J. Pharm. Sci. 2015;28(6S):2213–2220. [PubMed] [Google Scholar]
- Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam P., Siddiqui N.A., Al-Rehaily A.J., Alajmi M.F., Basudan O.A., Khan T.H. Stability-indicating densitometric high-performance thin-layer chromatographic method for the quantitative analysis of biomarker Naringin in the leaves and stems of Rumex vesicarius L. J. Planar Chromatogr. 2014;27:204–209. [Google Scholar]
- Barapatre A., Meena A.S., Mekala S., Das A., Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016;86:443–453. doi: 10.1016/j.ijbiomac.2016.01.109. [DOI] [PubMed] [Google Scholar]
- Basar M.H., Hossain S.J., Sadhu S.K., Rahman M.H. A comparative study of antioxidant potential of commonly used antidiabetic plants in Bangladesh. Orient. Pharm. Exp. Med. 2013;13:21–28. [Google Scholar]
- Benavente-Garia O., Castillo J., Marin F.R., Ortuno A., Del Rio J.A. Use and properties of citrus flavonoids. J. Agri. Food Chem. 1997;45:4505–4515. [Google Scholar]
- Bhandary B., Piao C.S., Kim D.S., Lee G.H., Chae S.W., Kim H.R., Chae H.J. The protective effect of rutin against Ischemia/reperfusion-associated Hemodynamic alteration through antioxidant activity. Arch. Pharm. Res. 2012;35:1091–1097. doi: 10.1007/s12272-012-0617-6. [DOI] [PubMed] [Google Scholar]
- Bouhlel I., Limem I., Skandrani I., Nefatti A., Ghedira K., Dijoux-Franca M.G., Leila C.G. Assessment of isorhamnetin 3-O-neohesperidoside from Acacia salicina: protective effects toward oxidation damage and genotoxicity induced by aflatoxin B1 and nifuroxazide. J. Appl. Toxicol. 2010;30(6):551–558. doi: 10.1002/jat.1525. [DOI] [PubMed] [Google Scholar]
- Calabro M.L., Tommasini S., Donato P., Stancanell R., Raneri D., Catania S., Costa C., Villari V., Ficarra P., Ficarra R. The rutin/beta-cyclodextrin interactions in fully aqueous solution: spectroscopic studies and biological assays. J. Pharm. Biomed. Anal. 2005;36:1019–1027. doi: 10.1016/j.jpba.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Chatti I.B., Boubaker J., Skandrani I., Bhouri W., Ghedira K., Chekir Ghedira L. Antioxidant and antigenotoxic activities in Acacia salicina extracts and its protective role against DNA strand scission induced by hydroxyl radical. Food Chem. Toxicol. 2011;49(8):1753–1758. doi: 10.1016/j.fct.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Derbel S., Noumi Z., Werner Anton K., Chaieb M. Life cycle of the coleopter Bruchidius raddianae and the seed predation of the Acacia tortilis Subsp. raddiana in Tunisia. CR Biol. 2007;330:49–54. doi: 10.1016/j.crvi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Deschner E.E., Ruperto J., Wong G. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–1196. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- Hall, N., Boden, R., Christian, C.S., Condon, R., Dale, F., Hart, A., Leigh, J., Marshall, J., McArthur, A., Russel, V., Turnbull, J., 1972. The use of trees and shrubs in the dry country of Australia. Australian Government Publishing Service, Canberra, Australian Capital Territory, Australia, pp 558.
- Hall N., Johnson L. Royal Botanic Gardens; Sydney: 1993. The Names of Acacias of New South Wales – With A Guide to Pronunciation of Botanical Names. [Google Scholar]
- Hamouz K., Lachman J., Pazderu K., Toma sek J., Hejtmankova K., Pivec V. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh color. Plant Soil Environ. 2011;57:478–485. [Google Scholar]
- Hodnick W.F., Kung F.S., Roettger W.J., Bohmont C.W., Pardini R.S. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem. Pharmacol. 1986;35:2345–2357. doi: 10.1016/0006-2952(86)90461-2. [DOI] [PubMed] [Google Scholar]
- Huang W., Niu H., Xue X., Li J., Li C. Robinetinidol-(4beta–>8)-epigallocatechin 3-O-gallate, a galloyl dimer prorobinetinidin from Acacia mearnsii De Wild, effectively protects human neuroblastoma SH-SY5Y cells against acrolein-induced oxidative damage. J. Alzheimers. Dis. 2010;21(2):493–506. doi: 10.3233/JAD-2010-090886. [DOI] [PubMed] [Google Scholar]
- Ibrahim Ahmed A.M., Aref Ibrahim M. Host status of thirteen Acacia species to Meloidogyne javanica. J. Nematol. 2000;32(4S):609–613. [PMC free article] [PubMed] [Google Scholar]
- Ilyas U.K., Katare D.P., Aeri V. Densitometric validation and optimization of polyphenols in ocimum sanctum linn by high performance thin-layer chromatography. Phytochem. Anal. 2015;26(4):237–246. doi: 10.1002/pca.2550. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Text and Methodology Q2 (R1), Complementary Guideline on Methodology incorporated in November 2005 by the ICH Steering Committee, IFPMA, Geneva.
- Kamalakkannan N., Stanely- Mainzen P.P. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell Biochem. 2006;293:211–219. doi: 10.1007/s11010-006-9244-1. [DOI] [PubMed] [Google Scholar]
- Kedare S.B., Singh R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Tech. Mys. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B.P., Singh R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. Biomed. Res. Int. 2014;2014:572013. doi: 10.1155/2014/572013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Kim D.B., Lee J., Cho J.H., Kim B., Choi H.S., Lee B.Y., Lee O.H. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18:12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M.F., Alrumman S.A., Hesham Ael-L. Biological activities of some Acacia spp. (Fabaceae) against new clinical isolates identified by ribosomal RNA gene-based phylogenetic analysis. Pak. J. Pharm. Sci. 2016;29(1):221–229. [PubMed] [Google Scholar]
- Mauludin R., Muller R.H., Keck C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009;370:202–209. doi: 10.1016/j.ijpharm.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Miller H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971;48(2):91. [Google Scholar]
- Mohammad Alharbi W.D., Azmat A. Anticonvulsant and neuroprotective effects of the Acacia tortilis growing in KSA. Pak. J. Pharm. Sci. 2015;28(2):531–534. [PubMed] [Google Scholar]
- Muhaisen Hasan M.H., Ilyas M., Mushin P.M. Flavonoids from Acacia tortilis. J. Chem. Res. (S) 2002:276–278. [Google Scholar]
- Muhetaer T., Resalat Y., Chu G., Yin X., Munira A. Determination of rutin, quercetin and kaempferol in Althaea rosea (L) Gavan for Uyghur medicine by high performance liquid chromatography. Se Pu. 2015;33(12):1269–1273. doi: 10.3724/sp.j.1123.2015.05008. [DOI] [PubMed] [Google Scholar]
- Mulaudzi R.B., Ndhlala A.R., Kulkarni M.G., Finnie J.F., Van Staden J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011;135(2):330–337. doi: 10.1016/j.jep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Figueira P., Figueira J.A., Pereira J.A., Camara J.S. A fast and innovative microextraction technique, μSPEed, followed by ultra high performance liquid chromatography for the analysis of phenolic compounds in teas. J. Chromatogr. A. 2015;1424:1–9. doi: 10.1016/j.chroma.2015.10.063. [DOI] [PubMed] [Google Scholar]
- Seigler D.S. Phytochemistry of Acacia sensu lato. Biochem. Syst. Ecol. 2003;31:845–873. [Google Scholar]
- Soponar F., Mot A.C., Sarbu C. High-performance thin-layer chromatography and three-dimensional image analysis for the determination of rutin in pharmaceutical preparations. J. AOAC Int. 2010;93(3):804–810. [PubMed] [Google Scholar]
- Toth G., Barabas C., Toth A., Kery A., Beni S., Boldizsar I., Varga E., Noszal B. Characterization of antioxidant phenolics in Syringa vulgaris L. flowers and fruits by HPLC-DAD-ESI-MS. Biomed. Chromatogr. 2016;30(6):923–932. doi: 10.1002/bmc.3630. [DOI] [PubMed] [Google Scholar]
- Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT - Food Sci. Technol. 2008;41:1060–1066. [Google Scholar]