Abstract
Purpose
To evaluate and compare outcome of stereotactic body radiation therapy (SBRT), yttrium-90 radioembolization, radiofrequency ablation (RFA), or transarterial chemoembolization (TACE) as bridge to liver transplant (LT) in patients with hepatocellular carcinoma.
Methods and materials
We retrospectively reviewed patients treated at our institution with SBRT, TACE, RFA, or yttrium-90 as bridge to LT between 2006 and 2013. We analyzed radiologic and pathologic response and rate of failure after bridge therapy. Toxicities were reported using Common Terminology Criteria for Adverse Events, 4.0. Kaplan-Meier method was used to calculate disease-free survival (DFS) and overall survival after LT.
Results
Sixty patients with a median age 57.5 years (range, 44-70) met inclusion criteria. Thirty-one patients (50.7%) had hepatitis C cirrhosis, 14 (23%) alcoholic cirrhosis, and 8 (13%) nonalcoholic steatohepatitis cirrhosis. Patients received a total of 79 bridge therapies: SBRT (n = 24), TACE (n = 37), RFA (n = 9), and Y90 (n = 9). Complete response (CR) was 25% for TACE, 8.6% for SBRT, 22% for RFA, and 33% for Y90. Grade 3 or 4 acute toxicity occurred following TACE (n = 4) and RFA (n = 2). Transplant occurred at a median of 7.4 months after bridge therapy. Pathological response among 57 patients was 100% necrosis (n = 23, 40%), >50% necrosis (n = 20, 35%), <50% necrosis (n = 9, 16%), and no necrosis (n = 5, 9%). Pathologic complete response was as follows: SBRT (28.5%), TACE (41%), RFA (60%), Y90 (75%), and multiple modalities (33%). At a median follow-up of 35 months, 7 patients had recurrence after LT. DFS was 85.8% and overall survival was 79% at 5 years.
Conclusion
All bridge therapies demonstrated good pathological response and DFS after LT. SBRT and Y90 demonstrated significantly less grade ≥3 acute toxicity. Choice of optimal modality depends on tumor size, pretreatment bilirubin level, Child-Pugh status, and patient preference. Such a decision is best made at a multidisciplinary tumor board as is done at our institution.
Summary.
Liver transplantation has become the treatment of choice for patients with hepatocellular carcinoma and end-stage liver disease. Bridge therapies such as radiofrequency ablation, trans-arterial chemoembolization, stereotactic body radiation therapy, and yttrium-90 can be used to decrease tumor progression and dropout rate from the transplant waiting list. We report our center’s experience in using these therapies, and evaluate and compare the treatment effect and outcomes before and after liver transplantation.
Introduction
Hepatocellular carcinoma (HCC), the most common primary malignancy of the liver, is the sixth most common cancer worldwide and the third leading cause of cancer mortality.1 In 2014, there were 33,190 new cases of liver cancer and 23,000 resultant deaths in the United States.2
Liver transplantation (LT) offers the best results in terms of overall survival (OS) and disease-free survival (DFS) for selected patients with HCC.3 After the Milan criteria were established (single nodule <5 cm or 3 nodules <3 cm), promising results have been reported after LT with survival in the range of 60% to 70% at 5 years.4 However, a major disadvantage with LT is that many patients faced a long waiting time for donor organs. This resulted in the development of an allocation schema so that the priority for donor organs is given to the most severely ill patients. In the United States, this is based on the “model for end-stage liver disease” (MELD) score, a statistical model predicting survival in patients with cirrhosis.5, 6 Patients with early HCC accrue MELD exception points based on waitlist time. Nationally, the median time from HCC exception to transplant is 77 days, though this varies greatly by United Network for Organ Sharing region; a recent policy change delaying the award of exception points will unquestionably extend this interval.7
Several studies have investigated the effect of locoregional therapies, such as radiofrequency ablation (RFA),8 transarterial chemoembolization (TACE),9 sorafenib,10 stereotactic body radiation therapy (SBRT),11 and yttrium-90 (Y90) radioembolization.12 Choice of the appropriate type of therapy is based on different parameters such as size of the tumor, location, bilirubin level, institutional expertise, and patient preference.
RFA involves the local application of radiofrequency thermal energy in which a probe emitting a high-frequency alternating current is inserted into the tumor to destroy it.13, 14 The best outcomes for RFA have been demonstrated in patients with a single tumor <4 cm in diameter.15
Several studies have shown that TACE is effective in terms of achieving tumor necrosis as demonstrated on explant analysis.16 TACE can be performed by injection of a chemotherapeutic agent, with or without lipiodol, or using drug-eluting beads (DEBs) which can be loaded with cytotoxic drugs such as doxorubicin. DEB-TACE provides significantly better tumor response compared with conventional TACE, with a similar safety profile.17
Radioembolization is a form of brachytherapy in which Y90 microspheres are injected via the hepatic artery and trapped within the microvasculature of the tumor. Benefits over other forms of local therapy include relatively low toxicity and the potential to treat patients with significant tumor burden.18 Y90 radioembolization is most commonly used as a treatment for patients with advanced HCC not eligible for transplantation. However, there is an increasing role for Y90 radioembolization in downstaging or as a bridge therapy for patients awaiting LT.12
SBRT is a technique in which a single or limited number of high-dose fractions are delivered to a small precisely defined target, allowing for the treatment of HCC while limiting damage to the normal liver parenchyma.19
In our study, we report our center’s experience in using TACE, SBRT, Y90, and RFA. We evaluate and compare the treatment effect and outcomes of these therapies before and after LT.
Methods and Materials
We conducted an institutional review board–approved retrospective review of the transplant surgery database and identified all transplanted patients treated at our institution with SBRT, TACE, RFA, or Y90 as bridge to LT between 2006 and 2013. We analyzed radiologic and pathologic response as well as rate of failure after bridge therapy. The Kaplan-Meier method was used to calculate OS and DFS after LT.
Study population
In our study, 60 patients received 79 therapies with SBRT, TACE, RFA, or Y-90 as bridge to LT. Diagnosis of HCC was based on biopsy sample or American Association for the Study of Liver Disease imaging criteria.20 The strategy for managing HCC patients at our institute is determined at a multidisciplinary tumor board including transplant surgeons, medical and radiation oncologists, interventional radiologists, and pathologists.
SBRT technique
All patients were immobilized using a vacuum cushion device during simulation and treatment. An end-expiratory shallow breath-hold technique was used in conjunction with the ExacTrac patient positioning platform (BrainLAB AG, Heimstetten, Germany). The gross tumor volume was contoured on treatment planning computed tomography (CT) fused with contrast enhanced CT or magnetic resonance imaging. No margin was added for the clinical target volume, but a minimum of 10 mm was added to the craniocaudal margin, and 7 mm to the other margins for the planning target volume.
Treatment was prescribed to the 100% isodose line, with the 80% isodose line covering the planning target volume, and delivered using conformal arcs or multiple fixed coplanar beams. A normal liver volume of 1000 mL, defined as the volume of liver not involved by gross tumor, was required. The dose to 70% of the normal liver was not to exceed 27 Gy.
Radiologic response and toxicity evaluation
All patients in the study were assessed for radiological response and toxicity. We assessed first radiological response and acute toxicity either after the initial or subsequent bridge therapies before transplant. Radiological response was assessed using Response Evaluation and Criteria in Solid Tumors.21 Tumor response was evaluated by CT or magnetic resonance imaging. Downstaging was defined as regression of tumor from stage T3/4 to T1/2 after treatment. Toxicity data were reviewed from hospital records and laboratory data. Toxicities during and after treatment were reported using National Cancer Institute Common Toxicity Criteria, version 4.0.
Pathologic response
We evaluated the effect of treatment on the tumor by histological assessment of tumor necrosis relative to the total tumor volume. Pathologic complete response (pCR) was 100% tumor necrosis and the absence of any viable tumor; significant pathologic response was >50% tumor necrosis in cross-section; minimal pathologic response was <50% tumor necrosis in cross-section; and viable tumor was no tumor necrosis present.
Statistics
Statistical analysis was performed using SPSS software (version 17.0; SPSS, Chicago, IL). Descriptive statistics were performed on sociodemographic and disease-specific variables. OS and DFS were obtained by Kaplan-Meier survival analyses (log-rank). A P value of less than .05 was considered statistically significant. Treatment outcomes and toxicity in different treatment groups was done using χ2 tests or the analysis of variance test.
Results
Sixty patients received a total of 79 therapies with SBRT (n = 24), TACE (n = 37), RFA (n = 9), and Y90 (n = 9) as bridge to transplant. HCC diagnosis was confirmed by biopsy in 27 patients (45%) and by American Association for the Study of Liver Disease criteria in 33 patients (55%). Median age at diagnosis was 57.5 years (range, 44-70.2) with a male predominance (88%). Child Pugh class A, B, C, and unknown at diagnosis were 22 (37%), 23 (38%), 6 (10%), and 9 (15%) patients, respectively. Main causes for cirrhosis were hepatitis C virus infection (31 patients, 51%), alcohol use (14 patients, 23%), and nonalcoholic steatohepatitis (8 patients, 13%). All patients had Karnofsky score >60 before treatment.
Of the 60 subjects, 37 patients (61.7%) presented with 1 lesion. The primary bridge therapy they received was TACE (n = 22), SBRT (n = 9), RFA (n = 5), and Y90 (n = 1). Eleven patients received further bridge therapies either from recurrence after initial response (n = 6) or progressive or persistent disease (n = 5).
Twenty-three patients (38.3%) had 2 or more lesions at presentation. The primary bridge therapy for this group was TACE (n = 10), SBRT (n = 8), RFA (n = 1), and Y90 (n = 4). Eight patients received further bridge therapies either from recurrence after initial response (n = 2), progressive or persistent disease (n = 2) or for continuation (for other lesions) (n = 4).
Forty-seven patients (78.3%) met Milan criteria at diagnosis and received bridge therapy to prevent progression before transplant, whereas 13 patients (21.7%) were outside of the Milan criteria at diagnosis and required downstaging.
Of the 13 patients outside of the Milan criteria at diagnosis, 6 received SBRT as primary bridge therapy, 5 received TACE, and 2 received Y90. Ten patients successfully underwent downstaging before transplant, whereas 3 went to transplant based on their MELD score without downstaging.
Treatment details and toxicity evaluation
Our patients received TACE on 37 occasions (33 conventional TACE and 4 DEBs). The most commonly used chemotherapy agents were cisplatin (n = 22) and doxorubicin (n = 9). Four treatments were combined with cryotherapy. The most common postprocedure toxicity was postembolization syndrome, consisting of fatigue, mild nausea, low-grade fever, and abdominal pain. Four patients (11%) developed G3 toxicity posttreatment adverse reactions included: acute renal impairment (n = 2), high fever and severe pain (n = 1), and bleeding and thrombocytopenia (n = 1). Grade 4 toxicity was not observed.
SBRT was administered on 24 occasions. Median dose of radiation was 50 Gy (range, 45-60), median number of fractions was 5. All patients tolerated treatment well. The most common side effect was grade 1-2 fatigue, (n = 6), and no grade 3 or 4 toxicity was seen.
Seven patients received Y90 therapy on nine occasions. The average dose of radiation was 109 Gy. Grade 1-2 fatigue was observed after three treatments in 3 patients and elevated liver enzymes after 1 treatment. No grade 3 or 4 toxicities were observed posttreatment.
Of the 9 RFA treatments, 2 grade 3 toxicities were observed: pneumothorax and severe pain. There was a significant association between type of bridge therapy received and occurrence of grade 3-4 toxicity (P = .046) (Table 1).
Table 1.
Patients, tumor characteristics, and toxicity evaluation
| No. of treatments | No. of lesions treated | Median diameter of lesion | Median bilirubin before treatment | CTP A-B before treatment (known patients), % | G3 toxicity after treatment, % | |
|---|---|---|---|---|---|---|
| TACE | 37 | 49 | 2.6 cm | 1 | 96 | 11 |
| SBRT | 24 | 36 | 3 cm | 2.8 | 70 | 0 |
| RFA | 9 | 10 | 2.5 cm | 1.3 | 100 | 22 |
| Y90 | 9 | 14 | 3.4cm | 1.8 | 100 | 0 |
RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; Y90, yttrium 90.
Radiological response after bridge therapies
Evaluation of response was based on the first CT/magnetic resonance imaging scan done after all bridge therapy received. First radiological assessment was done at a median of 2.4 months after 77 bridge therapies. Complete response (CR) was reported after 16 treatments (20.8%), partial response (PR) after 34 treatments (44%), stable disease after 23 treatments (29.8%), and progressive disease was reported after only 4 treatments (5.1%).
The number of patients who achieved a significant response (CR or PR) was 61% for TACE (22/36), 65% for SBRT (15/23), 67% for RFA (6/9), and 67% for Y90 (6/9) (Table 2). There was no significant correlation between type of bridge therapy and the radiological response achieved. Recurrence after initial response was observed in 8 of 60 patients after primary bridge therapy (13.3%), with 4 after TACE, 2 after RFA, and 2 after Y90. Local control rate after bridge therapy was 80.6% for TACE, 91.4% for SBRT, 77.8% for Y90, and 77.8% for RFA.
Table 2.
Radiological response after bridge therapy
| SBRT (N = 23) | TACE (n = 36) | RFA (n = 9) | Y90 (n = 9) | |
|---|---|---|---|---|
| CR (n = 16) | 2 (8.6%) | 9 (25%) | 2 (22.2%) | 3 (33.3%) |
| PR (n = 34) | 13 (56.5%) | 13 (36.1%) | 4 (44.4%) | 3 (33.3%) |
| SD (n = 23) | 8 (34.7%) | 12 (33.3%) | 3 (33.3%) | 2 (22.2%) |
| PD (n = 4) | 1 (4.3%) | 2 (5.5%) | 0 (0%) | 1 (11.1%) |
| Total | 23 (100%) | 36 (100%) | 9 (100%) | 9 (100%) |
CR, complete response; PD, progressive disease; PR, particle response; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; SD, stable disease; TACE, transarterial chemoembolization; Y90, yttrium 90.
Liver transplantation and pathologic response
Our patients received a transplant after a median waiting time on the transplant list of 7.4 months after bridge therapy; 5.7 months for SBRT, 10 months for TACE, 7.5 months for RFA, 9.1 months for RFA, and 7.4 months for multiple therapies. At the time of surgery, 57 patients (95%) met Milan criteria, whereas 3 patients (5%) did not. Ten of 13 patients (77%) who were outside Milan at diagnosis were successfully downstaged.
Histopathological analysis of the explanted livers showed 100% tumor necrosis in 40% of patients, 50% to 99% necrosis in 35%, <50% necrosis in 16%, and no necrosis in 9%. All patients who had complete necrosis originally met the Milan criteria before transplantation. Mean pathological necrosis for the whole group was 68% (95% confidence interval [CI], 58-77); 56% for patients who received SBRT only (95% CI, 35-77), 68% for TACE only (95% CI, 54-82), 70% for RFA only (95% CI, 14-125), 94% for Y90 only (95% CI, 73-113), and 73% for multiple therapies (95% CI, 45-102), (P = .55) (Table 3).
Table 3.
Pathological response
| Bridge therapy received | Pathological response |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 100% necrosis (n = 23) |
50%-99% necrosis (n = 20) |
<50% necrosis (n = 9) |
No necrosis (n = 5) |
Total (n = 57) | |||||
| No. | % | No. | % | No. | % | No. | % | ||
| SBRT | 4 | 28.5 | 6 | 42.8 | 2 | 14.2 | 2 | 14.2 | 14 (100%) |
| TACE | 10 | 41.7 | 8 | 33.3 | 5 | 20.8 | 1 | 4.1 | 24 (100%) |
| RFA | 3 | 60 | 1 | 20 | 0 | 0.0 | 1 | 20.0 | 5 (100%) |
| Y90 | 3 | 75 | 1 | 25 | 0 | 0.0 | 0 | 0.0 | 4 (100%) |
| Multiple therapies | 3 | 30 | 4 | 40 | 2 | 20 | 1 | 10 | 10 (100%) |
RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; Y90, yttrium 90.
Follow-up and survival analysis
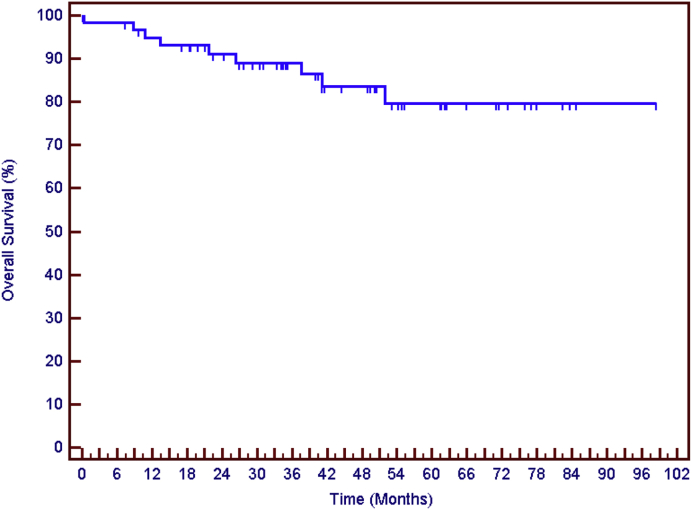
The median follow-up was 41 months (7.3-77.9) after transplantation. Seven patients recurred after transplantation (11.6%); 4 had local (hepatic) recurrence, 2 manifested extrahepatic distant metastasis, and 1 had both local and distant recurrence. Five-year DFS was 86%. The total number of deaths was 8 (13.3%) and 5-year OS 79% (Fig 1). Patients who had a pCR (vs no pCR) in the explanted liver were less likely to experience disease recurrence (32% pCR in patients with no recurrence vs 14% pCR in patients with recurrence) after transplant (P = .14).
Figure 1.
Overall survival for the whole group at 5 years: 79%.
None of the patients who received Y90, RFA, or multiple therapies recurred or died during the time of the study. One of the 17 patients who received SBRT only recurred after transplantation, and 4 of them died. Five of the 24 patients who received TACE only recurred after transplantation, and 4 had died by the end of study. Five-year DFS for SBRT was 91% vs 73% for TACE. Five-year OS for SBRT and TACE was 73% and 72%, respectively.
Discussion
LT is a curative treatment for early-stage HCC. However, many patients face the problem of organ availability and may drop off the waiting list because of tumor progression. Different locoregional therapies have been used as bridge to transplant. The choice of the optimal modality is usually undertaken through multidisciplinary tumor boards based on numerous parameters.
We analyzed and compared the first radiological response, acute toxicity, pathological response, and long-term outcomes after LT among 4 of the most commonly used bridging therapies. There are limited studies that compare the outcomes of these modalities, especially for SBRT and Y90, which are relatively new compared with TACE and RFA (Table 4).
Table 4.
Summary of most important studies of bridge therapies for HCC before liver transplantation
| Reference | Treatment | Tumor stage | Transplanted patients | Tumor progression | Waiting Time (months) | Survival after LT | |
|---|---|---|---|---|---|---|---|
| Present series | SBRT, TACE, Y90, RFA | 47 inside Milan 13 outside |
60 (100%) | 4 | 7.4 | 79% at 5 years | |
| Mazzafero et al, 200422 | RFA | 40 inside 10 outside |
50 (100%) | 0 (0%) | 9.5 | 83% at 3 years | |
| Katz et al, 201211 | SBRT | 15 inside 3 outside |
11 with 1 resection (66.6%) | 3 | 6.3 | NA | |
| Tohme et al, 201312 | Y90 | 14 inside 6 outside |
20 (100%) | 0 | 3.5 | 79% at 5 years | |
| Maddala et al, 200429 | TACE | 47 inside 7 outside |
46 (85%) | 6 (11%) | 7.0 | 74% at 5 years | |
| Yao et al, 200330 | TACE, RFA, PEI, resection | 70 inside | 38 (54%) | 18 (26%) | 6.1 | NA | |
HCC, hepatocellular carcinoma; LT, liver transplantation; NA, not available; PEI, percutaneous ethanol injection; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; Y90, yttrium 90.
In our study, the choice of the bridging therapy was affected by several patient and tumor characteristics. Patients with high pretreatment bilirubin level and Child C status received SBRT, whereas RFA was directed at smaller lesions (mean diameter, 2.5 cm). The ideal indications for RFA include tumor size <3 cm, <3 nodules, and no major vascular or biliary structure near the target lesions.14 Y90 has the advantage of covering areas with high tumor burden and is widely used in the nontransplant setting.22 In this study, the median diameter for Y90 was the highest among the 4 therapies (3.4 cm).
Major toxicities which required hospitalization were reported only after TACE (4/37) (all with the conventional method) and RFA (2/9). None of our patients who received SBRT or Y90 developed major toxicities. Mazzafero et al14 reported the results of 40 transplant candidates who received a single session of RFA before LT, of whom 4 developed major toxicities. A Chinese study23 of 20 patients showed no grade 3 or 4 toxicity after SBRT, although this study was conducted in patients whose cases were inoperable or who had refused LT/surgery.
In this cohort, the radiological CR-PR rate was comparable among the 4 modalities (ranges between 61% and 67%) when taking into consideration the differences in mean tumor diameters. As for pathologic response, we report promising results in patients who received Y90 or RFA only before transplant. Tohme et al12 reported complete tumor necrosis in 25% of 20 explanted livers who received Y90 as bridge to transplant. Also among 16 patients who had radiological follow up, 56% of them had significant response (CR, PR).
Lewandowski et al24 demonstrated that transarterial Y90 radioembolization was associated with greater partial response rates compared with chemoembolization in downstaging HCC from United Network for Organ Sharing stage T3 to T2. Some studies which studied RFA as bridge to transplant have reported complete tumor necrosis at histopathological analysis after LT in 47% to 75% of cases.25, 26 These results were consistent with our findings.
Our experience with SBRT showed radiological CR/PR of 65% (15/23). Similar results were reported from Andolino et al27 who demonstrated 75% CR/PR rate (18/24), with median tumor size of 3 cm. SBRT is being recognized as an effective therapy for HCC either alone or in combination with other modalities, especially in large tumors not suitable for other types of locoregional therapies. Although its clinical experience is still limited, SBRT is a promising option for bridging prior to transplant (Table 4).
TACE was delivered in our study either by conventional means (33 treatments) or DEBs (4 treatments). Radiological CR-PR was 61%. An important point to mention is that the CR-PR rate was 100% after the 4 treatments of DEBs, and no grade 3 toxicity was reported. The PRECISION trial which compared conventional TACE vs DEB showed that the [CR + PR + stable disease] rate was superior for DEBs (63.4% vs 51.9%), in 212 intermediate staged HCCs in the nontransplant setting.28 Interestingly, in this study, only 1 of the patients who achieved radiological CR and 1 patient who had complete tumor necrosis after transplant developed recurrence after surgery, which shows that response after bridging therapy can be used as a prognostic tool for recurrence after transplantation.
In conclusion, the 4 bridge treatment modalities used on the 60 patients in our study were effective as demonstrated by good pathological response and DFS after LT. Radiological response after bridge therapy was comparable among the 4 therapies, though SBRT and Y90 were superior to TACE and RFA in terms of acute toxicity. Choice of the optimal modality depends on various factors such as tumor size, pretreatment bilirubin level, Child-Pugh score, and patient preference. We recommend that this decision be made at a multidisciplinary tumor board including medical and radiation oncologists, transplant surgeons, transplant hepatologists, and interventional radiologists.
Although the results of this study were decidedly encouraging, there are some limitations that need to be considered when reviewing this study. One of the limitations may be the relatively modest sample size that was 60 patients and that only 4 modalities of treatment were investigated. This may limit withdrawing comparative conclusions about treatment and toxicity. Larger samples are needed to further validate our findings. Another limitation for this study might be the fact that several patients received more than one treatment which might further weaken the decisive results for the effect of each treatment.
Acknowledgments
The authors thank Mrs Laura Finger for editorial assistance.
Footnotes
Conflicts of interest: None.
References
- 1.Bosch F.X., Ribes J., Diaz M., Cleries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127(Suppl):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Ma J., Zou Z. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz M. Liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2004;10(Suppl 1):S81–S85. doi: 10.1002/lt.20048. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V., Regalia E., Doci E. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Malinhoc M., Kamath P.S., Gordon F.D. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 6.Kamath P.S., Wiesner R.H., Malinchoc M. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Samoylova M.L., Dodge J.L., Yao F.Y., Roberts J.P. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2014;20:937–944. doi: 10.1002/lt.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu D.S., Yu N.C., Raman S.S. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 9.Graziadei I.W., Sandmueller H. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557–563. doi: 10.1053/jlts.2003.50106. [DOI] [PubMed] [Google Scholar]
- 10.Vitale A., Volk M.L., Pastorelli D. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: A cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165–173. doi: 10.1002/hep.23260. [DOI] [PubMed] [Google Scholar]
- 11.Katz A., Chawla S., Qu Z. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: Clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895–900. doi: 10.1016/j.ijrobp.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Tohme S., Sukato D., Chen H. Yttrium-90 radio-embolization as a bridge to liver transplantation: A single institution experience. J Vasc Interv Radiol. 2013;24:1632–1638. doi: 10.1016/j.jvir.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 13.McGahan J.P., Brock J.M., Tesluk H. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291–297. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferro V., Battiston C., Perrone S. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: A prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins H., Berger D. RFA for liver tumors: Does it really work? Oncologist. 2006;11:801–808. doi: 10.1634/theoncologist.11-7-801. [DOI] [PubMed] [Google Scholar]
- 16.Majno P.E., Adam R., Bismuth H. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang K., Zhou Q., Wang R. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920–925. doi: 10.1111/jgh.12439. [DOI] [PubMed] [Google Scholar]
- 18.Salem R., Lewandowski R.J., Mulcahy M.F. Radio-embolization for hepatocellular carcinoma using yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Lo S.S., Dawson L.A., Kim E.Y. Stereotactic body radiation therapy for hepatocellular carcinoma. Discov Med. 2010;9:404–410. [PubMed] [Google Scholar]
- 20.Bruix J., Sherman M. Practice Guidelines Committee for the American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1209–1236. [Google Scholar]
- 21.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Kulik L.M., Atassi B., vanHolsbeeck L. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: Downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 23.Choi O., Jang H.S., Kang K.M. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154–158. doi: 10.1093/jjco/hyi236. [DOI] [PubMed] [Google Scholar]
- 24.Lewandowski R.J., Kulik L.M., Riaz A. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: Chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 25.Brillet P.Y., Paradis V., Brancatelli G. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: A prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186:S296–S305. doi: 10.2214/AJR.04.1927. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Sanjuán J.C., González F., Juanco C. Radiological and pathological assessment of hepatocellular carcinoma response to radiofrequency: A study on removed liver after transplantation. World J Surg. 2008;32:1489–1494. doi: 10.1007/s00268-008-9559-z. [DOI] [PubMed] [Google Scholar]
- 27.Andolino D.L., Maluccio M., Calley C. Stereotactic body radiotherapy (SBRT) for hepatocellular carcinoma (HCC): The Indiana University experience. Int J Radiat Oncol Biol Phys. 2010;78:S74. [Google Scholar]
- 28.Lammer J., Malagari K., Vogl T. Prospective randomized study of doxorubicin eluting- bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maddala Y.K., Stadheim L., Andrews J.C. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation: Outcome with chemoembolization. Liver Transpl. 2004;10:449–455. doi: 10.1002/lt.20099. [DOI] [PubMed] [Google Scholar]
- 30.Yao F.Y., Bass N.M., Nikolai B. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: Implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]