Abstract
Purpose/Objective(s)
We sought to assess the utility of docetaxel administered concurrently with salvage radiation therapy (SRT) following postprostatectomy biochemical failure (BF).
Methods and materials
Men with postprostatectomy BF were accrued on a single-arm phase 2 clinical trial. SRT doses ranged from 64.8 to 70.2 Gy and were delivered in 1.8-Gy fractions to the prostate bed alone as the clinical target volume with a +1-cm uniform planning target volume expansion. The primary endpoint was progression-free survival at 4 years compared with the Stephenson nomogram estimate. Kaplan-Meier methods were used to assess late toxicity, BF, and distant metastases. An unplanned matched-pair analysis was performed with 19 patients treated with SRT alone.
Results
Nineteen men were accrued and treated. Median follow-up was 4.8 years. Median pre-RT prostate-specific antigen level was 0.7 ng/mL (interquartile range, 0.4-1.3 ng/mL). All 8 cycles of docetaxel were completed in 17 (89%) patients. Acute grade 1-4 toxicities were observed in 79%, 50%, 58%, and 11%, respectively. A total of 68% of acute grade 1 toxicities were related to fatigue, urinary, or bowel symptoms. For grade 2 toxicities, 76% were related to neutropenia, fatigue, or urinary symptoms. Acute grade 3 and 4 toxicities were most commonly neutropenia (84% and 100%, respectively). All late toxicities were grade 1 to 2 with 89% related to bowel or urinary function. Predicted 4-year progression-free survival was 39% and observed was 42% (90% confidence interval [CI], 24-60). Matched-pair analysis demonstrated no significant improvement in BF (P = .96, hazard ratio, 0.98; 90% CI, 0.4-2.3) or distant metastases (P = .09; hazard ratio, 0.3; 90% CI, 0.07-1.2), and no difference between late bowel (P = .60) or urinary toxicity (P = .41).
Conclusions
Docetaxel can safely be administered concurrently with SRT without significantly impacting posttreatment toxicity. Neutropenia was the most significant acute toxicity. Given the small sample size, no clear clinical benefit was observed. Larger studies are needed to determine the efficacy of concurrent docetaxel in this setting.
Summary.
We hypothesized that, for men with a rising prostate-specific antigen level postprostatectomy, weekly concurrent docetaxel combined with salvage radiation therapy could be administered safely and improve upon progression-free survival. We present the results of a single-arm phase II clinical trial demonstrating that docetaxel can safely be administered in this setting; however, larger studies are needed to evaluate efficacy.
Introduction
Prostatectomy is the most common initial treatment for men with localized prostate cancer. Although many men have excellent long-term disease control following prostatectomy, up to approximately 30% of men will have a rising prostate-specific antigen (PSA) level postoperatively.1 For men with a rising PSA level following prostatectomy without evidence of metastatic progression, salvage radiation therapy (SRT) is a standard treatment option. Unfortunately, rates of recurrence following SRT remain high with almost half of men who receive SRT eventually experiencing disease progression,2 likely secondary either to the presence of micrometastatic disease at the time of radiation therapy (RT) or to the failure of RT to eradicate all disease within the defined treatment field.
Docetaxel, a taxane mitotic inhibitor, has been demonstrated to improve overall survival for men with androgen-independent metastatic prostate cancer and more recently in men with newly diagnosed metastatic prostate cancer.3, 4, 5 Additionally, docetaxel is a known radiosensitizer. Radiosensitization is achieved through docetaxel's mechanism of action, which is to promote the formation of stable microtubules and inhibit their disassembly. This disruption of the normal microtubule function causes G2-M cell-cycle arrest, the most radiosensitive phase of the cell cycle, and preclinical evidence supports docetaxel as a favorable radiosensitizing agent.6
Given the role for docetaxel in treating metastatic prostate cancer and its additional functionality as a radiosensitizer, we hypothesized that docetaxel administered concurrently with SRT would improve the 4-year progression-free survival (PFS) for men who experience a rising PSA level postprostatectomy. On the basis of this hypothesis we initiated a single-arm phase 2 clinical trial assessing the safety and efficacy of concurrent weekly docetaxel combined with SRT for men who experienced a rising PSA level postprostatectomy. Here, we report the findings for treatment related toxicity, PFS, metastasis-free survival, and quality of life (QOL).
Methods and Materials
Patient selection
This prospective single-arm phase 2 clinical trial assessing concurrent docetaxel with SRT for men experiencing a rising PSA level after prostatectomy was performed at a single institution (registered with clinical trials.gov, Identifier: NCT00480857) and was approved through the local institutional review board. All patients provided written informed consent. Patients were enrolled from March 2007 through May 2009; at that time, because of a change in industry support, accrual was closed. Men eligible for enrollment had a Karnofsky performance status >80% with pathologically proven prostate adenocarcinoma from a prostatectomy specimen. Additionally, patients were required to have biochemical evidence of PSA recurrence following prostatectomy, as demonstrated by a rising PSA level of at least 0.1 ng/mL between 2 consecutive measurements, and a serum PSA value ≥0.3 ng/mL. Hematologically, patients were required to have an absolute neutrophil count >1500 cells/mm3, platelets >100,000 cells/mm3, and hemoglobin >8.0 g/dL. Men with evidence of metastatic disease present on computed tomography or magnetic resonance imaging or a positive radionuclide bone scan were excluded from this trial, as were any patients receiving pharmacologic androgen ablation following prostatectomy.
Treatment and follow-up
SRT was delivered to the prostate bed only as the clinical target volume with a +1-cm uniform expansion for the planning target volume using 3-dimensional conformal RT with ≥6 MV photons. No patients received additional treatment to the pelvis. SRT doses ranged from 64.8 to 70.2 Gy and were delivered in 1.8-Gy fractions. SRT dose was determined from the pre-SRT PSA levels (<0.4 ng/mL, 0.4-1 ng/mL, and ≥1 ng/mL were treated with 64.8 Gy, 68.4 Gy, and 70.2 Gy, respectively). Docetaxel was supplied by Sanofi-Aventis and administered intravenously weekly at a dose of 20 mg/m2 in concurrent fashion with daily SRT for a total of 8 weeks. Patients received dexamethasone premedication before each administration of docetaxel. Docetaxel was held if the absolute neutrophil count was <1500 cells/mm3 or platelets were <100,000 cells/mm3. Liver function was assessed on weeks 2, 4, 6, and 8 of treatment.
Patients were seen in follow-up 1 and 3 months after completion of treatment, and every 3 months thereafter for the first year, every 6 months for the next 2 years, and yearly thereafter. PSA values were collected at each follow-up visit, and patients were also requested to complete an Expanded Prostate Index Composite (EPIC) questionnaire7 at follow-up appointments as per standard clinical practice.
Primary and secondary outcomes
The planned primary outcome was to assess the 4-year progression-free proportion of patients treated with concurrent weekly docetaxel and SRT and to compare this proportion with the expected 4-year PFS as estimated by prostatectomy Gleason score, pre-SRT PSA, surgical margin status, and postprostatectomy PSA doubling time as described by Stephenson et al.8, 9 Biochemical failure (BF) was defined as any PSA measurement after the completion of SRT that was greater than the post-SRT PSA nadir plus 0.2 ng/mL (post-SRT nadir + 0.2 ng/mL). Secondary outcomes included rates of complete biochemical response, as defined by the achievement of a post-SRT PSA nadir <0.1 ng/mL, rates of acute and late toxicities, rates of local recurrence-free survival, freedom from distant metastases (DM), as well as prostate cancer–specific mortality and overall mortality. Acute toxicities were defined as having occurred within 90 days of treatment end; late toxicities were all toxicities present from this time point forward. Toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 3.0. All recurrence and survival rates were calculated from the end date of SRT. QOL was assessed using the EPIC questionnaire.
Statistical analysis
Planned study characteristics included an enrollment size of n = 44, with an estimated 20% improvement in PFS at 4 years. This would have resulted in >80% power to detect a difference of this magnitude using a 1-sided test with an alpha of 0.05. However, accrual was halted early with enrollment of 19 patients. Kaplan-Meier methods were used to determine a 4-year estimate of BF as well as to assess rates of DM, prostate cancer–specific mortality, and overall mortality and late toxicity.
In addition to planned analyses, an unplanned matched pair analysis was performed. Nineteen patients treated with SRT alone at the same institution were selected from an institutional database of patients treated with SRT between 1995 and 2007. These patients were matched on Gleason score, surgical margin status, postprostatectomy PSA doubling time, pre-SRT PSA, and age at SRT, while blinding for all clinical outcomes. Patients who could not be matched exactly were matched to remain in the same terminal node from the Stephenson PFS estimate diagram such that the resulting estimated 4-year PFS would be the same for each patient's match. Patient characteristics were compared between the enrolled and matched patients using the paired samples t test and McNemar χ2 test for continuous and categorical variables, respectively. Kaplan-Meier methods were used to plot BF, DM, and late toxicities between the enrolled and matched patients. Proportional hazard models with sandwich estimates were used to test and provide hazard ratios (HR) and confidence intervals (CI) comparing the treatment groups.
Last, QOL data were available for 16 of the 19 enrolled patients on this trial. Results from the EPIC questionnaire at approximately 2 years posttreatment were compared between enrolled patients and all available patients treated at the same institution with SRT alone during the same period for whom 2-year posttreatment QOL data were available (n = 29). The independent samples t test was used to compare QOL between the enrolled and historical patients. Baseline QOL data were not available, as this was not recorded as part of this clinical trial.
Results
Patient characteristics
Nineteen men were treated with concurrent docetaxel and SRT. Median follow-up was 4.8 years (interquartile range [IQR], 4.6-5.3). Complete patient characteristics for the cohort of enrolled men as well as matched pairs can be found in Table 1. Matched-pair patients were similar to their enrolled counterparts across clinical, pathologic, and treatment-related variables. Matched pairs had longer follow-up than patients enrolled on the clinical trial; however, this is not surprising given the longer period over which matched patients were treated.
Table 1.
Patient characteristics
| Enrolled patients (SRT + docetaxel, n = 19) | Matched pairs (SRT alone, n = 19) | P value | |
|---|---|---|---|
| Age at SRT | |||
| Median (minimum-maximum) | 66.6 (43.6-78.9) | 66.9 (49.3-76.1) | .17 |
| Follow-up post-SRT, y | |||
| Median (IQR) | 4.7 (4.6-5.3) | 6.1 (4.5-7.1) | .04 |
| Pre-SRT PSA (ng/mL) | |||
| Median (IQR) | 0.7 (0.4-1.3) | 0.5 (0.4-1.5) | .07 |
| Pre-SRT PSA doubling time, mo | |||
| Median (IQR) | 9.5 (5.2-15.1) | 11.8 (4.9-21.5) | .6 |
| Post-SRT PSA nadir (ng/mL) | |||
| Median (IQR) | 0 (0-0.3) | 0.1 (0.0-0.6) | .3 |
| PSA nadir ≤0.1 ng/dL | |||
| No. of patients (%) | 12 (63.2%) | 11 (57.9%) | 1.0 |
| SRT dose (Gy) | .5 | ||
| 64.8 | 10.5% | 26.3% | |
| 68.4 | 57.9% | 47.4% | |
| 0.2 | 31.6% | 26.3% | |
| Gleason score | .17 | ||
| 2-6 | 5.3% | 21.0% | |
| 7 | 52.6% | 47.4% | |
| 8-10 | 42.1% | 31.6% | |
| +SM | 26.3% | 31.6% | 1.0a |
| SVI | 15.8% | 15.8% | 1.0 |
| ECE | 21.1% | 36.8% | .4 |
| +SM or SVI or ECE | 50.0% | 61.1% | .6 |
| +LN | 5.3% | 0.0% | .9 |
ECE, extracapsular extension; IQR, interquartile range; LN, lymph node; PSA, prostate-specific antigen; SM, surgical margin; SRT, salvage radiation therapy; SVI, seminal vesicle invasion.
1 SRT + docetaxel patient is missing these data, but this did not impact the Stephenson calculation. This pair is excluded from the testing.
Toxicity
Seventeen of 19 patients (89%) completed all 8 cycles of docetaxel. All but 1 of these patients received the full dose of docetaxel at each cycle. For the 2 patients who did not complete all 8 cycles, 1 patient completed 6 (possibly related upper gastrointestinal bleed), whereas the other completed 4 cycles (persistent neutropenia). Six patients (32%) had documented serious adverse events. Four were grade 3 lymphopenia, 1 was a grade 3 upper gastrointestinal bleed, and the last was grade 2 hematochezia. A complete list of the types of acute toxicities experienced by enrolled patients broken down by grade can be found in Table 2. Overall, 79% of patients experienced at least 1 grade 1 toxicity during treatment, 50% experienced at least 1 grade 2 toxicity, 58% had at least 1 grade 3 toxicity, and 2 patients experienced a grade 4 toxicity, both of which were grade 4 neutropenia.
Table 2.
SRT + docetaxel acute toxicity summary by grade
| Grade 1 (63%) | Grade 2 (18%) | Grade 3 (16%) | Grade 4 (3%) |
|---|---|---|---|
| Fatigue (n = 12, 26%) | Neutropenia (n = 5, 38%) | Neutropenia (n = 11, 84%) | Neutropenia (n = 2, 100%) |
| Bowel frequency/urgency/form (n = 9, 20%) | Fatigue (n = 3, 23%) | Bowel frequency/urgency/form (n = 1, 8%) | |
| Urinary frequency/urgency (n = 6, 13%) | Urinary frequency/urgency (n = 2, 15%) | Upper GI bleed (n = 1, 8%) | |
| Other (n = 6, 13%) | Anemia (n = 1, 8%) | ||
| Hematochezia (n = 4, 9%) | Rectal (n = 1, 8%) | ||
| Neuropathy (n = 4, 9%) | Weight loss (n = 1, 8%) | ||
| Thrombocytopenia (n = 3, 7%) | |||
| Anemia (n = 2, 4%) |
GI, gastrointestinal; SRT, salvage radiation therapy.
Late toxicities (>90 days from treatment end) were less common, with the majority of late toxicities being continuation of acute toxicities >90 days. All late toxicities were either grade 1 or 2 in severity (68% grade 1). A listing of late toxicities can be found in Table 3. Bowel and urinary toxicities accounted for 88% of the late toxicities.
Table 3.
SRT + docetaxel late toxicity (no grade >2 toxicities observed)
| Toxicity | Grade 1, n (% of patients) | Grade 2, n (% of patients) |
|---|---|---|
| Bowel | ||
| Bowel frequency/urgency/form | 6 (31.6) | 1 (5.3) |
| Proctitis | 1 (5.3) | 3 (15.8) |
| Rectal incontinence | 1 (5.3) | 1 (5.3) |
| Urinary | ||
| Worsening urinary incontinence | 3 (15.8) | 0 (0) |
| Cystitis | 0 (0) | 2 (10.5) |
| Urinary hesitancy | 1 (5.3) | 1 (5.3) |
| Urinary urgency | 2 (10.5) | 0 (0) |
| Urinary stricture | 0 (0) | 1 (5.3) |
| Worsening erectile dysfunction | 2 (10.5) | 0 (0) |
| Peripheral neuropathy | 1 (5.3) | 0 (0) |
SRT, salvage radiation therapy.
PFS
Based on Gleason score, pre-SRT PSA, surgical margin status, and PSA doubling time, the 4-year PFS estimate per Stephenson et al8 for the patients enrolled was 39.8%. Actual 4-year PFS was 42% (90% CI, 24-60). There was no difference in rates of 4-year PFS or metastases among patients with 1 or more of the features seminal vesicle involvement, extracapsular extension, or positive surgical margins compared with patients without any of these features (48.8% vs 41.1%, P = .75, and 73.9% vs 82.4%, P = .34, respectively). Twelve of 19 patients (63.2%) obtained a complete biochemical response (PSA nadir ≤0.1 ng/mL). To date, 8 patients have not experienced a biochemical recurrence. All 7 patients who did not obtain a PSA nadir ≤0.1 ng/mL have had a biochemical recurrence. No patients had evidence of a local recurrence, and there were no clinical failures. Only 1 patient enrolled on this trial has since died; this was not related to prostate cancer. As such, prostate cancer–specific and overall survival at 4 years following treatment were 100% and 94.7%, respectively.
Matched-pair analysis
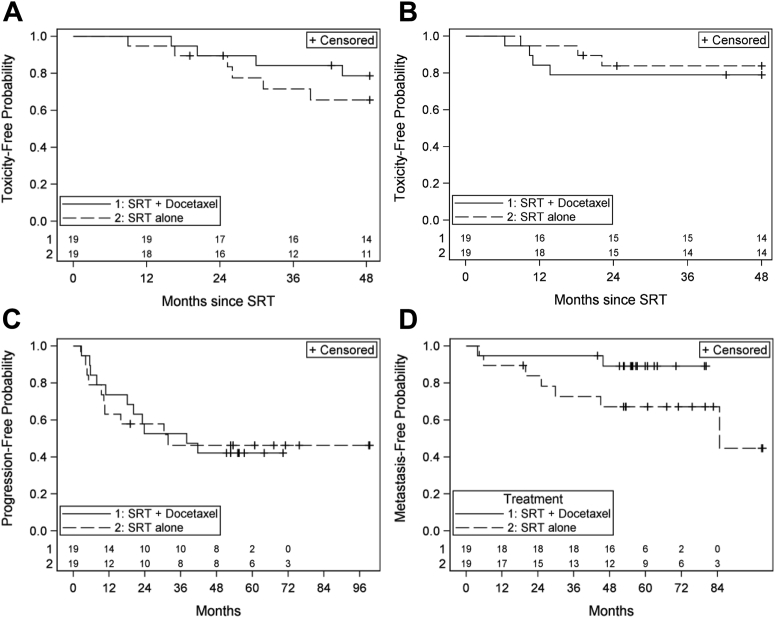
We next compared clinical outcomes and rates of late grade 2+ toxicities between enrolled men and their matched pairs (Fig 1 A-D). There was no statistically significant difference between the 2 groups with regard to either late grade 2+ bowel or urinary toxicity (both P > .4). For rates of BF and DM, there was no statistically significant difference between treatment groups for either endpoint, although there appeared to be a trend favoring docetaxel with regards to DM (P = .09; HR, 0.29 [90% CI, 0.07-1.22]).
Figure 1.
(A) Freedom from grade 2+ late urinary toxicity (P = .41) and (B) late rectal toxicity (P = .60). Freedom from (C) biochemical progression (HR, 098 [90% CI, 0.41-2.35], P = .9648) and (D) from metastasis (HR 0.29 [90% CI, 0.07-1.22], P = .918. CI, confidence interval; HR, hazard ratio; SRT, salvage radiation therapy.
Quality of life
Sixteen of 19 patients enrolled on the trial completed posttreatment EPIC questionnaires during the course of their follow-up, with all patients completing a questionnaire approximately 2-years posttreatment. Pretreatment QOL was not obtained. These results were compared with an institutional cohort of 29 patients treated with SRT to the prostate bed alone during the same period who had completed an EPIC questionnaire approximately 2 years posttreatment (Table 4). Patients treated with docetaxel did report significantly decreased sexual scores compared with the historical cohort treated without docetaxel (P = .03). However, overall docetaxel did not seem to significantly alter 2-year posttreatment QOL. Urinary function scores, urinary bother scores, bowel scores, hormone scores, and overall scores were nearly identical, with no statistically significant difference identified between the 2 groups with respect to these domains.
Table 4.
EPIC 2-y posttreatment quality of life scores
| SRT + docetaxel (n = 16) | SRT alone (n = 29) | P value | |
|---|---|---|---|
| Years post-RT for QOL | |||
| Median (IQR) | 2.2 (2.1-3.1) | 2.3 (2.0-2.9) | .6 |
| Year of RP | |||
| Median (IQR) | 2003 (1999-2006) | 2003 (2002-2007) | .4 |
| SRT dose | |||
| Median (IQR) | 68.4 (68.4-70.2) | 68.4 (64.8-68.4) | .01a |
| Urinary function score (out of 100) | |||
| Median (IQR) | 66.8 (52.8-79.3) | 69.9 (52.3-100) | .5 |
| Urinary bother score (out of 100) | |||
| Median (IQR) | 93.8 (87.5-100) | 93.8 (92.2-100) | .6 |
| Bowel score (out of 100) | |||
| Median (IQR) | 97.9 (90.0-100) | 100 (90.6-100) | .9 |
| Sexual score (out of 100) | |||
| Median (IQR) | 5.0 (0-24.5) | 36.1 (20.8-70.8) | .03 |
| Hormone score (out of 100) | |||
| Median (IQR) | 100 (91.3-100) | 100 (85.0-100) | .8 |
| Overall QOL score (out of 100) | |||
| Median (IQR) | 78.4 (68.1-89.6) | 82.1 (71.0-91.6) | .3 |
IQR, interquartile range; QOL, quality of life; RP, radical prostatectomy; SRT, salvage radiation therapy.
Statistically significant difference between mean SRT dose (68.5 Gy for SRT + docetaxel vs 66.9 Gy for SRT alone).
Discussion
The use of docetaxel has been evaluated broadly in prostate cancer, originally in men with advanced androgen-independent disease and more contemporarily in earlier disease stages. Initial enthusiasm for docetaxel in the treatment of prostate cancer stemmed from 2 prospective randomized trials demonstrating improved overall survival among men with metastatic androgen-independent disease with docetaxel-based therapy.4, 5 Recently, preliminary results from the Eastern Cooperative Oncology Group ChemoHormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer revealed that upfront use of docetaxel combined with androgen deprivation therapy (ADT) in men with newly diagnosed metastatic prostate cancer improved overall survival compared with ADT alone,3 although the same results were not obtained in a smaller French trial.10
Given the benefits seen in the metastatic setting, multiple early-phase clinical trials have assessed the addition of docetaxel to definitive RT plus ADT for the treatment of localized high-risk prostate cancer because these men have poor long-term outcomes even with the addition of ADT to radiation. Results from these single-arm studies demonstrate acceptable rates of early and late toxicity and some preliminary evidence for improved biochemical control compared with expected PSA relapse rates based on historical controls.11, 12, 13, 14, 15, 16 The true efficacy of a combination of docetaxel, definitive RT, and ADT is being assessed in the phase 3 randomized Radiation Therapy Oncology Group (RTOG) 0521 trial with a control arm of radiation plus ADT and a primary outcome of overall survival.17 The trial met its accrual goal in 2009; preliminary results demonstrated an improvement in 4-year overall survival (HR, 0.70; P = .04) and decreased cumulative incidence of metastases (P = .05) in favor of the addition of docetaxel, without demonstrating any benefit in rates of BF (P = .19) (NCT00288080).18
Men with rising PSA levels after prostatectomy are also at high risk for biochemical recurrence and metastatic progression.19 To our knowledge, our findings are the first dedicated report of docetaxel in combination with SRT for men with a rising postprostatectomy PSA level. We demonstrated that, although acute treatment-related toxicities were common, grade 1 toxicities were most commonly related to either fatigue, change in bowel frequency/urgency/form, or urinary frequency/urgency, and grade 2 toxicities were related to neutropenia, fatigue, or change in urinary habits. The majority (87%) of grade 3-4 acute toxicities was related to neutropenia and was transient in nature. There were no cases of febrile neutropenia. Late toxicities were less common and most frequently were secondary to acute toxicities persisting >90 days. More important, the rate of late urinary toxicities do not appear to be significantly increased with the addition of docetaxel compared with previous reports at 2 years posttreatment, with patients in our trial having a 10% rate of grade 2 or greater urinary toxicity at 2 years compared with 7% as reported by Feng et al in a large multi-institutional analysis of late toxicities following postprostatectomy radiation.20 Bowel toxicity was increased in our study with an estimated 22% of patients having a grade 2 or greater toxicity at 2 years compared with 3% as reported by Feng et al.20 The use of postprostatectomy docetaxel in combination with RT has also recently been assessed in a small cohort of men receiving adjuvant RT by Gutilla et al, who similarly showed an acceptable toxicity profile.13 Finally, 2-year posttreatment QOL was comparable to patients treated at the same institution over the same time frame except perhaps with decreased sexual QOL; however, the lack of pretreatment QOL significantly limits the strength of this conclusion and prospective validation is required. The impact of the addition of docetaxel to ADT on sexual QOL has been assessed in a group of men with hormone-naïve metastatic prostate cancer and was not shown to have a negative impact21; however, it is possible that in the absence of concurrent ADT that docetaxel may have an impact on sexual QOL. Future studies are needed to assess the impact of docetaxel on QOL for men receiving postprostatectomy RT.
Though we were unable to demonstrate a statistically significant improvement in the rate of biochemical recurrence compared with our 4-year predicted rate, this may be in large part because our study was significantly underpowered to detect differences in rates of BF of our expected magnitude, which was a 20% reduction in 4-year rates of BF. Although we did not demonstrate a statistically significant difference in rates of distant metastasis (0.3 [0.07-1.2]), our findings appear suggestive that an impact of concurrent radiation and docetaxel on development of metastatic disease may be present and suggest further evaluation in a larger trial is warranted. Given the findings of improved overall survival with decreased rates of metastatic disease without affecting rates of BF observed in the preliminary results of RTOG 0521, it is possible that our observed lack of benefit with regards to BF and trend toward decreased rates of metastasis are characteristic of treatment with combined RT and docetaxel.
Recently, the RTOG reported the results of a study combining salvage radiation, concurrent 6-month androgen ablation, and sequential adjuvant docetaxel (RTOG 0621). This single-arm multi-institutional trial of 76 patients demonstrated an approximate 20% improvement in the 3-year rate of freedom from BF compared with historical controls.22 There were several key differences between the designs of RTOG 0621 and our presented trial. RTOG 0621 treated men with adjuvant radiation combined with 6 months of concurrent ADT, followed by sequential docetaxel, at a higher dosing than we treated our patients with concurrently (75 mg/m2 every 21 days [RTOG 0621] vs 20 mg/m2 every week). Although median pre-RT PSA levels were similar in both studies (median 0.7 vs 0.6 ng/mL), 58% of patients in ROTG 0621 had positive surgical margins compared with only 26% in our study, and as such may have been more likely to have disease localized to the prostate bed alone. Additionally, multiple research groups have proposed that treatment with taxanes impairs androgen receptor activity23, 24, 25; therefore, it is possible that there is a synergistic effect when treating with the combination of ADT and docetaxel and that this interaction drove the improvement in freedom from BF observed in RTOG 0621. Although we were unable to demonstrate any improvement in rates of BF secondary to a lack of statistical power, given the impressive rates of biochemical control in RTOG 0621, future trials of docetaxel combined with salvage RT for recurrent prostate cancer postprostatectomy are warranted.
A notable finding in our analysis is that all men with a PSA nadir >0.1 ng/mL experienced biochemical recurrence. The importance of reaching an undetectable nadir following SRT has previously been highlighted, with patients not achieving an undetectable nadir having decreased overall survival.26, 27
The major limitation of our study is the small sample size which resulted in a lack of power to fully assess our primary outcome of improvement in 4-year rate of BF. Additionally, lack of baseline QOL data prevents complete assessment of changes in QOL associated with docetaxel therapy.
In closing, we demonstrate that concurrent docetaxel with salvage radiation for men with a rising PSA level following prostatectomy appears to be associated with acceptable rates of acute toxicity, of which the most significant was neutropenia, low rates of late toxicity, and minimal changes in overall QOL at 2-years posttreatment compared with historical controls. We were unable to demonstrate improved efficacy of concurrent docetaxel in this setting, possibly because of early closure and a small sample size. Nevertheless, the safety of this regimen as well as the encouraging results with docetaxel combined with ADT in the ChemoHormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer and radiation and ADT in RTOG 0621 suggest that larger clinical trials may be warranted to assess the efficacy of docetaxel when administered concurrently with salvage radiation following prostatectomy.
Footnotes
Conflicts of interest: Docetaxel was supplied by Sanofi-Aventis, and this trial was supported by a grant from Sanofi-Aventis to The University of Michigan Medical Center for the performance of this investigator-initiated trial.
References
- 1.Han M., Partin A.W., Pound C.R. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Jackson W., Hamstra D.A., Johnson S. Gleason pattern 5 is the strongest pathologic predictor of recurrence, metastasis, and prostate cancer-specific death in patients receiving salvage radiation therapy following radical prostatectomy. Cancer. 2013;119:3287–3294. doi: 10.1002/cncr.28215. [DOI] [PubMed] [Google Scholar]
- 3.Christopher S, Chen Y.-H, Carducci MA, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. ASCO Annual Meeting, 2014; Chicago, IL.
- 4.Petrylak D.P., Tangen C.M., Hussain M.H. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock I.F., de Wit R., Barry R. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Milas L., Milas M.M., Mason K.A. Combination of taxanes with radiation: Preclinical studies. Semin Radiat Oncol. 1999;9(Suppl 1):12–26. [PubMed] [Google Scholar]
- 7.Wei J.T., Dunn R.L., Litwin M.S. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson A.J. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25(15):2035–2041. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson A.J., Shariat S.F., Zelefsky M.J. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 10.Gravis G., Fizazi K., Joly F. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 11.Bolla M., Hannoun-Levi J.M., Ferrero J.M. Concurrent and adjuvant docetaxel with three-dimensional conformal radiation therapy plus androgen deprivation for high-risk prostate cancer: Preliminary results of a multicentre phase II trial. Radiother Oncol. 2010;97:312–317. doi: 10.1016/j.radonc.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Chen R.C., Rosenman J.G., Hoffman L.G. Phase I study of concurrent weekly docetaxel, high-dose intensity-modulated radiation therapy (IMRT) and androgen-deprivation therapy (ADT) for high-risk prostate cancer. BJU Int. 2012;110:E721–E726. doi: 10.1111/j.1464-410X.2012.11536.x. [DOI] [PubMed] [Google Scholar]
- 13.Guttilla A., Bourtolus R., Ciannarini G. Multimodal treatment for high-risk prostate cancer with high-dose intensity-modulated radiation therapy preceded or not by radical prostatectomy, concurrent intensified-dose docetaxel and long-term androgen deprivation therapy: Results of a prospective phase II trial. Radiat Oncol. 2014;9:24. doi: 10.1186/1748-717X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P., Perrotti M., Weiss R. Phase I trial of weekly docetaxel with concurrent three-dimensional conformal radiation therapy in the treatment of unfavorable localized adenocarcinoma of the prostate. J Clin Oncol. 2004;22:1909–1915. doi: 10.1200/JCO.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Marshall D.T., Ramey S., Golshavan A.R. Phase I trial of weekly docetaxel, total androgen blockade, and image-guided intensity-modulated radiotherapy for localized high-risk prostate adenocarcinoma. Clin Genitourin Cancer. 2014;12:80–86. doi: 10.1016/j.clgc.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Perrotti M., Doyle T., Kumar P. Phase I/II trial of docetaxel and concurrent radiation therapy in localized high risk prostate cancer (AGUSG 03-10) Urol Oncol. 2008;26:276–280. doi: 10.1016/j.urolonc.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Patel A.R., Sandler H.M., Pienta K.J. Radiation Therapy Oncology Group 0521: A phase III randomized trial of androgen suppression and radiation therapy versus androgen suppression and radiation therapy followed by chemotherapy with docetaxel/prednisone for localized, high-risk prostate cancer. Clin Genitourin Cancer. 2005;4:212–214. doi: 10.3816/cgc.2005.n.035. [DOI] [PubMed] [Google Scholar]
- 18.Sandler HM, Hu C, Rosenthal SA, et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521). 2015 ASCO Annual Meeting, 2015; Chicago IL.
- 19.Johnson S., Jackson W., Speers C. A comprehensive assessment of the prognostic utility of the Stephenson nomogram for salvage radiation therapy postprostatectomy. Pract Radiat Oncol. 2014;4:422–429. doi: 10.1016/j.prro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Feng M., Hanlon A.L., Pisansky T.M. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1417–1423. doi: 10.1016/j.ijrobp.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Joly F, Gravis G, Oudard S, et al. Patients' self-assessment of tolerance and quality of life during docetaxel-based chemotherapy: Results from a phse III trial in hormone-naive metastatic prostate cancer patients (GETUG-AFU 15/0403). ASCO Annual Meeting, 2010.
- 22.Hurwitz M, Sartor AO, Zhang Q, et al. Adjuvant radiation, androgen deprivation, and docetaxel for highrisk prostate cancer postprostatectomy: Results of RTOG 0621. ASTRO Annual Meeting, 2014; San Francisco, CA.
- 23.Darshan M.S., Lofts M.S., Thadani-Mulero M. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick J.M., de Wit R. Taxane mechanisms of action: Potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:1198–1204. doi: 10.1016/j.eururo.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Gan L., Chen S., Wang Y. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69:8386–8394. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geinitz H., Riegel M.G., Thamm R. Outcome after conformal salvage radiotherapy in patients with rising prostate-specific antigen levels after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82:1930–1937. doi: 10.1016/j.ijrobp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Jackson W.C., Johnson S.B., Foster B. Combining prostate-specific antigen nadir and time to nadir allows for early identification of patients at highest risk for development of metastasis and death following salvage radiation therapy. Pract Radiat Oncol. 2014;4:99–107. doi: 10.1016/j.prro.2013.05.008. [DOI] [PubMed] [Google Scholar]