Abstract
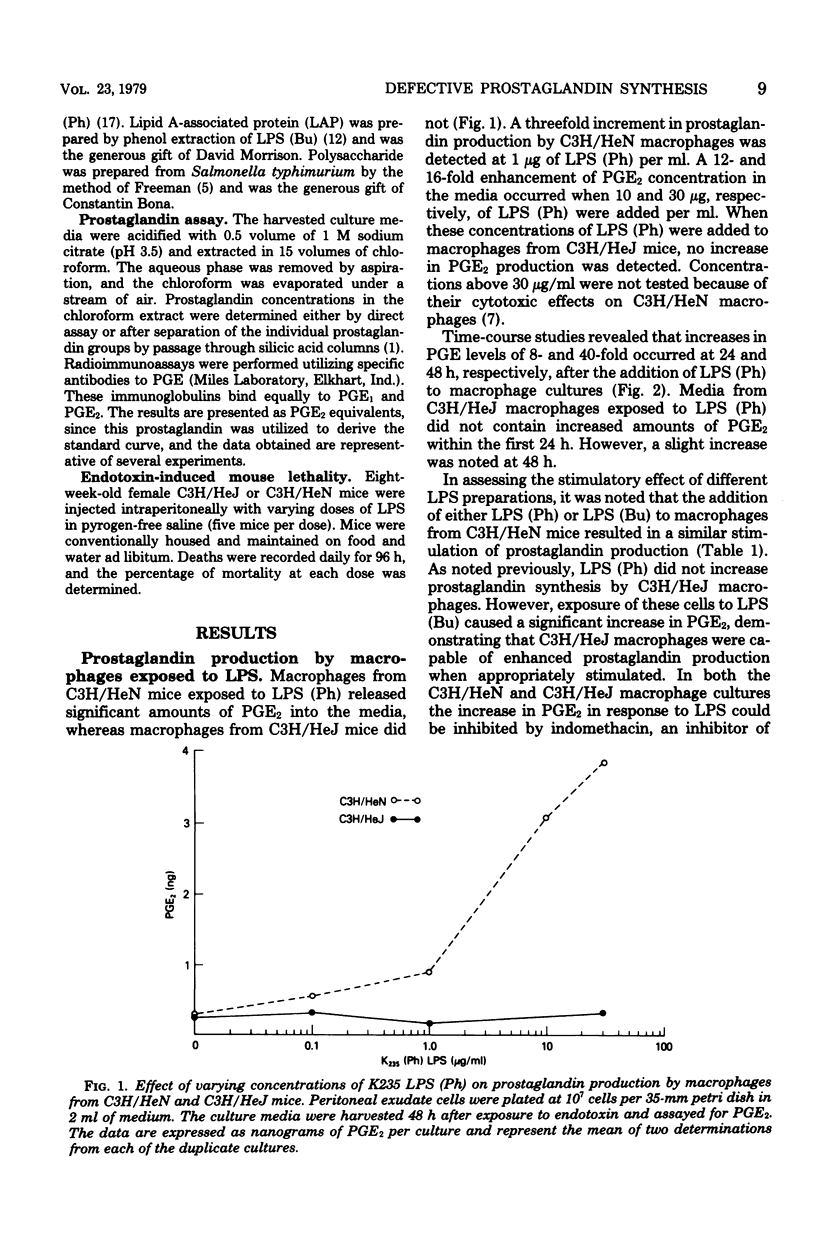
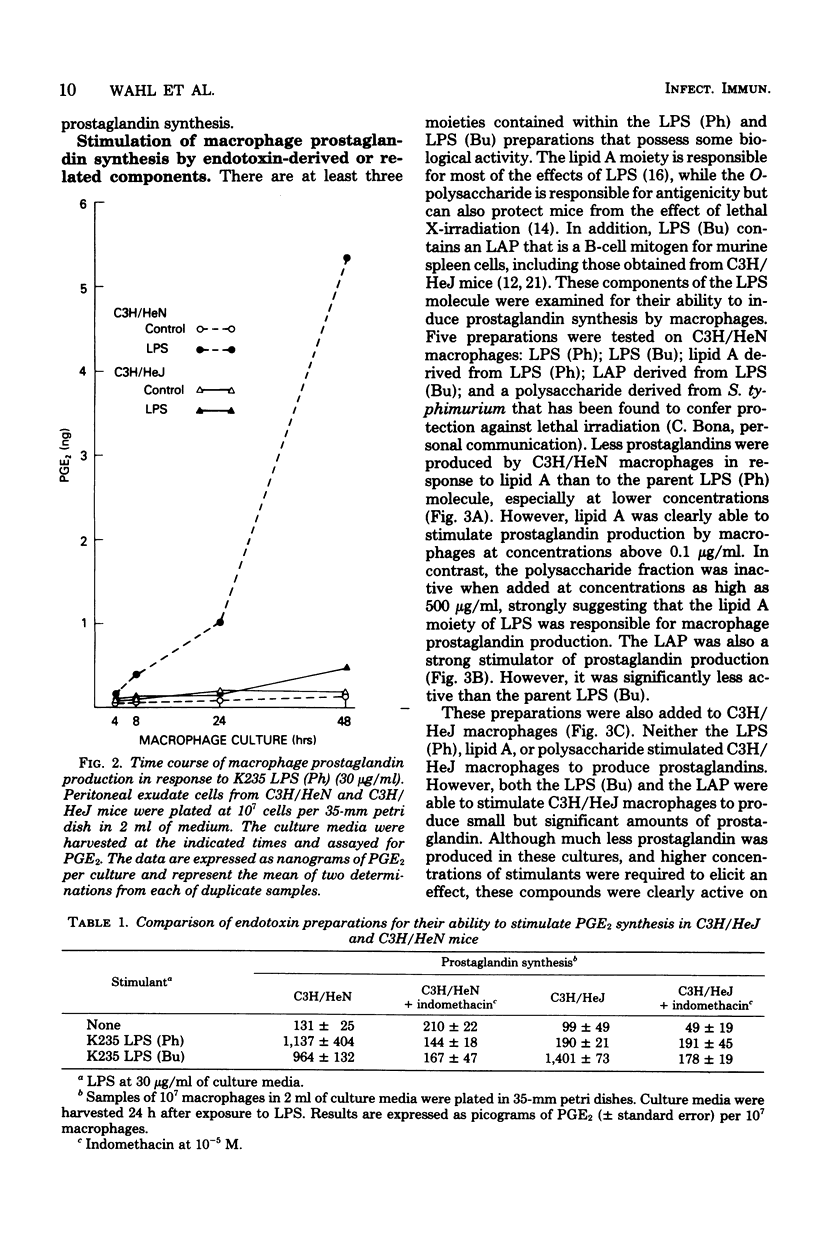
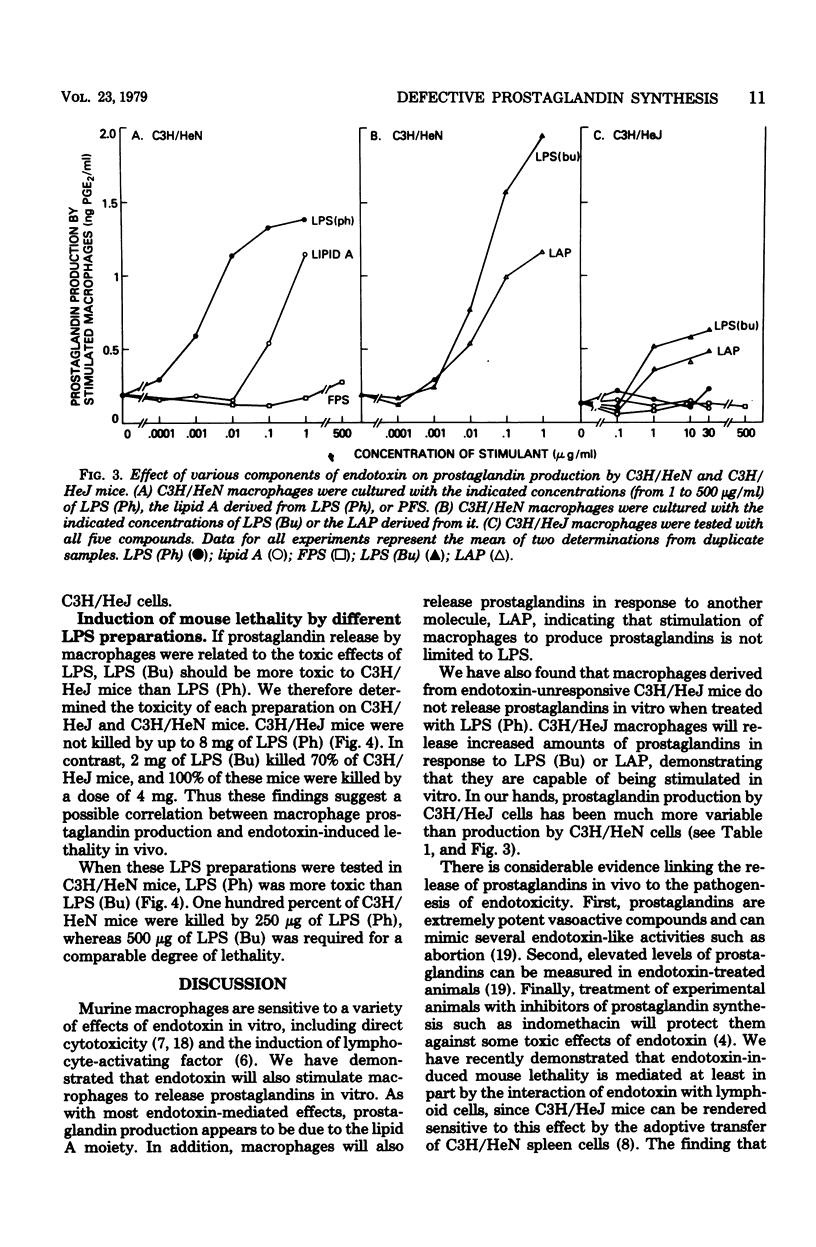
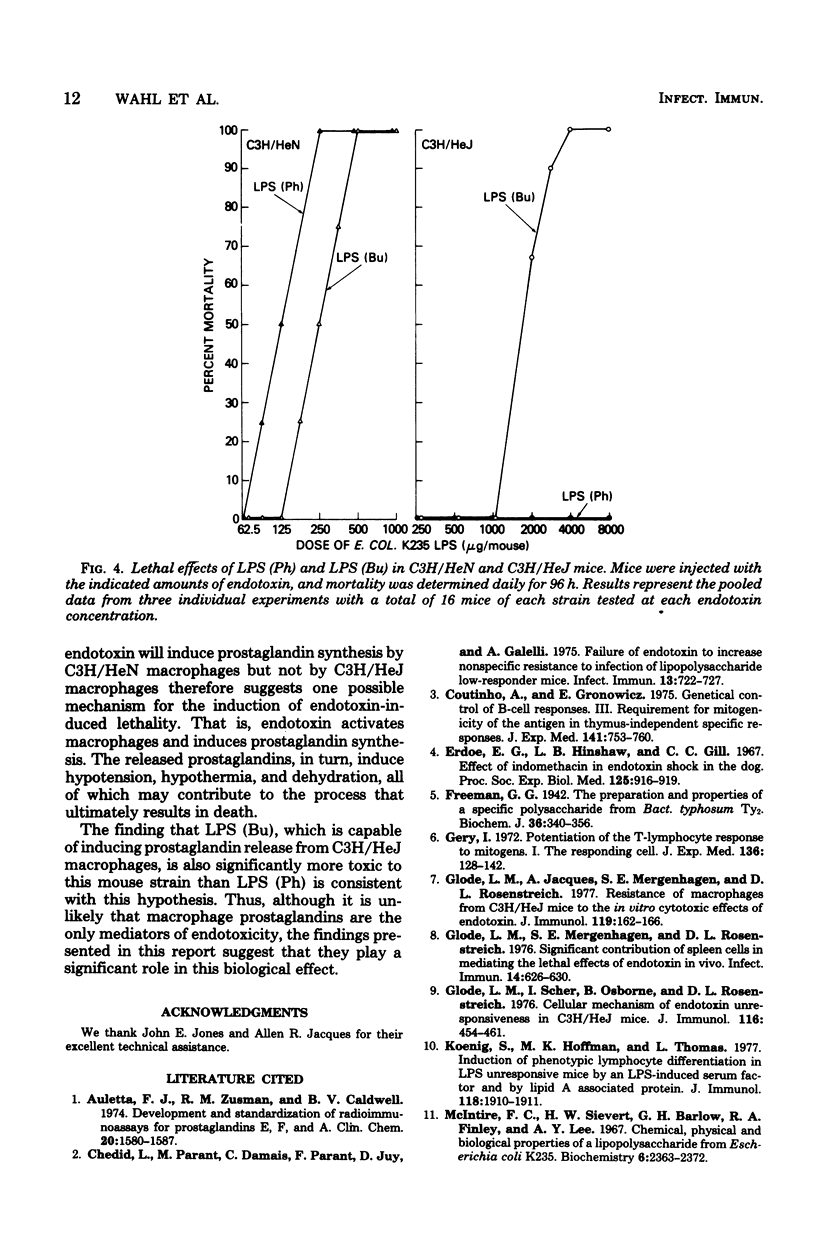
Macrophages obtained from C3H/HeN mice produced significant amounts of prostaglandin E when exposed to phenol-extracted lipopolysaccharides (LPS), whereas macrophages from C3H/HeJ mice were unresponsive. The lipid A fraction from phenol-extracted LPS was an effective inducer or prostaglandin synthesis by macrophages from C3H/HeN mice. The polysaccharide portion of the LPS molecule had no effect. In contrast, the C3H/HeJ macrophages did not produce prostaglandin E in response to the lipid A moiety of phenol-extracted LPS. LPS prepared by butanol extraction stimulated the production of prostaglandin E by macrophages from both C3H/HeN and C3H/HeJ mice. The component of butanol-extracted LPS that stimulated the C3H/HeJ macrophages was shown to be a lipid A-associated protein. Further studies demonstrated a correlation between prostaglandin production by the macrophages of these two strains of mice in response to butanol- and phenol-extracted LPS and the lethal effects of the endotoxin preparations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auletta F. J., Ausman R. M., Caldwell B. V. Development and standardization of radioimmunoassays for prostaglandins E, F, and A. Clin Chem. 1974 Dec;20(12):1580–1587. [PubMed] [Google Scholar]
- Chedid L., Parant M., Damais C., Parant F., Juy D., Galelli A. Failure of endotoxin to increase nonspecific resistance to infection of lipopolysaccharide low-responder mice. Infect Immun. 1976 Mar;13(3):722–727. doi: 10.1128/iai.13.3.722-727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E. Genetical control of B-cell responses. III. Requirement for functional mitogenicity of the antigen in thymus-independent specific responses. J Exp Med. 1975 Apr 1;141(4):753–760. [PMC free article] [PubMed] [Google Scholar]
- Erdös E. G., Hinshaw L. B., Gill C. C. Effect of indomethacin in endotoxin shock in the dog. Proc Soc Exp Biol Med. 1967 Jul;125(3):916–919. doi: 10.3181/00379727-125-32239. [DOI] [PubMed] [Google Scholar]
- Freeman G. G. The preparation and properties of a specific polysaccharide from Bact. typhosum Ty(2): With an addendum by J. St L. Philpot, From the Department of Biochemistry, Oxford. Biochem J. 1942 Apr;36(3-4):340–356. doi: 10.1042/bj0360340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Jacques A., Mergenhagen S. E., Rosenstreich D. L. Resistance of macrophages from C3H/HeJ mice to the in vitro cytotoxic effects of endotoxin. J Immunol. 1977 Jul;119(1):162–166. [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Scher I., Osborne B., Rosenstreich D. L. Cellular mechanism of endotoxin unresponsiveness in C3H/HeJ mice. J Immunol. 1976 Feb;116(2):454–461. [PubMed] [Google Scholar]
- Koenig S., Hoffmann M. K., Thomas L. Induction of phenotypic lymphocyte differentiation in LPS unresponsive mice by an LPS-induced serum factor and by lipid A-associated protein. J Immunol. 1977 May;118(5):1910–1911. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Nowotny A., Behling U. H., Chang H. L. Relation of structure to function in bacterial endotoxins. VIII. Biological activities in a polysaccharide-rich fraction. J Immunol. 1975 Jul;115(1):199–203. [PubMed] [Google Scholar]
- Rosenstreich D. L., Glode L. M. Difference in B cell mitogen responsiveness between closely related strains of mice. J Immunol. 1975 Sep;115(3):777–780. [PubMed] [Google Scholar]
- Rosenstreich D. L., Nowotny A., Chused T., Mergenhagen S. E. In vitro transformation of mouse bone-marrow-derived (B) lymphocytes induced by the lipid component of endotoxin. Infect Immun. 1973 Sep;8(3):406–411. doi: 10.1128/iai.8.3.406-411.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Gormus B. J., McGraw J. In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun. 1974 Jan;9(1):106–112. doi: 10.1128/iai.9.1.106-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C., Harper M. J. Relationship between endotoxin-induced abortion and the synthesis of prostaglandin F. Prostaglandins. 1972 Mar;1(3):191–203. doi: 10.1016/0090-6980(72)90004-4. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]



