Abstract
Background:
Overview of clinical impact of positron emission tomography-computed tomography (PET-CT) scans in patients with head and neck carcinomas at our center.
Methods:
Retrospective review of posttreatment 18F-fluorodeoxyglucose (18F-FDG) PET-CT scans in patients with head and neck carcinomas with risk of residual disease. Clinical outcome served as the reference standard.
Results:
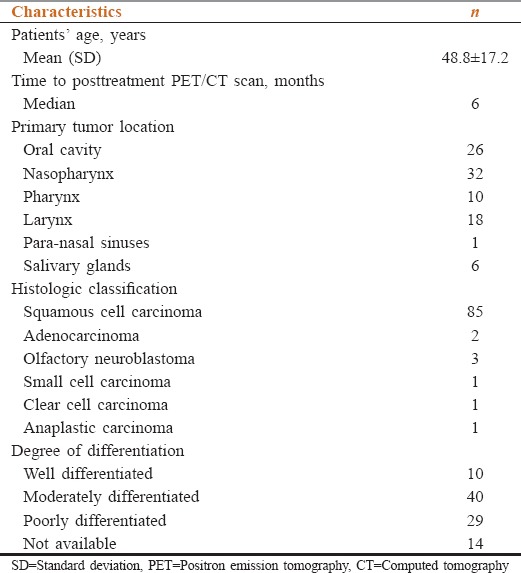
This study included 93 patients (65.6% males, mean age: 48.8 years ± 17.2 standard deviation) with squamous cell carcinoma as most frequent histopathology (91.4%). PET-CT scans were performed on average 6 months posttreatment. Diagnostic accuracy, positive predictive value, and negative predictive value of PET-CT for disease were found to be 88%, 88%, and 92%, respectively. A median follow-up of 24 months was available for 91 patients. Kaplan–Meier curves showed significantly higher disease-free survival with negative PET-CT as compared to positive PET-CT (P = 0.01) and maximum standardized uptake values of <5.0 (P = 0.01).
Conclusion:
FDG PET-CT has diagnostic and prognostic implications in treated patient of head and neck cancers.
Keywords: 18F-fluorodeoxyglucose positron emission tomography-computed tomography scan, disease-free survival, head and neck cancer, maximum standardized uptake values, squamous cell carcinoma
Introduction
Approximately, 550,000 cases of head and neck carcinomas are reported annually worldwide.[1] The diagnostic workup for head and neck cancers is accomplished with computed tomography (CT) and magnetic resonance imaging delineating the anatomical details and extent of tumor. The metabolic and functional imaging with positron emission tomography-CT (PET-CT) is usually reserved for the assessment of nodal involvement, distant metastasis, treatment response evaluation, and disease recurrence.[2]
The accuracy of hybrid PET-CT imaging is superior to the conventional anatomic imaging for restaging and radiotherapy planning in squamous cell carcinomas. A negative fluorodeoxyglucose (FDG) PET-CT scan acquired 2–3 months postchemoradiotherapy has high-negative predictive value (NPV). This prevents overtreatment with neck dissection and unnecessary diagnostic workup.[3]
This study was conducted to review and analyze the clinical impact of the FDG PET-CT scans in carcinomas of head and neck in our oncological referral.
Methods
A consecutive, retrospective cohort of database was reviewed comprising 18F-FDG PET-CT scans acquired in patients with carcinomas of head and neck with high clinical risk of residual or recurrent disease after chemoradiotherapy during. Electronic data of 93 patients, from January’ 2010 to June 2014, were analyzed, available on institutional medical record system. At our institute, PET-CT is only employed in cases when there is a question of residual/recurrent disease on conventional imaging at least 4–6 months after the completion of chemoradiotheray. Median time for PET-CT scans from completion of chemoradiotherapy was 6 months, excluding the chances of posttherapy metabolic changes. PET images were quantitatively (visual appearance and intensity of FDG activity in the lesion under question, lesser or greater than that of the liver) and qualitatively (maximum standardized uptake values [SUVmax]) were assessed by the qualified nuclear physicians and radiologists with at least 5 years’ experience at hand.
SUVmax values were compared amongst different histopathologies, degree of differentiation, and primary tumor location to identify any statistical correlation using Pearson's correlation coefficient. In all cases, statistical significance was accepted for P < 0.05.
The data set were evaluated for the presence or absence of metabolically active residual disease on 18F-FDG PET-CT scans in terms of frequency, identifying true positive, true negative, false positive, and false negative based on the clinical outcome and histopathological correlation as available. The data were sub-classified as posttreatment PET-CT positive and PET-CT negative groups.
Kaplan–Meier survival analyses curves were generated using SPSS program (SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago) to assess disease-free survival (DFS), in terms of PET-CT outcome and SUVmax values. Survival differences between groups were evaluated by the log-rank test with a significance level of P < 0.05.
Results
A total of 93 patients with head and neck cancer underwent 18F-FDG PET-CT during the study period. Of these, 61 (65.6%) were males and 32 (34.4%) females [Table 1].
Table 1.
Patients’ and disease characteristics (n=93)
18F-fluorodeoxyglucose positron emission tomography-computed tomography scan outcome
About 68/93 had positive posttreatment PET-CT scans. In sixty patients, metabolically active residual disease (true positive) was identified (active residual n = 33; disease progression n = 27). One PET-CT scan revealed false positive FDG uptake in cervical node in a case of carcinoma buccal mucosa, which turned out to be a benign histopathology on biopsy. Similarly, FDG uptake without corresponding morphologic abnormality was reported in seven scans, correlating with nonspecific postradiotherapy changes.
About 25/93 had negative posttreatment PET-CT scans. FDG nonavid morphologic residual was reported in five cases (true negative) while 18 were metabolically and morphologically unremarkable. False negative results were reported in two cases. An ill-defined soft tissue in a case of nasopharyngeal carcinoma with FDG uptake, which was labeled as reactive, however it turned out to be to be active disease on postsurgical histopathology. Similarly, FDG nonavid residual in one case of laryngeal carcinoma showed disease on postsurgical histopathology.
Accuracy, positive predictive value (PPV), and NPV of PET-CT for the evaluation of persistent disease were found to be 88%, 88%, and 92%, respectively.
On bivariate analysis, no significant correlation was found between the histopathology and SUVmax (P = 0.001, insignificant as the data set was skewed) or degree of differentiation and the SUVmax of the primary tumor (P = 0.690).
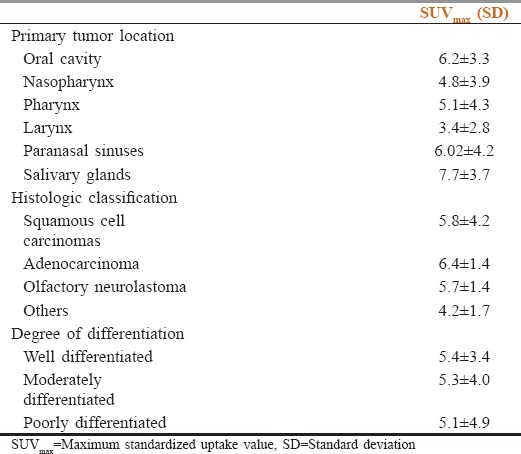
No correlation was seen between the SUVmax of the primary lesion and the location of tumor (P = 0.142). The SUV ranges in reference to the tumor location and histopathology are reported in Table 2.
Table 2.
Standardized uptake value ranges in reference to the tumor location and histopathology
The patients with FDG avid residual disease were further treated with chemoradiation (39.2%), surgical resection (10%), or palliative management (50.7%). Patients with negative PET-CT scans were put on follow-up.
Disease-free survival based on positron emission tomography-computed tomography findings and maximum standardized uptake values
DFS patients with posttreatment PET-CT scans was estimated over a median duration of 24 months, with maximum available duration of 57 months after PET-CT scan. Out of sixty patients whose posttreatment PET-CT scan demonstrated avid disease, 11 got cured and fifty were not cured (35 alive with disease, 15 died during the study period). No follow-up is available for seven patients.
Of 25 patients whose posttreatment PET-CT scan demonstrated no disease, 17 are alive, and cured, 5 alive with disease, and 3 patients died during the study period Figure 1.
Figure 1.
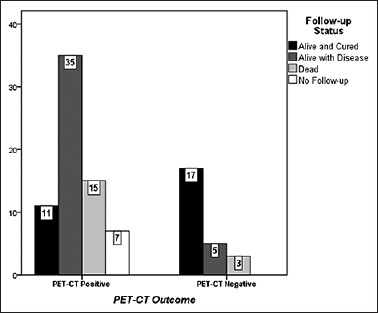
Comparison of follow-up status between posttreatment fluorodeoxyglucose positron emission tomography-computed tomography positive and negative groups
The overall 3-year DFS was found to be higher (71%) in patients with negative PET-CT scan as compared to positive PET-CT scan (11%) after treatment. The difference in survival between the two groups was statistically significant (log-rank test; P < 0.01). The Kaplan–Meier survival curve is shown in Figure 2.
Figure 2.
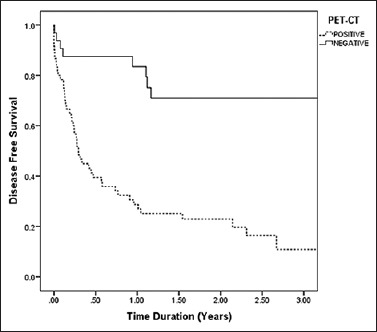
Kaplan–Meier 3-year disease free survival curve in patients with posttreatment positron emission tomography-computed tomography findings (positive positron emission tomography-computed tomography scan = 11%, negative positron emission tomography-computed tomography scan = 71%). The log-rank test shows a significant difference in the percent survival (P < 0.001)
Based on SUVmax values on PET-CT scans, DFS rates were SUVmax <5 = 62%, SUVmax5–10 = 42%, and SUVmax >10 = 6%. The log-rank test showed a statistically significant difference in the percent survival (P < 0.01) Figure 3.
Figure 3.
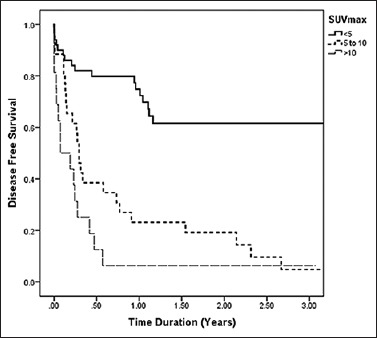
Kaplan–Meier 3-year disease free survival curve based on maximum standardized uptake values on positron emission tomography-computed tomography (<5 = 62%, 5–10 = 42%, >10 = 6%). Log-rank test P < 0.001
Discussion
Head and neck tumors demonstrate heterogeneity in terms of location and histopathology. However, squamous cell carcinoma is the most frequently encountered histopathology in head and neck cancers, which has been reported in approximately more than 90% of cases.[4] Similar high frequency (91.4%) of squamous cell carcinomas was observed in our data group. Furthermore, varied histology such as adenocarcinoma, neuroblastoma, small cell, clear cell, and anaplastic carcinomas enabled us to evaluate FDG avidity in these not very frequently seen tumors. Standardized uptake value, taken as an indicator for the degree of metabolic activity, remained inconclusive in terms of identifying any statistically significant difference between histopathological classification, degree of differentiation (P = 0.690), or tumor location (P = 0.142). Overall, 26.5% response rate was seen on postchemoradiotherapy PET-CT scans. We found 88% accuracy of PET-CT in detecting the viable residual disease. The PPVs and NPVs were 77% and 92%, respectively. Gupta et al. in a meta-analysis review reported the similar low PPV (58.6%) and exceptionally high NPV (95.1%). This lower PPV is the result of posttherapy inflammatory changes frequently observed in head and neck cancer patients.[5]
In our center, we perform posttreatment PET-CT scan at least 4–6 months after the completion of chemoradiotherapy. Average time observed in this data group was 6 months. This complies with the general consensus that this optimum time before 18F-FDG PET-CT allows the posttherapy changes to settle down, thereby reducing false positive results.[3]
The high NPV of FDG PET-CT, ruling out the presence of viable disease, helps in decision making regarding further treatment or surveillance. As suggested by Porceddu et al. and Ware et al., PET-CT has added value in restaging patients with morphological residual disease after definitive treatment, modifying decision regarding surgical interventions.[6,7]
Despite high NPV, the controversies still exist where there is a question of microscopic disease. In our cohort, 32% of patients with negative posttherapy PET-CT scan developed recurrent disease within 6–12 months. All these patients had higher stage with moderate to poorly differentiated histology, to begin with. This fact highlights that FDG PET-CT cannot be the sole factor in decision making and other factors such as initial stage and histology of the primary tumor remain the mainstay in prognostication.
We found favorable prognosis in patients with negative posttreatment PET-CT scan. Overall, 3-year DFS rate for negative PET-CT (71%) was higher than positive PET-CT scan (11%).
Ito et al. and Torizuka et al. has reported that SUVmax can provide prognostic survival estimates of at least 2 years.[8,9] Similarly, in our analysis, better prognosis was observed in patients with lower SUVmax values as compared to those with higher SUVmax values (P < 0.01). Metabolic response indicates the aggressive behavior of the disease, necessitating aggressive treatment approach. As most of the recurrent disease is seen during 1st year posttreatment,[3] induction of PET-CT as follow-up after treatment can provide early indication of metabolically active residual or recurrent disease. This coupled with histopathological evidence has a better prognostic implication.
Limitations of the study
A small cohort database was the major limitation of our study. We had statistically significantly comparable groups in terms of location and degree of differentiation of the primary head and neck carcinoma. However, based on the histopathological classification, our groups were not statistically comparable, since 91% of the patients had squamous cell carcinoma versus other histology seen.
Conclusion
18F-FDG PET-CT has diagnostic and prognostic implications in patients treated for head and neck cancer. With results of our study clearly showing a high NPV in treated patients with head and neck cancer, role of PET-CT is strongly advocated. Statistically significant DFS period also noted among patients with negative posttreatment scans.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Abraham J. Imaging for head and neck cancer. Surg Oncol Clin N Am. 2015;24:455–71. doi: 10.1016/j.soc.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Castaldi P, Leccisotti L, Bussu F, Miccichè F, Rufini V. Role of (18) F-FDG PET-CT in head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2013;33:1–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Sanderson RJ, Ironside JA. Squamous cell carcinomas of the head and neck. BMJ. 2002;325:822–7. doi: 10.1136/bmj.325.7368.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: A systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–95. doi: 10.1007/s00259-011-1893-y. [DOI] [PubMed] [Google Scholar]
- 6.Ware RE, Matthews JP, Hicks RJ, Porceddu S, Hogg A, Rischin D, et al. Usefulness of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with a residual structural abnormality after definitive treatment for squamous cell carcinoma of the head and neck. Head Neck. 2004;26:1008–17. doi: 10.1002/hed.20097. [DOI] [PubMed] [Google Scholar]
- 7.Porceddu SV, Pryor DI, Burmeister E, Burmeister BH, Poulsen MG, Foote MC, et al. Results of a prospective study of positron emission tomography-directed management of residual nodal abnormalities in node-positive head and neck cancer after definitive radiotherapy with or without systemic therapy. Head Neck. 2011;33:1675–82. doi: 10.1002/hed.21655. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Shimoji K, Miyata Y, Kamiya K, Minamimoto R, Kubota K, et al. Prognostic value of post-treatment (18) F-FDG PET/CT for advanced head and neck cancer after combined intra-arterial chemotherapy and radiotherapy. Chin J Cancer Res. 2014;26:30–7. doi: 10.3978/j.issn.1000-9604.2014.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torizuka T, Tanizaki Y, Kanno T, Futatsubashi M, Naitou K, Ueda Y, et al. Prognostic value of 18F-FDG PET in patients with head and neck squamous cell cancer. AJR Am J Roentgenol. 2009;192:W156–60. doi: 10.2214/AJR.08.1429. [DOI] [PubMed] [Google Scholar]


