Abstract
A strain-induced copper-free click reaction mediated by a new and easily prepared cyclooctyne derivative was used to efficiently assemble a DOTA–biotin adduct capable of radionuclide (68Ga) uptake. This synthetic strategy offers a potentially general and convenient means of preparing targeted radiolabeling and radiotherapeutic agents.
Graphical abstract
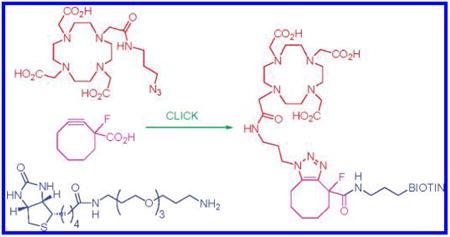
Developing methods to efficiently construct bifunctional materials featuring radionuclide chelators conjugated to specific bioligands is an important objective in contemporary bioconjugate chemistry. The impetus for much of this work derives from the recognition that techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) offer unparalleled sensitivity for in vivo diagnostic imaging of cancer and other diseases. Additionally, targeted cell-specific delivery of radionuclides presents opportunities to establish new radiopharmaceuticals for cancer therapy.1
Derivatives of DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, 1) are metal chelators commonly employed in radionuclide chemistry. Attachment of DOTA to biomolecules is often accomplished through amide bond formation via selective manipulation of one of the acetic acid arms.2 Such preparative routes necessitate the extensive use of protecting groups, resulting in added manipulation (protection/deprotection) and purification steps that decrease overall synthetic efficiency. In this context, an attractive, versatile, and modular approach toward DOTA bioconjugation involves application of high-yielding and bio-orthogonal “click” reactions.
The prototypical click reaction advanced by Sharpless and Meldal is a Cu(I)-catalyzed cycloaddition between azides and terminal alkynes to afford 1,4-disubstituted triazoles.3 The reaction offers a convenient means of conjoining two molecules through functional groups (azide and alkyne) that are easily incorporated into biomolecules and generally compatible with existing biomolecular functionality (i.e., bioorthogonal). As a result, the use of Cu-catalyzed click chemistry in biochemical settings is rapidly increasing.4
Several reports have appeared describing reactions of alkyne-modified DOTA derivatives with various azide-functionalized bioprobes (e.g., azido peptides) in the presence of suitable Cu(I) catalysts.5 In these cases, the metal-chelating ability of the DOTA macrocycle complicates the click cycloaddition by sequestering the Cu catalyst. Additionally, formation of DOTA–Cu adducts also interferes with subsequent radionuclide uptake and binding. These problems have been addressed by performing click reactions on DOTA derivatives protected as tert-butyl esters (which exhibit attenuated metal binding ability) and removing residual copper salts from triazole products by precipitation with Na2S.
An alternative solution that has not been extensively explored is the application of Cu-free variants of click reactions to achieve DOTA bioconjugation.6 While thermally activated cycloadditions between alkynes and azides are well-established, these reactions require prohibitively high temperatures and long reaction times.7 Strained alkynes, however, are known to be much more reactive in [3 + 2] cycloadditions, and Bertozzi et al. have developed several bifunctional cyclooctyne reagents for in vitro and in vivo biomolecular applications (Figure 1).8 Inconveniently slow cycloaddition rates were encountered between cyclooctyne 2 and azide-modified biomolecules,8a prompting development of the difluorinated cyclooctyne 3 as a more reactive analogue that exhibits a kinetic profile well-suited for use in chemical biology settings.8c The dibenzocyclooctyne 4 has also been employed by Boons and co-workers to assemble glycoconjugates for live cell imaging studies.9 Finally, oxanorbornadienes (e.g., 5) have been found to react with azides under physiological conditions via tandem [3 + 2] cycloaddition/retro Diels–Alder sequences, thus providing another approach to Cu-free click chemistry.10
Figure 1.
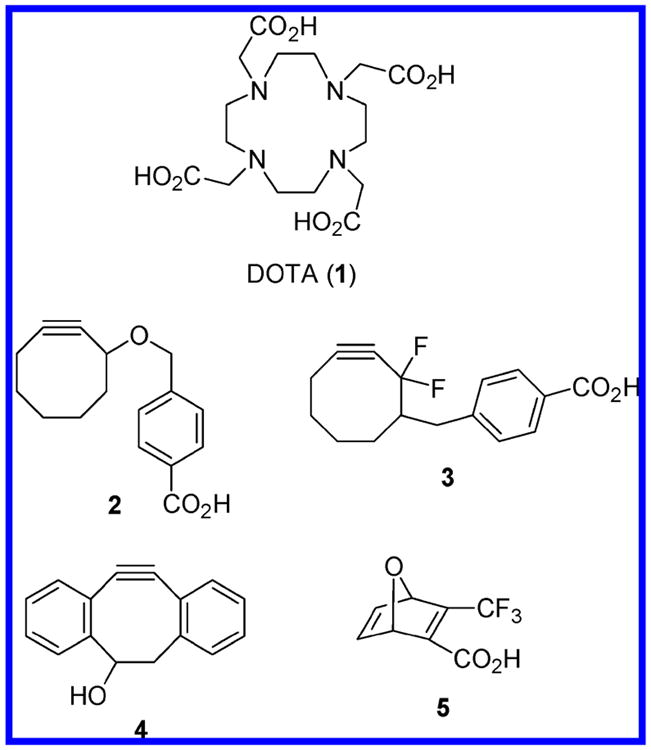
Examples of reagents (2–5) used in Cu-free click chemistry.
We are interested in preparing a variety of DOTA bioconjugates for use as novel radioimaging and radiotherapeutic agents. Toward this end, it is envisioned that preparative methods based on Cu-free click reactions will offer concise and efficient synthetic routes to targeted DOTA derivatives. The strained cycloalkyne and oxanorbornadiene reagents described above, however, exhibit several shortcomings (for example, 4 possesses relatively hydrophobic arene rings, 5 gives rise to unwanted byproduct in the click reaction, and 3 is prepared through a long (∼12 step) synthesis) that may detract from their use in certain settings. Consequently, we have designed and synthesized a simpler bifunctional cyclooctyne derivative (9) and demonstrate its utility as a bioconjugate linchpin through construction of a DOTA–biotin assembly.
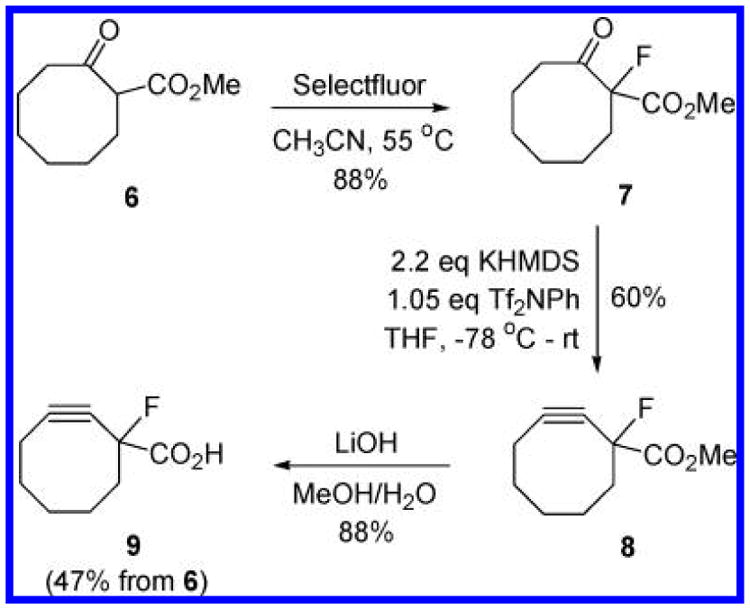
The synthesis of 9 begins with commercially available cyclooctanone 6 (Scheme 1).11 Treatment of 6 with Select-fluor resulted in smooth fluorination, and 7 was obtained in good yield. Exposure of 7 to excess KHMDS (∼2.2 equiv) and Tf2NPh (1.05 equiv) at −78 °C afforded the corresponding vinyl triflate in situ. Triflate elimination was found to occur upon warming the reaction mixture to room temperature, and cyclooctyne 8 was isolated in 60% yield. Saponification of the methyl ester then gave 9 as a clear oil after purification by column chromatography.
Scheme 1.
The reactivity of 9 was first probed in a model click reaction with benzyl azide. Gratifyingly, simply stirring an equimolar mixture of these two compounds in MeOH at room temperature afforded the expected triazole product 10 in excellent isolated yield (eq 1).12
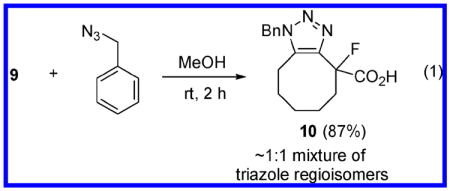 |
Next, the rate of cycloaddition was examined, again using benzyl azide as a model reactant. Bertozzi has established the beneficial effect of difluoro substitution in enhancing the rate of azide cycloaddition to cyclooctynes.8c Given that 9 possesses only a single fluoro group, we anticipated diminished rates of cycloaddition with this compound relative to 3. Nonetheless, monofluoro activation coupled with the presence of an electron-withdrawing carboxyl group was still expected to deliver a more reactive click reagent than parent cyclooctyne derivatives (e.g., 2). The second-order rate constant for the reaction of 9 with benzyl azide was determined by 1H NMR in CD3CN. A plot of 1/[azide] vs time was analyzed using linear regression methods, with the slope of the resulting line corresponding to the rate constant (k) (see the Supporting Information). The rate constant for reaction of 9 with benzyl azide under these conditions was determined to be (1.47 ± 0.21) × 10−2 M−1 s−1. As expected, this reaction is slower than a similar transformation involving difluorocyclooctyne 3 (k = 4.2 × 10−2)8c but is roughly 1 order of magnitude faster than reactions using cyclooctyne 2 (k = 2.4 × 10−3 M−1 s−1) as well as monofluoro analogues related to 2 but which lack a carboxyl substituent (k = 4.3 × 10−3 M−1 s−1).13
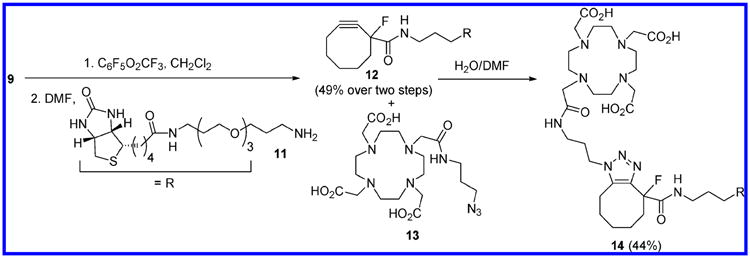
Having established the viability of 9 as a Cu-free click reagent, we then turned our attention to the preparation of potential radiolabeling agents. Biotin was chosen as a model biological probe molecule, and cyclooctyne 9 was attached to amine-functionalized biotin14 derivative 11 by first converting the carboxyl moiety to an activated pentafluorophenyl ester (Scheme 2). Without isolation, the PFE ester was treated with a slight excess of 11 in DMF, and the desired biotin–cyclooctyne derivative 12 was obtained in 49% isolated yield after purification by column chromatography. This material was then treated with azide-modified DOTA 13 in aqueous DMF.15 Click cycloaddition occurred smoothly, and DOTA-biotin conjugate 14 was isolated in 44% yield afer HPLC purification.
Scheme 2.
A noteworthy consequence stemming from the use of Cu-free click chemistry in the sequence described above is the ability to bioconjugate the DOTA chelator without prior protection of the carboxylic acid residues. This removes the burden of deprotecting DOTA–biomolecular constructs and allows for direct radiolabeling of click reaction products. In the present study, proof-of-concept radiolabeling of the DOTA–biotin conjugate demonstrates the utility of the approach. An acetate buffer solution containing generator-produced 68Ga (7.3 MBq; pH 3.8) was added to aliquots of 14 dissolved in the identical acetate buffer. The reaction was carried out at 99 °C for 20 min. After cooling, aliquots of the reaction mixtures were analyzed directly by radio-HPLC without further purification (Figure 2). Two major peaks were observed for the radiolabeled biotin–DOTA conjugate, reflecting the formation of two regioisomers in the click reaction leading to 14 as well as a minor peak that reflects possible stereoisomer formation (see Supporting Information). Significantly, the radiochemical purity in each solution was >97% with specific activity up to 1.45 MBq nmol−1.
Figure 2.
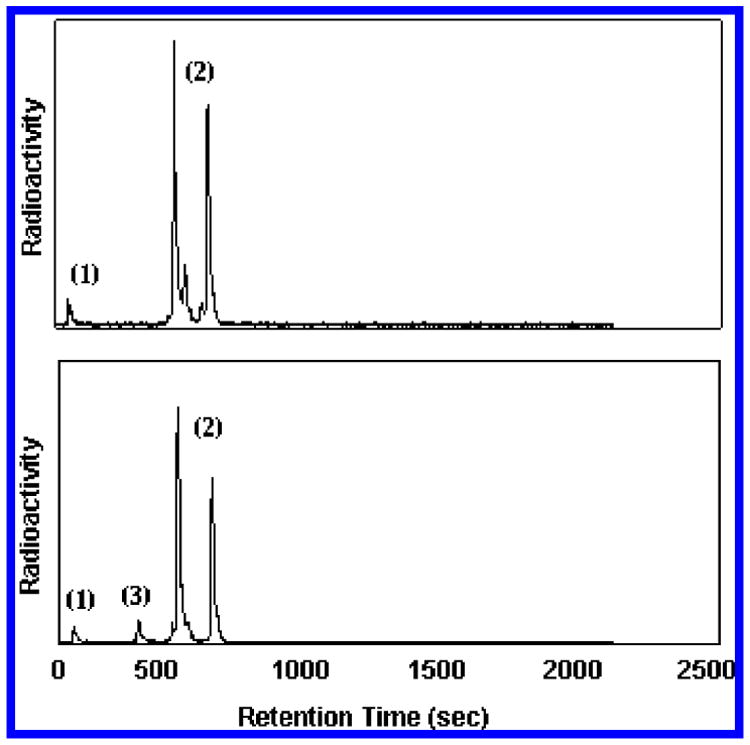
Radio-HPLC trace of 68Ga–biotin–DOTA reaction mixtures. Top: 68Ga labeling in the presence of 5 μmol of 14. Bottom: 68Ga labeling in the presence of 5 nmol of 14. Peak labeling: (1) “free” 68Ga; (2) 68Ga–14 complex as a mixture of two regioisomers; (3) unknown trace impurity.
In conclusion, we have designed and synthesized a new bifunctional cyclooctyne reagent suitable for use in Cu-free click chemistry. Significantly, 9 is easily prepared in only three steps from commercially available starting material in high (47%) overall yield. Attachment of 9 to bioligands can be accomplished through manipulation of the carboxyl residue, and a biotin–cyclooctyne derivative 12 was prepared to demonstrate this feature. The advantages of Cu-free click reactions in the construction of potential radiolabeling agents has been highlighted through formation of DOTA conjugate 14. This material was successfully labeled with 68Ga in >97% radiochemical purity and specific activity of ∼1.5 MBq nmol−1. This demonstrates the utility of 9 for the assembly of radionuclide-chelated bioconjugates. It is anticipated that high-specific activity agents can be prepared using this strategy, and the synthesis of other DOTA bioconjugates for targeted molecular imaging applications using this approach is currently being examined in our laboratories.
Acknowledgments
We thank the American Cancer Society, The University of Iowa Holden Comprehensive Cancer Center (M.K.S.), and the Carver Charitable Trust (F.C.P., S.G.P.) for generous financial support. We also thank Garry Kiefer and Paul Jurek of Macrocyclics, Inc., for their assistance in obtaining 13.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Volkert WA, Hoffman TJ. Chem Rev. 1999;99:2269. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]; (b) Liu S. Chem Soc Rev. 2004;33:445. doi: 10.1039/b309961j. [DOI] [PubMed] [Google Scholar]; (c) Shokeen M, Anderson CJ. Acc Chem Res. 2009;42:832. doi: 10.1021/ar800255q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, Hennig M. Chem—Eur J. 1999;5:1974. [Google Scholar]; (b) Eisenwiener KP, Prata MIM, Buschmann I, Zhang HW, Santos AC, Wenger S, Reubi JC, Mäcke HR. Bioconjugate Chem. 2002;13:530. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]; (c) Wängler B, Beck C, Wagner-Utermann U, Schirrmacher E, Bauer C, Rösch F, Schirrmacher R, Eisenhut M. Tetrahedron Lett. 2006;47:5985. [Google Scholar]; (d) De León-Rodríguez LM, Kovacs Z. Bioconjugate Chem. 2008;19:391. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 3.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Tornøe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sletten EM, Bertozzi CR. Angew Chem, Int Ed. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tron GC, Pirali T, Billington RA, Canonico PL, Sorba G, Genazzani AA. Med Res Rev. 2007;28:278. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]; (c) Moses JE, Moorhouse AD. Chem Soc Rev. 2007;36:1249. doi: 10.1039/b613014n. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lebedev AY, Holland JP, Lewis JS. Chem Commun. 2010;46:1706. doi: 10.1039/b924784j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mindt TL, Müller C, Stuker F, Salazar JF, Hohn A, Mueggler T, Rudin M, Schibli R. Bioconjugate Chem. 2009;20:1940. doi: 10.1021/bc900276b. [DOI] [PubMed] [Google Scholar]; (c) Knör S, Modlinger A, Poethko T, Schottelius M, Wester HJ, Kessler H. Chem—Eur J. 2007;13:6082. doi: 10.1002/chem.200700231. [DOI] [PubMed] [Google Scholar]; (d) Dijkgraaf I, Rijnders AY, Soede A, Dechesne AC, van Esse GW, Brouwer AJ, Corstens FHM, Boerman OC, Rijkers DTS, Liskamp RMJ. Org Biomol Chem. 2007;5:935. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 6.For a review of Cu-free click reactions, see: Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39:1272. doi: 10.1039/b901970g.
- 7.Huisgen R. Angew Chem, Int Ed. 1963;2:565. [Google Scholar]
- 8.(a) Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; (b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci USA. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J Am Chem Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sletten EM, Bertozzi CR. Org Lett. 2008;10:3097. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci USA. 2010;107:1821. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning X, Guo J, Wolfert MA, Boons Gj. Angew Chem, Int Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) van Berkel SS, Dirks AJ, Debets MF, van Delft FL, Cornelissen JJLM, Nolte RJM, Rutjes FPJT. ChemBioChem. 2007;8:1504. doi: 10.1002/cbic.200700278. [DOI] [PubMed] [Google Scholar]; (b) van Berkel SS, Dirks AJ, Meeuwissen SA, Pingen DLL, Boerman OC, Laverman P, van Delft FL, Cornelissen JJLM, Rutjes FPJT. ChemBioChem. 2008;9:1805. doi: 10.1002/cbic.200800074. [DOI] [PubMed] [Google Scholar]; (c) Laverman P, Meeuwissen SA, van Berkel SS, Oyen WJG, van Delft FL, Rutjes FPJT, Boerman OC. Nucl Med Biol. 2009;36:749. doi: 10.1016/j.nucmedbio.2009.05.001. [DOI] [PubMed] [Google Scholar]; See also: Singh I, Vyle JS, Heaney F. Chem Commun. 2009:3276. doi: 10.1039/b904185k.
- 11.This compound can also be prepared in high yield by treating cyclooctanone with NaH and dimethyl carbonate: Frew AJ, Proctor GR. J Chem Soc, Perkin Trans. 1980;1:1245.
- 12.Compound 10 was produced as a ∼1:1 mixture of inseparable triazole regioisomers.
- 13.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. ACS chem Biol. 2006;1:644. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 14.Wilbur DS, Hamlin DK, Vessella RL, Stray JE, Buhler KR, Stayton PS, Klumb LA, Pathare PM, Weerawarna SA. Bioconjugate Chem. 1996;7:689. doi: 10.1021/bc9600628. [DOI] [PubMed] [Google Scholar]
- 15.Compound 13 was purchased from Macrocyclics, Inc. (www.macrocyclics.com). This material can be prepared from DOTA-mono-NHS-tris-tert-butyl ester and 1-amino-3-azidopropane. Details of this synthesis will be reported elsewhere.


