Abstract
Background
Serious mental illness (SMI) with psychiatric instability accounts for disproportionately high use of emergency room visits and hospitalizations.
Aim
To evaluate the effectiveness of an automated telehealth intervention supported by nurse health care management for improving psychiatric illness management and reducing acute service use among individuals with SMI and psychiatric instability.
Methods
Thirty-eight individuals with SMI received the automated telehealth intervention for 6 months. Psychiatric symptoms, illness self-management, and self-reported service use (emergency room visits and hospital admissions) were collected at baseline, 3- and 6-months. Measures of quality of life, health indicators, and subjective health status were also collected.
Results
Participants demonstrated improvements in self-reported psychiatric symptoms and illness self-management skills, an 82% decrease in hospital admissions (from 76 to 14 hospitalizations, p<0.001) and a 75% decrease in emergency room visits (from 63 to 16 visits, p<0.001). Improvements were also observed in quality of life, severity of depressive symptoms, and mental health status.
Conclusion
These highly promising findings support the use of an automated telehealth device monitored by a nurse care manager for people with SMI, and highlight the potential for cost savings through reductions in acute health care utilization.
Keywords: Acute service use, automated telehealth, community mental health, mental health, psychiatric instability, serious mental illness
Introduction
Psychiatric instability contributes to poor functioning and high rates of acute health care use among individuals with serious mental illness (SMI) (Hackman et al., 2006). Relapses and hospitalizations are burdensome and highly disruptive for individuals and families, causing interruptions in work, housing, and pursuit of meaningful life goals. Interventions promoting psychiatric stability, such as illness management and recovery (IMR), skills training, and assertive community treatment (ACT), benefit individuals and payers by increasing community tenure, reducing relapse, and preventing re-hospitalization. However, there are challenges in routinely implementing and sustaining these face-to-face interventions including staffing requirements, need for ongoing supervision and training for providers, and costs (Bond et al., 2014).
Remote monitoring devices and automated illness management programs are increasingly being used as alternatives to face-to-face services to follow key health indicators and functional status for individuals with unstable medical illnesses. Automated programs have the advantage of conducting daily assessments of high-risk individuals in their homes, and engaging costly health professionals only when immediate attention is necessary. These devices are also effective at obtaining daily clinical information on isolated individuals who live in rural areas, or are reluctant or unable to leave their homes.
In contrast to an extensive literature on remote monitoring and automated telehealth for general medical conditions, much of the research on telehealth for mental illness has focused on providing real-time communication with a clinician using telephonic or video-based systems. Although effective in reducing travel time, this approach still requires expensive professional involvement and is impractical for decreasing risk of relapse and hospitalization among unstable, high-risk individuals. Among telehealth approaches for treating SMI, a mobile phone-based program that involved weekly completion of a 10-item early warning sign questionnaire, and online alerts sent to the treating psychiatrist if scores exceeded a given threshold, was shown to reduce hospitalizations in people with schizophrenia (Spaniel et al., 2008). Other telehealth interventions targeting this vulnerable group include video-conferencing (Nieves et al., 2009), informational websites (van der Krieke et al., 2012), telephone-based therapy (Cook et al., 2008), and smartphone-based interventions in early development that consist of frequent real-time symptom self-assessment and monitoring (Ben-Zeev et al., 2013).
Despite these varied efforts, few studies have evaluated automated telehealth programs designed to monitor daily symptoms and prevent relapse and acute hospitalization. Three recent pilot studies demonstrated the acceptability and potential effectiveness of “Health Buddy”, an automated telehealth device that provides daily monitoring of symptoms and self-management prompting, supported by a remote nurse. Use of this device among older veterans with a mental health diagnosis contributed to reductions in acute care service use (Godleski et al., 2012), and more rapid remission of suicidality symptoms among veterans with schizophrenia (Kasckow et al., 2013). Most recently, among adults with SMI and a comorbid chronic medical condition receiving care in a community-based setting, use of the device was associated with improvements in self-efficacy for management of depression and blood pressure, increased understanding of medical condition, and reductions in urgent care and primary care visits among those with diabetes and co-occurring depression or bipolar disorder (Pratt et al., 2013).
These studies have demonstrated the feasibility and initial effectiveness of this automated telehealth device for delivering in-home daily sessions that: (1) provide education about illness; (2) collect information about key symptoms; (3) train users on illness self-management, providing direction on actions to take when symptoms increase; and (4) identify at-risk users requiring clinical contact by a nurse who monitors responses to the device. The current study expands on prior evaluations of automated telehealth among veterans by including a sample with a more balanced distribution of gender and psychiatric diagnoses receiving services in a community mental health center. We hypothesized that automated telehealth would be associated with decreased acute health care use and improvements in psychiatric illness self-management and illness severity.
Methods
This single-arm pilot trial assessed the effectiveness of the Health Buddy automated telehealth device with an adapted content library supported by a nurse care manager, enrolling participants at a community mental health center in Concord, New Hampshire between October 2011 and April 2013. Participants were recruited through self-referral and clinician referrals, and were paid for completing assessments but not for participation. Informed consent was obtained through procedures approved by the Dartmouth College and New Hampshire Bureau of Behavioral Health Committees for the Protection of Human Subjects.
Participants
Eligible individuals met the following inclusion criteria: age ≥ 18; SMI defined by at least moderate impairment in multiple domains of life functioning and a primary Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, axis I diagnosis, based on chart review and confirmation by the community mental health center team psychiatrist, of schizophrenia, schizoaffective disorder, bipolar disorder, post-traumatic stress disorder or major depression; psychiatric instability defined as at least two emergency room visits or hospitalizations within the past year, or over 10 calls to a crisis line within the past 3 months; ability to read; and voluntary informed consent for participation. All participants had a landline telephone or had one installed in their residence. Exclusion criteria were: terminal illness expected to result in death within 1 year; plans to relocate out of Concord, New Hampshire within 1 year; and primary diagnosis of dementia or significant cognitive impairment defined as a Mini Mental Status Examination (MMSE) score<24 (Folstein et al., 1975).
Automated telehealth intervention
Automated telehealth device
The Health Buddy automated telehealth device, developed by Robert Bosch Healthcare, Palo Alto, CA is about the size of a clock radio with four buttons and a high-resolution liquid-crystal display screen. It was connected to a home telephone line and programed with content written at a fourth-grade level. Participants were assigned to psychiatric content matching their primary behavioral health condition. The content was specifically developed for this study by incorporating elements of previously evaluated self-management training interventions for SMI, including Integrated IMR (Bartels et al., 2014) and Helping Older People Experience Success (Bartels et al., 2013).
Users participate in daily sessions with the automated telehealth device that last 5–10 min. Users begin their daily session by pushing the “start” button, which is followed by a personalized greeting (e.g. “Welcome back, Mary.”), and then questions about key symptoms and suggestions for proactively managing them, reminders, and questions designed to provide education about the user’s illness. Participants press one of four buttons to answer the questions. The device automatically provides specific instructions (e.g. “call your doctor right away”) to participants demonstrating signs of imminent relapse (e.g. endorsement of suicidal thoughts). The content also includes branching logic where additional questions or feedback appear depending on the user’s response to an initial question. For example, if a participant endorses medication non-adherence, a subsequent question appears asking why medications were not taken. Daily sessions end with coping tips and trivia questions to enhance motivation to complete the session. Responses are transmitted over the phone line and uploaded to a secure server each night.
Telehealth nurse
The telehealth nurse received training in the technical aspects of using the automated telehealth device and the desktop application where responses to daily sessions were reviewed. The nurse visited all participants in their homes to install the unit and provide orientation surrounding its use. Based on a programed disease management algorithm developed using standard clinical practice guidelines, participant responses are hierarchically arranged into three risk levels (red, yellow, or green) and are reviewed by the nurse several times daily during work hours Monday–Friday. The nurse instructed participants that the device does not serve as an emergency response system and that they should access crisis services between 5 pm and 9 am and on weekends if a psychiatric emergency should arise. She followed an established protocol for responding to early signs of relapse. For example, the nurse called or sent a text message to participants if more than 24 h elapsed without completion of a session. Participants assigned to the high-risk (red) category, were contacted to discuss the need for immediate mental health treatment. Participants sorted to the medium risk (yellow) category were contacted to prompt use of coping strategies and to assess psychiatric status. Most importantly, the nurse notified the treatment team about all participants in high and medium categories to ensure that team support was engaged.
Measures
Psychiatric symptom severity was assessed using the Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, 1962). Negative symptoms were measured using the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983). Psychiatric illness self-management skills (e.g. medication adherence, having a relapse prevention plan, knowledge of symptoms, and coping strategies) were measured using the 15-item IMR scale (Mueser & Gingerich, 2005). Ability to perform a variety of general health behaviors (e.g. exercise, diet) was assessed using the Self-Rated Abilities for Health Practices scale (Becker et al., 1993). The nine-item Patient Health Questionnaire (PHQ-9) assessed depressive symptoms (Löwe et al., 2004). Mental Component and Physical Component scores from the 12-item Short Form Health Survey (SF-12) assessed subjective mental health and physical health well-being (Ware et al., 1998). Trained research interviewers completed measures at baseline and at 3- and 6-months follow-up.
Service use (hospital admissions and emergency room visits) was self-reported by participants at baseline and at 3- and 6-months. Participant adherence was calculated for each individual as the total number of telehealth sessions completed divided by the number of possible sessions over the 6-month study duration (7 sessions per week).
Statistical analyses
We used general linear mixed models with unstructured covariance to compare differences over time in self-reported measures and acute care service use. Time was entered into the model as a continuous variable. Mixed-effects models accommodate missing data and allow inclusion of all subjects with data in the analyses. Treatment effect sizes are reported for each outcome, and were considered small (0.20), moderate (0.50) or large (0.80). Statistical significance was set at p ≤ 0.05.
Results
Eighty-seven potential participants were screened, of which 81 were eligible and 51 were invited to participate. We were not able to obtain permission from clinical providers at the community mental health center to contact the remaining 30. Forty consumers agreed to participate, and 38 completed baseline measures and were enrolled. Over 6 months, participants completed a mean of 128.5 (±19.2) sessions (71.4%) out of a possible 180. Participants were predominantly female (N=27; 69%) and psychiatric diagnoses were relatively evenly distributed. Fifty percent (N=19) of participants had comorbid borderline personality disorder. Baseline characteristics are listed in Table 1.
Table 1.
Baseline demographic characteristics.
| Characteristic | Total sample (N =38)
|
|
|---|---|---|
| N | % | |
| Age (M±SD) | 46.4 ± 11.7 | |
| Gender | ||
| Male | 11 | 29 |
| Female | 27 | 71 |
| Ethnicity | ||
| White | 37 | 97 |
| American Indian | 1 | 3 |
| Latino | ||
| Yes | 2 | 5 |
| No | 36 | 95 |
| Marital status | ||
| Married | 5 | 13 |
| Never married | 12 | 32 |
| Separated/Divorced | 16 | 42 |
| Widowed | 5 | 13 |
| Education | ||
| ≤ High school | 20 | 53 |
| >High school | 18 | 47 |
| Residential | ||
| Supervised/supported housing | 2 | 5 |
| Independent | 36 | 95 |
| Psychiatric diagnosis (N =38) | ||
| Schizophrenia | 4 | 10 |
| Schizoaffective | 6 | 16 |
| Bipolar Disorder | 8 | 21 |
| Major Depression | 8 | 21 |
| PTSD | 12 | 32 |
| Co-occurring disorders | ||
| Borderline Personality Disorder | 19 | 50 |
Results from baseline to 6-months are listed in Table 2. Improvements were observed for measures of psychiatric illness symptoms, including significant decreases in psychiatric symptom severity on the BPRS. Participants demonstrated significant increases in psychiatric illness self-management on the IMR scale and health self-efficacy on the Self-Rated Abilities for Health Practices scale. Depressive symptoms, ability to perform general health behaviors, and subjective mental health well-being, but not physical health well-being, also improved.
Table 2.
Psychiatric and physical health outcomes at baseline, 3-months and 6-months.
| Measure | Baseline | 3-months | 6-months | ES | df | F | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |||||
| BPRS totala | 68.6 | 13.1 | 61.9 | 16.9 | 53.6 | 12.4 | −1.04 | 1, 35 | 37.1 | <0.001 |
| SANSb | 2.6 | 0.6 | 2.3 | 0.6 | 2.3 | 0.5 | −0.63 | 1, 35 | 12.6 | 0.001 |
| IMR Scalec | 2.8 | 0.4 | 3.3 | 0.47 | 3.4 | 0.4 | 1.61 | 1, 32 | 103.1 | <0.001 |
| Total Abilities for Health Practices Scaled | 50.8 | 15.2 | 60.4 | 17.3 | 64.3 | 14.6 | 0.64 | 1, 34 | 15.9 | <0.001 |
| Exercise abilities | 9.1 | 7.4 | 12.1 | 8.0 | 13.2 | 7.2 | 0.33 | 1, 34 | 4.4 | 0.044 |
| Health abilities | 16.2 | 4.5 | 18.8 | 4.2 | 19.0 | 4.3 | 0.43 | 1, 34 | 5.7 | 0.022 |
| Nutrition abilities | 15.2 | 6.1 | 16.2 | 5.8 | 17.9 | 4.6 | 0.37 | 1, 33 | 6.2 | 0.018 |
| Psychological Wellbeing abilities | 10.4 | 4.6 | 13.3 | 5.5 | 14.2 | 5.3 | 0.69 | 1, 32 | 18.3 | <0.001 |
| PHQ-9e | 17.5 | 5.5 | 12.9 | 6.1 | 10.4 | 5.7 | −1.15 | 1, 33 | 43.7 | <0.001 |
| SF-12 Mentalf | 26.1 | 6.4 | 32.5 | 10.7 | 37.9 | 12.1 | 0.99 | 1, 35 | 35.4 | <0.001 |
| SF-12 Physicalg | 40.3 | 12.5 | 45.0 | 13.2 | 43.6 | 12.6 | 0.23 | 1, 34 | 2.1 | 0.156 |
Possible scores range from 24 to 168. Higher scores indicate greater psychiatric symptom severity.
Possible scores range from 0 to 5. Higher scores indicate greater negative symptoms.
Possible scores range from 1 to 5. Higher scores indicate greater psychiatric illness self-management self-efficacy.
Possible scores range from 0 to 112. Higher scores indicate greater self-rated abilities for health practices. Subscale scores range from 0 low to 28 high.
Possible scores range from 0 to 27. Higher scores indicate greater depressive symptoms.
Possible scores range from 0 to 100. Higher scores indicate greater subjective mental health well-being.
Possible scores range from 0 to 100. Higher scores indicate greater subjective physical health well-being.
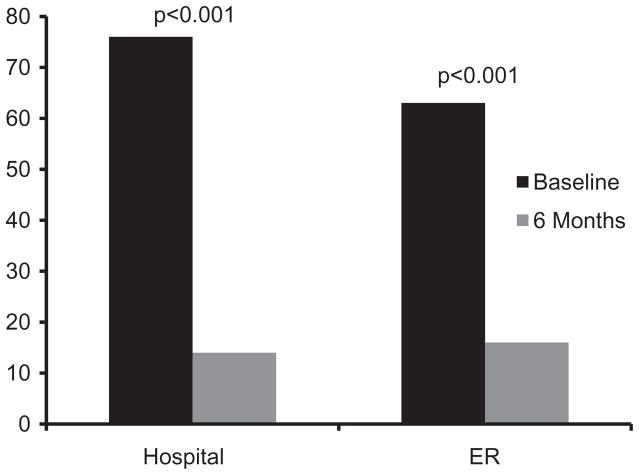
Participants experienced an 82% decrease in hospital admissions, from 76 hospitalizations during the 6 months prior to baseline to 14 hospitalizations at 6-months (df=1, 32; F=24.5; p<0.001) and a 75% decrease in emergency room visits, from 63 visits during the 6 months prior to baseline to 16 visits at 6-months (df=1, 32; F=29.6; p<0.001) (see Figure 1).
Figure 1.
Mean hospital admissions and emergency room visits before and after receiving the automated telehealth intervention.
Given that about half of our sample had comorbid borderline personality disorder, we conducted exploratory analyses to see whether this diagnosis was associated with differences in outcomes. With the exception of baseline depressive symptoms, which were higher in people with borderline personality disorder, this diagnosis was not associated with any differences in outcomes.
Discussion
This pilot study suggests that frequent monitoring and automated self-management instruction to individuals with SMI may improve psychiatric symptoms while reducing the need for daily in-person visits and decreasing acute psychiatric relapses requiring hospitalization. Feasibility and acceptability of the automated telehealth device were supported by high willingness to participate among eligible individuals who were invited, as well as telehealth session adherence exceeding 70%. Our study also demonstrates that automated telehealth supported by a nurse care manager can be delivered within a public sector mental health setting, and is potentially useful for improving mental health outcomes within this high-risk population.
As this intervention consists of multiple components, we are unable to determine which aspect of the program is most associated with improvements in psychiatric symptoms and stability. It is noteworthy that half our sample, which was selected on the basis of psychiatric instability, had a co-occurring diagnosis of borderline personality disorder, commonly associated with high rates of relapse and acute service use. Outcomes for this group were no different from those without this diagnosis. Reductions in emergency room visits and hospitalizations suggest that automated telehealth may confer particular benefits among complex psychiatric conditions that typically respond poorly to conventional case management outreach services. Daily interactions with an automated telehealth device (in contrast to less frequent but more intensive interactions with clinicians) may provide consistent prompting of adaptive coping skills while minimizing challenges associated with negotiating interpersonal relationships.
Our positive outcomes suggest potential applications to other disadvantaged, low-income health disparity populations; for example, individuals with SMI who are less likely to engage clinic-based services, those who are isolated because they live in rural areas or because they are reluctant or unable to leave their homes, or individuals who receive treatment in primary care settings in which psychiatric expertise is limited. Our findings may also support the use of automated telehealth in behavioral health homes as an efficient, easy to implement, consumer-centered approach to managing unstable psychiatric symptoms for individuals with SMI at greatest risk for institutional care and excess morbidity.
Several limitations warrant consideration. First, there was no comparison group and the sample size was small. Second, reduced acute service use might represent regression to the mean, though large effect sizes combined with positive results from prior evaluations of this telehealth device are encouraging. Also, as this was a pilot study, we were unable to evaluate cost-savings, though we anticipate reduced costs given savings reported in other trials using this automated telehealth device including a study involving 3534 Medicare recipients with chronic diseases showing cost reductions of $312–$542 per person per quarter over a 2-year period (Baker et al., 2011). The sample was predominantly white, limiting generalizability of the results to ethnically diverse populations. However, the community based setting of this trial may be generalizable to other community mental health centers given health system similarities. The intervention consisted of automated telehealth supported by a nurse, thus it was not possible to determine the relative effectiveness of either component alone. The use of a nurse potentially limits generalizability of this intervention across settings due to cost and staffing requirements, highlighting the need to evaluate whether a lower cost provider could effectively support this intervention. Our research group has such a study under review at the National Institute of Mental Health that would replace the nurse with a master’s level mental health clinician. Finally, longer follow-up is required to establish long-term feasibility and effectiveness of this intervention for managing psychiatric instability in this at-risk group.
Conclusions
This pilot study contributes to a growing body of literature highlighting the value of emerging technologies and remote monitoring devices for use among individuals with SMI (Bauer & Moessner, 2012; Jones et al., 2011). We found that automated telehealth supported by a nurse care manager may lead to better mental health outcomes and decreased use of acute and crisis-based mental health care services. These results combined with those from prior evaluations of this device support the feasibility and acceptability of using automated telehealth across diverse mental health patient populations. Our promising findings support the need for a future large scale randomized study in an ethnically diverse sample of people with SMI and psychiatric instability to confirm the effectiveness and evaluate potential cost savings associated with automated telehealth. If proven effective, automated telehealth could transform care by providing daily self-management support to large numbers of psychiatrically unstable individuals, increasing both the reach and effectiveness of community-based services for the highest-risk, highest cost adults with complex mental health conditions.
Footnotes
Declaration of interest
This work was supported by a grant from Robert Bosch Healthcare, Palo Alto, CA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article. The authors report no competing interests.
References
- Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City (IA): University of Iowa; 1983. [Google Scholar]
- Baker LC, Johnson SJ, Macaulay D, Birnbaum H. Integrated telehealth and care management program for Medicare beneficiaries with chronic disease linked to savings. Health Affairs. 2011;30:1689–97. doi: 10.1377/hlthaff.2011.0216. [DOI] [PubMed] [Google Scholar]
- Bartels SJ, Pratt SI, Mueser KT, et al. Long-term outcomes of a randomized trial of integrated skills training and preventive healthcare for older adults with serious mental illness. Am J Geriatr Psychiatr. 2013 doi: 10.1016/j.jagp.2013.04.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels SJ, Pratt SI, Mueser KT, et al. Integrated IMR for psychiatric and general medical illness for adults aged 50 or older with serious mental illness. Psychiatr Serv. 2014;65:330–7. doi: 10.1176/appi.ps.201300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Moessner M. Technology-enhanced monitoring in psychotherapy and e-mental health. J Ment Health. 2012;21:355–63. doi: 10.3109/09638237.2012.667886. [DOI] [PubMed] [Google Scholar]
- Becker H, Stuifbergen A, Oh HS, Hall S. Self-rated abilities for health practices: A health self-efficacy measure. Health Values: J Health Behav, Educ Promot. 1993;17:42–50. [Google Scholar]
- Ben-Zeev D, Kaiser SM, Brenner CJ, et al. Development and usability testing of FOCUS: A smartphone system for self-management of schizophrenia. Psychiatr Rehabil J. 2013;36:289–96. doi: 10.1037/prj0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond GR, Drake RE, McHugo GJ, et al. Long-term sustainability of evidence-based practices in community mental health agencies. Admin Pol Mental Health Mental Health Serv Res. 2014;41:228–36. doi: 10.1007/s10488-012-0461-5. [DOI] [PubMed] [Google Scholar]
- Cook PF, Emiliozzi S, Waters C, El Hajj D. Effects of telephone counseling on antipsychotic adherence and emergency department utilization. Am J Managed Care. 2008;14:841–6. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Godleski L, Cervone D, Vogel D, Rooney M. Home telemental health implementation and outcomes using electronic messaging. J Telemed Telecare. 2012;18:17–19. doi: 10.1258/jtt.2011.100919. [DOI] [PubMed] [Google Scholar]
- Hackman A, Goldberg R, Brown C, et al. Brief reports: Use of emergency department services for somatic reasons by people with serious mental illness. Psychiatr Serv. 2006;57:563–6. doi: 10.1176/ps.2006.57.4.563. [DOI] [PubMed] [Google Scholar]
- Jones S, Beck A, Deville M. Enhancing self-management for service users and carers: How can technology help? J Mental Health. 2011;20:505–8. doi: 10.3109/09638237.2011.608751. [DOI] [PubMed] [Google Scholar]
- Kasckow J, Zickmund S, Rotondi A, et al. Development of telehealth dialogues for monitoring suicidal patients with schizophrenia: Consumer feedback. Commun Mental Health J. 2013;50:339–42. doi: 10.1007/s10597-012-9589-8. [DOI] [PubMed] [Google Scholar]
- Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: Sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81:61–6. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Gingerich S. Illness management and recovery (iMR) scales. In: Campbell-Orde T, Chamberlin J, Carpenter J, Leff HS, editors. Measuring the Promise: A Compendium of Recovery Measures. II. Cambridge (MA): Human Services Research Institute; 2005. pp. 124–32. [Google Scholar]
- Nieves JE, Godleski LS, Stack KM, Zinanni T. Videophones for intensive case management of psychiatric outpatients. J Telemed Telecare. 2009;15:51–4. doi: 10.1258/jtt.2008.080706. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pratt SI, Bartels SJ, Mueser KT, et al. Feasibility and effectiveness of an automated telehealth intervention to improve illness self-management in people with serious psychiatric and medical disorders. Psychiatr Rehabil J. 2013;36:297–305. doi: 10.1037/prj0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniel F, Vohlidka P, Hrdlicka J, et al. ITAREPS: Information technology aided relapse prevention programme in schizophrenia. Schizophrenia Res. 2008;98:312–17. doi: 10.1016/j.schres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- van der Krieke L, Emerencia AC, Aiello M, Sytema S. Usability evaluation of a web-based support system for people with a schizophrenia diagnosis. J Medical Internet Res. 2012;14:91–101. doi: 10.2196/jmir.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. The SF-12 users manual: How to score the SF-12 physical and mental health summary scales. 3. Boston (MA): Quality Metric Inc; 1998. [Google Scholar]