Abstract
Background
Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is associated with significant morbidity and mortality. Similarities have been observed between patients with idiopathic pulmonary fibrosis (IPF) and the UIP (usual interstitial pneumonia) form of RA-ILD. The GAP (gender, age, physiology) model has been shown to predict mortality in patients with IPF, but its ability to predict mortality in RA-ILD is not known.
Methods
We identified 309 patients with RA-ILD at 4 academic centers with ongoing longitudinal cohorts of patients with ILD. The primary endpoint was mortality. To handle missing data (n=219 subjects with complete dataset), multiple imputation by iterative chained equations was used. Using the GAP model as a baseline, we assessed improvements in mortality risk prediction achieved by incorporating additional variables. Model discrimination was assessed using the c-index, and calibration was checked by comparing observed and expected incidence of death.
Results
Patients had a mean age of 65 years and were predominantly female (54%). The mean forced vital capacity (FVC) % predicted was 73 and the mean diffusing capacity for carbon monoxide (DLCO) % predicted was 55. Twenty-four percent of the 236 patients with a high-resolution computed tomography scan available for review had a definite UIP pattern. The original GAP model, including gender, age, FVC%, and DLCO%, had a c-index of 0.746 in our cohort. Calibration of this model was satisfactory at 1, 2 and 3 years. Model discrimination was not meaningfully improved by adding other clinical variables.
Conclusion
The GAP model that was derived for IPF performs similarly as a mortality risk prediction tool in RA-ILD.
Keywords: rheumatoid arthritis, interstitial lung disease, prognosis, risk assessment
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that is common in the United States with an estimated prevalence of 0.5 – 1%1. Although the principal manifestation of RA is inflammatory arthritis, extra-articular organ involvement is often observed2–4. Interstitial lung disease (ILD) constitutes the most frequent pulmonary manifestation of RA2. Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is associated with high morbidity and mortality5–9.
Previous studies have attempted to identify factors that can predict mortality in RA-ILD10–15. Variables such as age, gender, diffusion capacity of the lung for carbon monoxide (DLCO), extent of fibrosis, and presence of the usual interstitial pneumonia (UIP) pattern have been shown to be associated with mortality in this population. A recent systematic review highlighted the lack of methodologic quality and the inconsistent results between several of these studies16.
Clinical and prognostic similarities have been observed between patients with idiopathic pulmonary fibrosis (IPF) and the UIP form of RA-ILD14,17. The GAP model (gender, age, physiology) is a validated risk prediction model for mortality among patients with IPF20. Its ability to predict mortality among patients with RA-ILD is not known. Given the shared attributes between RA-ILD and IPF, we assessed the ability of the GAP model to predict mortality in a cohort of patients with RA-ILD and explored whether its performance could be improved by adding clinical variables.
Methods
Study population
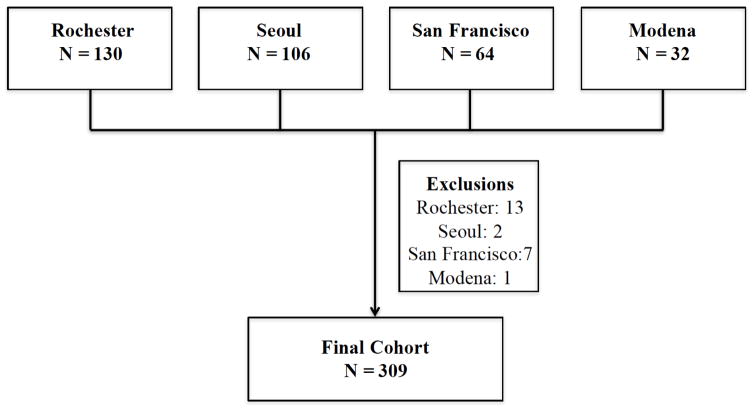
We used retrospectively collected patient data from longitudinal databases of ILD centers from the Mayo Clinic Rochester (USA), the University of Ulsan, Seoul (Korea), the University of California, San Francisco (USA) and the University of Modena and Reggio Emilia (Italy). To be included in the study, patients were required to have a rheumatologist-confirmed diagnosis of RA 21 and a confirmed diagnosis of ILD on high-resolution computed tomography (HRCT) and/or surgical lung biopsy. Patients with no ILD on HRCT were excluded (Figure 1). The final cohort included 309 patients. The institutional review boards at the four sites (Mayo Clinic Institutional Review Board (#12-009206), Institutional Review Board of Asan Medical center (#2013-0433), University of California, San Francisco Institutional Human Subject Review Committee (#10-01592) and the ethics committee of the University of Modena and Reggio Emilia (#2636)) approved the parent studies and patients provided written informed consent.
Figure 1.
Cohort formation
Patients were excluded if they did not have interstitial lung disease on high-resolution computed tomography and/or surgical lung biopsy
Measurements
Baseline demographic data (age, sex, ethnicity, smoking status, oxygen use and body mass index [BMI]) were obtained using structured questionnaires at the initial ILD clinic visit and medical record review. Results of pulmonary function tests (PFTs) performed within 6 months of the first visit were recorded. Medical chart review was used to obtain additional variables such as serologic profile (e.g rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (CCP)), surgical lung biopsy results, and immunosuppressive therapy.
HRCTs performed within 1 year of the initial visit in the ILD clinic were reviewed by an experienced thoracic radiologist (BME). Each HRCT was classified as having either a definite UIP, possible UIP or inconsistent UIP pattern, according to the published guidelines22. Time to mortality was the main endpoint. Death dates were obtained using medical chart review and the US. Social Security Death Index or national death registries. Lung transplantation was handled by censoring at the time of transplant.
Statistical analysis
To account for missing values, we used multiple imputation by iterative chained equations (e-Appendix 1). We first looked at the performance of the variables in the continuous GAP model (gender, age, DLCO % predicted and forced vital capacity (FVC) % predicted). The c-index of the GAP model was estimated in our cohort using 10-fold cross-validation in each of the 20 completed datasets, then averaged. A 95% bootstrap percentile confidence interval of this c-index was calculated.
Next, we explored if we could improve the performance of the GAP model by incorporating additional variables. Candidate variables included ethnicity, ever smoking history, BMI, RF, CCP, and definite UIP pattern on HRCT. We evaluated how the combination of the GAP variables with these additional predictors was associated with mortality using Cox models. To identify a prediction model without over-fitting, we exhaustively screened candidate models that included the GAP variables and one to four additional candidate variables. We calculated the c-index using cross-validation for each candidate model. Models were then ranked according to this optimism-corrected measure of discrimination. We then repeated this screening without requiring the inclusion of the four GAP variables.
We calculated the difference between the c-index of the original GAP and the expanded GAP. To better characterize the value of the additional variables, we calculated the p-values to evaluate the impact of these predictors in the Cox model including the original GAP variables. Sensitivity analyses were conducted using complete cases analysis.
Finally, we assessed the calibration of the final model by comparing the model-based estimates to nonparametric estimates of mortality at year 1, 2 and 3 in both the complete cohort (using the imputed data) and the complete case analysis.
All analyses were performed using STATA 14 (Stata Corp, College Station, Texas).
Results
Patient Characteristics
Table 1 presents the baseline characteristics of the complete cohort (n=309). Patients had an average age of 65, were predominantly female (54%), Caucasian (57%) and had a history of smoking (53%). On average, they had moderate physiologic impairment with an FVC % predicted of 73.4% and a DLCO % predicted of 55.1%. The majority of patients had a positive RF (89%) or CCP (71%) antibody. In patients with available results for both serologic tests (n=251), 64% had a positive RF and CCP antibody, while 9% had a negative RF and CCP antibody. Twenty-four percent of the 236 patients with HRCT scans available for review had a definite UIP pattern. Nineteen percent underwent a surgical lung biopsy. Of those, 61% had a UIP pattern on pathology. As noted in Table 1, there was some missing data in some of the baseline characteristics reported. The cohort of patients with complete data included 219 subjects. The baseline characteristics of patients by center are shown in e-Table 1.
Table 1.
Patient Characteristics
| Characteristics | n=309 |
|---|---|
| Center | |
| Rochester- n/total (%) | 117/309 (38) |
| Seoul- n/total (%) | 104/309 (34) |
| San Francisco- n/total (%) | 57/309 (18) |
| Modena- n/total (%) | 31/309 (10) |
| Age (SD), years | 64.9 (9.7)† |
| Female – n/total (%) | 167/309 (54) |
| Race and ethnicity | |
| White or Caucasian- n/total (%) | 177/309 (57) |
| Asian- n/total (%) | 110/309 (36) |
| African american- n/total (%) | 9/309 (3) |
| Native American - n/total (%) | 3/309 (1) |
| Hispanic/Latino - n/total (%) | 9/309 (3) |
| Ever smoker - n/total (%) | 162/308 (53) |
| BMI (SD), kg/m2 | 27.0 (6.2)‡ |
| Rheumatoid factor - n/total (%) | 222/266 (89) |
| Anti-cyclic citrullinated peptide antibody- n/total (%) | 186/261 (71) |
| FVC (SD), % predicted | 73.4 (20.1)§ |
| DLCO (SD), % predicted | 55.1 (21.3)ll |
| HRCT - n/total (%)* | 236/309 (76) |
| Definite UIP - n/total (%) | 56/236 (24) |
| Possible UIP – n/total (%) | 38/236 (16) |
| Inconsistent with UIP – n/total (%) | 142/236 (60) |
| Surgical lung biopsy - n/total (%) | 59/309 (19) |
| UIP pattern - n/total (%) | 36/59 (61) |
| NSIP pattern - n/total (%) | 13/59 (22) |
| Others – n/total (%) | 10/59 (17) |
According to the published guidelines
n = 309
n = 297
n = 300
n = 284
Abbreviations: UCSF = University of California, San Francisco; BMI = body mass index ; FVC = forced vital capacity; DLCO = diffusing capacity for carbon monoxide; HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia; NSIP = non-specific interstitial pneumonia.
Treatment and outcomes are summarized in table 2 and shown by center in e-Table 2. The majority of patients received immunosuppressive therapy (79%). The most frequently reported agents were prednisone (83%), methotrexate (40%), hydroxychloroquine (33%), and azathioprine (23%). Median follow-up time was 3.01 (range 0.03 – 18.8) years. During the follow-up period, 99 subjects died and 3 underwent lung transplantation.
Table 2.
Treatment and outcomes
| Characteristics | n=309 |
|---|---|
| Long-term oxygen therapy - n/total (%) | 70/302 (23.2) |
| Immunosuppressive therapy - n/total (%) | 243/309 (78.6) |
| Prednisone - n/total (%) | 255/308 (82.8) |
| Methotrexate - n/total (%) | 124/308 (40.3) |
| Hydroxychloroquine- n/total (%) | 98/302 (32.5) |
| Azathioprine- n/total (%) | 70/308 (22.7) |
| Leflunomide- n/total (%) | 43/300 (14.3) |
| Etanercept - n/total (%) | 44/308 (14.3) |
| Rituximab- n/total (%) | 16/309 (5.2) |
| Mycophenolate Mofetil - n/total (%) | 12/306 (3.9) |
| Deaths - n/total (%) | 99/309 (32) |
| Lung transplant - n/total (%) | 3/309 (1) |
Original and expanded GAP model (Imputed data)
Averaged over the imputed datasets, the cross-validated c-index of the continuous GAP model was 0.746 (95% CI: 0.733 – 0.756) (Table 3). We then analyzed the effect of incorporating additional predictor variables on model performance. After consideration of 6 new variables and exploration of all potential combination of variables in the candidate models, the model that performed best included a combination of the four GAP and 2 additional variables, definite UIP pattern on HRCT and a positive RF. The c-index of this new model was 0.749 (95% CI: 0.735 – 0.751). The difference between the c-index of the 2 models was 0.0029 (95% CI: 0.0006 – 0.0089). The two additional variables, definite UIP on HRCT (p = 0.06) and positive RF (p=0.10), were only borderline statistically significant in the multivariate Cox model. Last, we performed model screening without requiring the inclusion of the four GAP variables, and could not identify a model that performed better than the GAP model alone.
Table 3.
Mortality risk prediction models
| Imputed datasets (n = 309) | Complete case analysis (n = 219) | |
|---|---|---|
| c-index (95% CI) | c-index (95% CI) | |
| GAP | 0.746 (0.733 – 0.756) | 0.753 (0.668 – 0.800) |
| Expanded GAP: GAP + Definite UIP + RF | 0.749 (0.735 – 0.751) | 0.760 (0.678 – 0.812) |
| Difference between the 2 models | 0.0029 (0.0006 – 0.0019) | 0.0072 (−0.0233 – 0.0553) |
Abbreviations: GAP = gender-age-physiology; UIP = usual interstitial pneumonia; RF = rheumatoid factor
Sensitivity analysis (complete case analysis)
We repeated the same steps using data for patients with complete data. In this dataset, the c-index of the original GAP model was 0.753 (95% CI: 0.668 – 0.800) (Table 3). When looking at the effect of additional variables on the baseline GAP model, the same variables (definite UIP on HRCT and positive RF) were included in the model with the highest cross-validated c-index of 0.760 (0.678 – 0.812). However, these variables did not significantly change the discriminative ability of the model.
Final model choice and model calibration
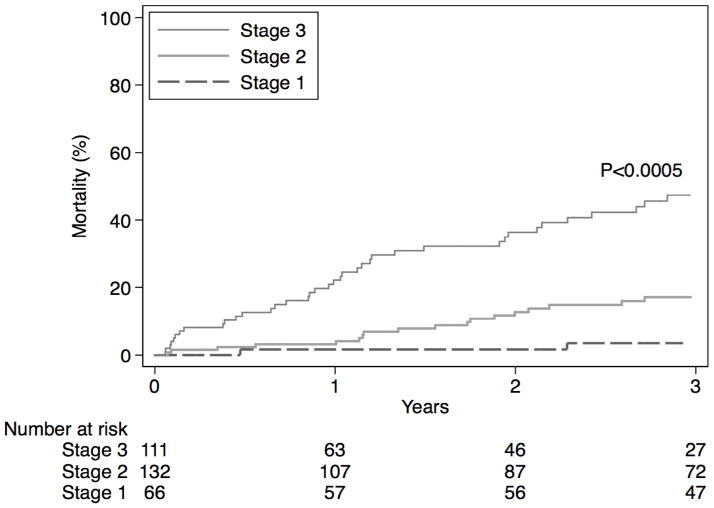
As illustrated above, the incorporation of additional clinical variables to the GAP model did not substantially improve its ability to predict survival in patients with RA-ILD. Using the original GAP model as the final model, we tested the performance of the GAP index and staging system that categorizes patients into 3 stages according to their 1-year mortality. The calibration of the model was satisfactory in both the imputed datasets and the complete case analysis (Table 4). Figure 2 and e-Figure 1 show the cumulative mortality difference when categorized into the 3 risk groups based on imputed and complete case data, respectively. In addition, the model calibration was similar within each center (e-Table 3).
Table 4.
Model performance by GAP stage
| Imputed data | Complete case analysis | |||
|---|---|---|---|---|
|
| ||||
| Predicted | Observed (95% CI) | Predicted | Observed (95% CI) | |
| 1-year mortality% | ||||
| Stage 1 | 2.1 | 1.6 (0.2–11.1) | 2.0 | 0.0 (−) |
| Stage 2 | 6.6 | 3.2 (1.2–8.3) | 6.4 | 4.1 (1.7–9.6) |
| Stage 3 | 17.9 | 22.1 (14.8–32.2) | 18.9 | 22.9 (15.4–33.3) |
| 2-year mortality% | ||||
| Stage 1 | 4.4 | 1.6 (0.2–11.1) | 4.2 | 0.0 (−) |
| Stage 2 | 13.7 | 12.7 (7.7–20.6) | 13.4 | 14.1 (8.7–22.3) |
| Stage 3 | 34.3 | 36.4 (27.0–47.7) | 36.0 | 37.5 (27.9–49.0) |
| 3-year mortality% | ||||
| Stage 1 | 6.3 | 3.5 (0.9–13.3) | 6.0 | 1.9 (0.3–12.9) |
| Stage 2 | 19.0 | 17.1 (11.1–25.9) | 18.8 | 17.6 (11.4–26.6) |
| Stage 3 | 44.9 | 47.4 (36.8–59.3) | 46.9 | 50.3 (39.4–62.3) |
Figure 2.
Cumulative mortality in the imputed dataset by risk category.
Discussion
In this study, we showed that the continuous GAP model, when applied to a large and diverse cohort of patients with RA-ILD, has a discrimination similar to what has been reported in patients with IPF20,23. The addition of other clinical variables, such as definite UIP pattern on HRCT and positive RF, did not meaningfully improve the discrimination of the prediction model. The GAP Index and Staging system also had satisfactory calibration in predicting mortality at year 1, 2 and 3 in our cohort of patients with RA-ILD.
The original GAP model was initially derived and validated in a population of IPF patients and had a c-index of 0.695 (95% CI: 0.656 – 0.727)20. It has since been studied in patients with scleroderma related ILD 24 and other chronic ILDs 25. While RA-ILD was included in the connective tissue disease (CTD) associated ILD group of a prior publication 25, their contribution to the study cohort was small (n= 56 out of 281 with CTD-ILD). This study specifically looks at the performance of the GAP model in a substantially larger cohort of RA-ILD patients from several different countries. Nonetheless, the general consistency of these results across different forms of ILD suggest that they may share similar risk factors for death14,17.
Definite UIP pattern on HRCT or surgical lung biopsy has been shown to be associated with mortality in patients with RA-ILD19,23,26. The prevalence of a definite UIP pattern on high-resolution computed tomography (HRCT) has been reported to vary between 24 and 41%12,18,19. When surgical lung biopsy is performed in patients with RA-ILD, UIP pattern is found in up to 56% of cases14,27. Similar to IPF, radiologic UIP pattern is specific for the finding of UIP pattern on pathology in RA-ILD28. In this study, the incorporation of the definite UIP pattern on HRCT did not significantly improve the c-index of our prediction model over the baseline GAP model, although 73 patients did not have HRCT scans available for review. Similar findings were reported in another smaller cohort of 137 RA-ILD patients. This single-center study demonstrated that UIP pattern was a predictor of survival on univariate analysis, but was not a significant predictor of mortality on multivariate analysis when adjusting for age, sex, smoking history and both baseline and change in FVC % predicted over time15.
In a previous cohort study of 309 RA patients without known ILD, baseline titers of RF were shown to be associated with mortality in a multivariate Cox model adjusting for age, sex and duration of disease29. Positivity of the RF alone was not a significant predictor of mortality. The authors hypothesized higher titers of RF could be associated with a more severe pathological process. In our study, a positive RF was included in the model with the best c-statistic, although it did not substantially improve the performance of the GAP model. Unfortunately, we could not include RF titer as a candidate variable in our model given the variability in the techniques and reporting of the RF titers across the 4 centers. This difference in RF measurement and titer positivity across centers is a limitation.
The strengths of this study include analysis of patients from 4 different centers in 3 different countries, comprising a diverse, international cohort with one of the largest samples sizes to date among studies investigating RA-ILD mortality. We used multiple imputation to deal with missing data, as well as performing complete case analyses; both yielded similar results, suggesting that the findings are robust. This study does have some limitations. The data was retrospectively analyzed from ongoing longitudinal databases. In addition, all patients were selected from ILD referral centers, and may differ from the general population of patients with RA-ILD, making our findings less generalizable.
In summary, our study suggests that the GAP model can be used as a mortality risk prediction tool in patients with RA-ILD. Although the performance of the model could not be meaningfully improved with the addition of common clinical variables, the role of other biologic markers and/or genetic variants on predicting mortality in RA-ILD should be explored.
Supplementary Material
RA-ILD is associated with high morbidity and mortality.
The GAP model that was derived for mortality risk prediction in IPF performs similarly in patients with RA-ILD.
The addition of other clinical variables did not meaningfully improve the performance of the model.
Acknowledgments
The authors thank the physician members and staff of the UCSF Interstitial Lung Disease Program, the Mayo Clinic, Asan Medical Center, University of Ulsan and the University of Modena & Reggio Emilia for their assistance in recruiting patients for this study and the many patients who generously agreed to participate in our longitudinal cohort study.
Financial Sources: This work was supported by the National Center for Advancing Translational Science, NIH, Grant Number UCSF-CTI KL2TR000143 and the Nina Ireland Program for Lung Health.
Footnotes
Author Contributions:
Involvement in conception, hypothesis and design of the study (J.M, E.V, P.J.W, T.E.K, H.R.C, J.S.L); acquisition of the data (J.M, B.Y.L, R.T, X.H, B.M.E, J.H.R, K.D.J, S.C, A.M, M.S, D.S.K J.S.L); analysis and interpretation of the data (J.M, E.V, B.M.E, B.L, P.J.W, T.E.K, H.R.C, J.S.L); substantial involvement in the writing and/or revision of the article (J.M, E.V, B.Y.L, R.T, X.H, J.H.R, S.C, A.M, M.S, B.L P.J.W, T.E.K, D.S.K, H.R.C, J.S.L)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722–727. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turesson C, Eberhardt K, Jacobsson LT, Lindqvist E. Incidence and predictors of severe extra-articular disease manifestations in an early rheumatoid arthritis inception cohort. Ann Rheum Dis. 2007;66(11):1543–1544. doi: 10.1136/ard.2007.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, Johnston SS, John AK. The incidence and prevalence of extra-articular and systemic manifestations in a cohort of newly-diagnosed patients with rheumatoid arthritis between 1999 and 2006. Curr Med Res Opin. 2008;24(2):469–480. doi: 10.1185/030079908x261177. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picchianti Diamanti A, Germano V, Bizzi E, Lagana B, Migliore A. Interstitial lung disease in rheumatoid arthritis in the era of biologics. Pulm Med. 2011;2011:931342. doi: 10.1155/2011/931342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443–448. doi: 10.1513/pats.200703-045MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136(5):1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe F, Caplan L, Michaud K. Rheumatoid arthritis treatment and the risk of severe interstitial lung disease. Scand J Rheumatol. 2007;36(3):172–178. doi: 10.1080/03009740601153774. [DOI] [PubMed] [Google Scholar]
- 11.Dixon WG, Hyrich KL, Watson KD, Lunt M, Symmons DP. Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2010;69(6):1086–1091. doi: 10.1136/ard.2009.120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 13.Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 2010;49(8):1483–1489. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 14.Solomon JJ, Ryu JH, Tazelaar HD, et al. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD) Respir Med. 2013;107(8):1247–1252. doi: 10.1016/j.rmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2015 doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 16.Assayag D, Lubin M, Lee JS, King TE, Collard HR, Ryerson CJ. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology. 2014;19(4):493–500. doi: 10.1111/resp.12234. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007;175(7):705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka N, Kim JS, Newell JD, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology. 2004;232(1):81–91. doi: 10.1148/radiol.2321030174. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya Y, Takayanagi N, Sugiura H, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J. 2011;37(6):1411–1417. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 20.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Annals of internal medicine. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim ES, Choi SM, Lee J, et al. Validation of the GAP score in Korean patients with idiopathic pulmonary fibrosis. Chest. 2015;147(2):430–437. doi: 10.1378/chest.14-0453. [DOI] [PubMed] [Google Scholar]
- 24.Ryerson CJ, O'Connor D, Dunne JV, et al. Predicting mortality in systemic sclerosis-associated interstitial lung disease using risk prediction models derived in idiopathic pulmonary fibrosis. Chest. 2015 doi: 10.1378/chest.15-0003. [DOI] [PubMed] [Google Scholar]
- 25.Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura Y, Suda T, Kaida Y, et al. Rheumatoid lung disease: prognostic analysis of 54 biopsy-proven cases. Respir Med. 2012;106(8):1164–1169. doi: 10.1016/j.rmed.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 28.Assayag D, Elicker BM, Urbania TH, et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology. 2014;270(2):583–588. doi: 10.1148/radiol.13130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chehata JC, Hassell AB, Clarke SA, et al. Mortality in rheumatoid arthritis: relationship to single and composite measures of disease activity. Rheumatology (Oxford) 2001;40(4):447–452. doi: 10.1093/rheumatology/40.4.447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.