Abstract
Gallium-68 (68Ga) is a generator-produced radionuclide with a short half-life (t½ = 68 min) that is particularly well suited for molecular imaging by positron emission tomography (PET). Methods have been developed to synthesize 68Ga-labeled imaging agents possessing certain drawbacks, such as longer synthesis time because of a required final purification step, the use of organic solvents or concentrated hydrochloric acid (HCl). In our manuscript, we provide a detailed protocol for the use of an advantageous sodium chloride (NaCl)-based method for radiolabeling of chelator-modified peptides for molecular imaging. By working in a lead-shielded hot-cell system, 68Ga3+ of the generator eluate is trapped on a cation exchanger cartridge (100 mg, ∼8 mm long and 5 mm diameter) and then eluted with acidified 5 M NaCl solution directly into a sodium acetate-buffered solution containing a DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) or DOTA-like chelator-modified peptide. The main advantages of this procedure are the high efficiency and the absence of organic solvents. It can be applied to a variety of peptides, which are stable in 1 M NaCl solution at a pH value of 3–4 during reaction. After labeling, neutralization, sterile filtration and quality control (instant thin-layer chromatography (iTLC), HPLC and pH), the radiopharmaceutical can be directly administered to patients, without determination of organic solvents, which reduces the overall synthesis-to-release time. This procedure has been adapted easily to automated synthesis modules, which leads to a rapid preparation of 68Ga radiopharmaceuticals (12–16 min).
Introduction
68Ga-labeled compounds for molecular PET imaging are of increasing interest in nuclear medicine. DOTA or NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid)-conjugated peptides have a particularly important role as precursor molecules for the labeling with 68Ga. The well-known 68Ga-labeled somatostatin analogs DOTATOC (DOTA-[Tyr3]-octreotide) and DOTATATE (DOTA-[Tyr3]-octreotate) are particularly well suited for PET imaging of neuroendocrine tumors expressing somatostatin receptors1.
The predominant advantage of 68Ga radiopharmaceuticals is that the synthesis is based on generator-produced 68Ga and that it can be performed on site (and on demand), without the need for a medical cyclotron. Nowadays, 68Ge/68Ga generator systems are widely available, and they consistently deliver high-purity 68Ga for radiolabeling reactions. The parent radionuclide 68Ge is accelerator-produced through the 69Ga(p,2n) reaction, whereby Ga2O3 is used as the target material2.
Several 68Ge/68Ga generators have been developed in which 68Ge is adsorbed as a tetravalent compound onto an inorganic or organic matrix. The parent radionuclide decays with a half-life of 271 d to the trivalent 68Ga3+ cation. Because of distinct chemical differences, the daughter nuclide 68Ga3+ can be easily separated by elution from the generator with aqueous HCl. Depending on the carrier material (generator stationary phase), the concentration of eluent HCl varies from 0.05 to 1.0 M. The concentration of the parent nuclide 68Ge in the eluent in currently available generators has been found to be acceptably low, and it has been thoroughly investigated3. These generators deliver 68Ga in a high chemical purity with a low concentration of foreign ions. Heavy-metal ions such as iron and copper ions have a high impact on the labeling efficiency, as does zinc. Most important are iron impurities in the generator eluate or in the labeling reagents, which therefore must be avoided.
Numerous methods have been developed to synthesize 68Ga-labeled peptides starting from 68Ga elution from the generators. These methods can be classified into six different categories (Table 1). All use the amphoteric behavior of 68Ga to form cationic and anionic species such as cationic 68Ga3+ and anionic tetrachlorogallate [68GaCl4]−. Here we briefly describe the approach of each of these five classes of 68Ga radiolabeling paradigms and we provide a detailed protocol for the use of a NaCl-based method (class 6) for radiolabeling of chelator-modified peptides and small molecules for molecular imaging applications.
Table 1.
Labeling methods.
| Method | Ion exchanger | Elution solution ion exchanger | Buffer | Advantages/limitations | References | |
|---|---|---|---|---|---|---|
| 1 | Fractional elution | _ | _ | HEPES | (+) No preconcentration is required | 4 |
| (−) Only ∼80% of activity is available for labeling | ||||||
| (−) Final purification is required | ||||||
| (−) The final product contains ethanol | ||||||
| 2 | Anionic method | SAX | ∼0.4 ml water | HEPES | (+) The method delivers 68Ga in high chemical purity | 5 |
| (−) Handling with concentrated HCl is necessary | ||||||
| (−) Final purification is required | ||||||
| (−) The final product contains ethanol | ||||||
| 3 | Cationic method (acetone) | SCX | 0.5 ml of acetone/HCl | HEPES | (+) The method is well established | 6 |
| (−) Final purification is required | ||||||
| (−) The final product contains ethanol and other side products | ||||||
| (±) The method is licensed for Eckert & Ziegler | ||||||
| 4 | Cationic method (ethanol) | SCX | 1 ml of ethanol/HCl | HEPES, ammonium acetate | (+) Because of the low concentration of foreign ions in the reaction mixture, high specific activities are achievable | 8–10 |
| (−) The final product contains ethanol | ||||||
| (±) The method is licensed for Eckert & Ziegler | ||||||
| 5 | Combined cationic-anionic method | (1) SCX(2) SAX | (1) 0.4 ml of 4 M HCl(2) ∼0.4 ml of water | HEPES | (+) Because of the low concentration of foreign ions in the reaction mixture, high specific activities are achievable | 11,12 |
| (−) Handling of 7 M HCl is required | ||||||
| (−) The final product contains ethanol | ||||||
| (−) Two steps of postprocessing 68Ga eluate are needed | ||||||
| 6 | Cationic method (NaCl) | SCX | 0.5 ml of 5 M NaCl/0.1 M HCl | Ammonium acetate, sodium acetate | (+) No organic solvents are found in the final product | 13,14 |
| (+) High specific activities are routinely achievable | ||||||
| (±) The reaction mixture usually contains 1 M sodium chloride, which could be disadvantageous for NaCl-sensitive peptides |
The first method known from literature is based on enrichment of 68Ga using a (tin(IV)-oxide) SnO2-based generator. The fraction of the eluate with the highest concentration of activity (e.g., in 1–2 ml) is buffered for labeling, and a final purification step using a solid-phase extraction (SPE; e.g., C18 cartridge) is required to trap the radiopeptide4. This purification step removes buffer compounds such as HEPES and unreacted ‘free’ 68Ga, which are unwanted in the final product, and it also reduces the 68Ge concentration resulting from small quantities of 68Ge breakthrough from the generator. The final (highly pure) product is elutable from the SPE cartridge, usually with a small volume of ethanol, and the mixture can be diluted with 0.9% (wt/wt) NaCl solution.
In a second approach, the labeling begins by eluting the generator and converting the eluted 68Ga3+ into the anionic [68GaCl4]− species by the addition of concentrated HCl (final HCl concentration 5.5 M) and trapping anionic 68Ga on an anion exchanger5. The excess of HCl is removed from the anion exchanger cartridge using a stream of inert gas. [68GaCl4]− can then be eluted with a small volume of water (0.2–0.4 ml) into the buffered precursor-containing reaction vial. Basically, the pH for the labeling of DOTA-conjugated, NOTA-conjugated or similarly conjugated peptides should be adjusted to between 3 and 4 (ref. 4). The labeling can be performed at 85–95 °C for DOTA-bearing peptides or at room temperature (21 °C) for, e.g., NOTA-conjugated compounds6. The use of concentrated HCl may be a disadvantage in the radiopharmaceutical practice.
A third approach to obtain 68Ga-labeled tracers uses a cation exchanger to trap cationic 68Ga3+ directly from the generator eluate. The adsorbed 68Ga can be eluted subsequently with mixtures of HCl-containing organic solvents (e.g., acetone/HCl) into the precursor peptide solution7,8. The organic solvent must be removed by heating the reaction mixture at 95 °C, and the labeled compound is separated using SPE cartridges. The resulting final product acetone concentrations are found to be well within regulatory limits for release. Labeling procedures using ethanol instead of acetone have also been published9,10. Because of the high concentration of ethanol in the radiopharmaceutical product, the determination of ethanol content is required in many countries. This method is patented and licensed for Eckert & Ziegler.
The fifth method for the utilization of 68Ga combines the high efficiency of the cationic concentration with the anionic purification. In this method, concentrated HCl is substituted by a small volume of diluted HCl. Organic solvents are not required11,12. This procedure delivers 68Ga in a high chemical and radiochemical purity, which allows the labeling of peptides at high specific activity. However, the requirement for two cartridges has limited the routine use of this method in practice.
All these methods are limited in their use for the routine production of radiopharmaceuticals, either because of the use of organic solvents or because of the use of semiconcentrated, corrosive-acting HCl.
In contrast, the procedure described in this protocol focuses on a 68Ga radiolabeling procedure (method 6 in Table 1), which requires the use of only one single cation exchanger cartridge, and purification of 68Ga for radiolabeling reactions is achieved just by manipulating cationic/anionic speciation of 68Ga. The method, pioneered by Mueller et al., uses the behavior of 68Ga to form anionic species present in concentrated NaCl solution13. In this method, 68Ga3+ of the generator eluate (usually in 0.1 M HCl or a lower concentration of HCl) is trapped on a cation exchange resin. Rinsing the column with acidified 5 M NaCl solution transforms the cationic 68Ga3+instantly and in situ, which results in desorption and elution of 68Ga as anionic [68GaCl4]−. This eluate is directed to a buffered solution that contains a chelator-modified DOTA-like peptide for labeling (see Fig. 1). The present protocol has been optimized for the use of DOTA- and NOTA-conjugated peptides12–16. The main advantages of this procedure are the high efficiency of desorption of the column and the absence of organic solvents.
Figure 1.
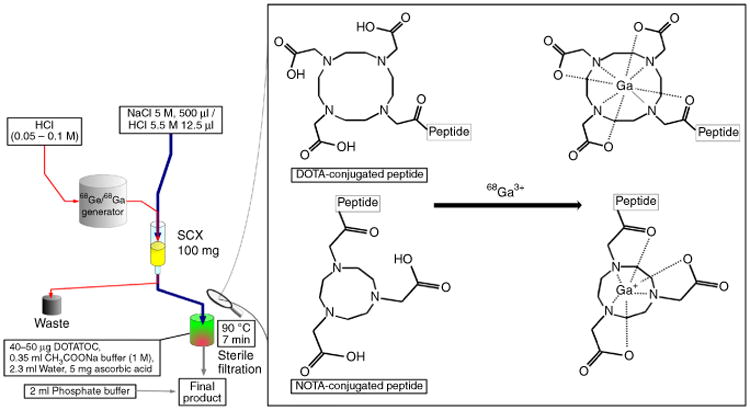
Schematic drawing of the NaCl-based labeling procedure for the labeling of DOTA- or NOTA-conjugated peptides with 68Ga.
Usually, the NaCl eluate of the cation-exchange resin is added to the reaction mixture, which contains 2–3 ml of water, and the NaCl concentration is therefore ∼1 M. Some proteins might be sensitive to high NaCl concentrations, and these usually precipitate by dehydration17. For many proteins and peptides, however, NaCl concentrations up to 1 M show stabilizing effects18–20. We recommend that preliminary experiments (i.e., appropriate biochemical assays) be performed to check that radiolabeling does not alter the biological activity of the peptides or proteins; if this labeling procedure is used to label compounds that are known to be sensitive, the binding affinity of the labeled products should be checked before routine production.
After labeling, neutralization and sterile filtration of the reaction mixture, quality control can be performed, and the 68Ga-labeled radiopharmaceutical can be directly administered to patients. Appropriate quality control for this process is iTLC, HPLC and measurement of pH value. It is not necessary to determine the concentration of organic solvents, which reduces the overall synthesis-to-release time and simplifies the overall process for radiolabeling and quality control for 68Ga-labeled radiopharmaceuticals. Furthermore, this procedure has been adapted easily to an automated synthesis module (Supplementary Fig. 1), thus leading to a rapid preparation of 68Ga-labeled radiopharmaceuticals (14 min; ref. 14).
Materials
Reagents
▲ CRITICAL Selection of ultra-high-purity reagents is crucial to the success of 68Ga labeling procedures. Practitioners should carefully select reagents with the lowest possible metal content (e.g., trace metal grade) to avoid unwanted interference in the 68Ga-DOTA labeling reaction. Examples of precise catalog numbers for trace metal grade reagents are given in this list of reagents. Reagents that are accompanied with a certificate of analysis stating metal concentrations at the parts per trillion level are preferred.
Water for injection (e.g., Fresenius Kabi, cat. no. B101216)
Hydrochloric acid (HCl) solution, >37% (e.g., TraceSELECT; Fluka, cat. no. 84415)
Sodium chloride (NaCl), 99.99% (e.g., Suprapur; EMD Millipore/Merck Millipore, cat. no. 106406)
Water, ultrapure (e.g., EMD Millipore/Merck Millipore, cat. no. 101262)
Sodium acetate, ≥99.999% (e.g., TraceSELECT; FLUKA, cat. no. 59929)
Acetic acid, 100% (e.g., EMD Millipore/Merck Millipore, cat. no. 100063)
Sodium di-hydrogen phosphate dihydrate for analysis (e.g., EMSURE Reag. Ph Eur; EMD Millipore/Merck Millipore, cat. no. 106342)
Di-sodium hydrogen phosphate dodecahydrate for analysis (e.g., EMSURE Reag. Ph Eur; EMD Millipore/Merck Millipore, cat. no. 106579)
l-Ascorbic acid ACS reagent, ≥99% (e.g., Sigma-Aldrich, cat. no. 255564)
Trifluoroacetic acid for spectroscopy (e.g., Uvasol (TFA); EMD Millipore/Merck Millipore, cat. no. 108262)
Acetonitrile for preparative chromatography (e.g., Prepsolv; EMD Millipore/Merck Millipore, cat. no. 113358)
Ammonium acetate for analysis (e.g., EMSURE Reag. Ph Eur; EMD Millipore/Merck Millipore, cat. no. 101116)
Methanol for analysis (e.g., EMSURE Reag. Ph Eur; EMD Millipore/Merck Millipore, cat. no. 106009)
Colorimetric iron test (e.g., Merck Millipore, cat. no. 114759)
▲ CRITICAL Use this test in parallel to check your reagents and equipment for the presence of iron contamination (Box 1).
-
Colorimetric copper test (e.g., Merck Millipore, cat. no. 114765)
▲ CRITICAL Use this test in parallel to check your reagents and equipment for the presence of copper contamination.
-
Colorimetric zinc test (e.g., Merck Millipore, cat. no. 114780)
▲ CRITICAL Use this test in parallel to check the eluate of the generator for the presence of zincions after the complete decay of 68Ga.
iTLC eluent (Box 2)
HPLC solvent A (Box 2)
HPLC solvent B (Box 2)
DOTA-like conjugated peptide (e.g., piChem, Bachem); DOTA-TOC, DOTA-NOC, DOTA-TATE, NODAGA-THERANOST and DOTA-SB3 are commonly used. Peptides carrying chelators that are different from DOTA or NOTA should be carefully checked for labeling efficiency when applying this protocol (e.g., for DATA-TOC, it was reported that this protocol led to incomplete labeling10).
Box 1. Determination of iron ● TIMING ∼6 min.
Radiolabeling of tracers with 68Ga requires that the reagents, tips, caps and reaction vessels used be as free as possible of trace metals, especially iron. If required, metal-free plastic needles must be used during the labeling reaction (Non-Vented Spike, e.g., Qosina, cat. no. 11757 or 11656). The determination of iron contaminations in reagents, generator eluate or other components using an atom absorption spectrometer is possible, but the use of the colorimetric test as mentioned above in Reagents is very helpful and easy to handle. The colorimetric test allows determination of iron concentrations between 0.1 and 5 μg/ml. The concentration of iron during the labeling should be <0.1 μg/ml.
Box 2. Measurement of radiolabeling efficiency ● TIMING iTLC (option a), ∼6 min HPLC (option B), 10–15 min.
The radiolabeling efficiency can be measured by iTLC (option A) or by HPLC (option B). The HPLC shows also potential side products that can be formed by radiolysis.
Additional materials
iTLC:
iTLC chamber
iTLC Paper (iTLC-SG; Agilent, cat. no. SGI001)
miniGITA positron Radio iTLC scanner (e.g., Raytest)
Eluent for 68Ga-labeled DOTA-like conjugated peptides used in this protocol: 1 M ammonium acetate/methanol (1:1 vol/vol)
HPLC
HPLC pump: Jasco PU-1580; quaternary gradient unit: Jasco LG-1580-04
HPLC radio detector: biostep IsoScan LC
RP-18 column (e.g., LiChrospher 100 RP-18 endcapped (5 μm) LiChroCART 250-4, EMD Millipore/Merck Millipore, cat. no. 150838)
HPLC eluents for 68Ga-labeled DOTA-like conjugated peptides used in this protocol: solvent A: 5% acetonitrile in water (0.1% TFA); and solvent B: 95% acetonitrile in water (0.01% TFA)
HPLC method:
Flow rate: 1.2 ml/min
| Equilibration time: | 2 min | A: 100% | B: 0% |
| Start method: | 0–2 min | A: 100% | B: 0% |
| Gradient: | 2–15 min | A: 100–0% | B: 0–100% |
| 15–17 min | A: 0% | B: 100% | |
| Gradient: | 17–19 min | A: 0–100% | B: 100–0% |
| 19–21 min | A: 100% | B: 0% |
Procedure
1. Measurement of radiolabeling efficiency by various techniques
-
Measurement of radiolabeling efficiency by itlc
Pour the iTLC eluent (Reagent Setup) into the iTLC chamber until it is 0.5 cm deep and covers the chamber.
Place a 1- to 2-μl spot of the sample of the final reaction mixture onto the start point of the iTLC strip (at the first centimeter of the iTLC strip above the level of the iTLC eluent in the chamber).
Place the iTLC strip upright in the iTLC chamber gently. Cover the chamber and allow the solvent to run up the support material.
When the solvent is ∼1 cm from the top of the strip, remove it from the chamber and dry the strip.
Place the strip into a thin plastic sheet (e.g., Glad Clingwrap) and lay it onto the radio-iTLC scanner in the appropriate orientation for the scan.
Scan the radio-iTLC and calculate the amount of unchelated 68Ga (Rf = 0–0.1) and the 68Ga-labeled peptide (Rf = 0.8–1.0).
-
Measurement of radiolabeling efficiency by Hplc
Take a sample (10–20 μl) of the final reaction solution and inject it on the RP-HPLC using the HPLC method as mentioned in this box.
Preliminary step. Usually, the sample can be injected directly without dilution using the HPLC radio detector, as mentioned in the Equipment Setup. Because of the pH of the reaction mixture (3μ4), a neutralization of the HPLC sample is not required. Care should be taken to optimize the aliquot volume and radioactivity concentration of the radio-HPLC aliquot to ensure that the radioactivity concentration does not overwhelm the radioactivity detector at the outflow of the radio-HPLC system. Preliminary experiments should be conducted to develop an understanding of detection efficiency and upper threshold of the detector before the preparation of a dose that will be used for important biological experiments or clinical work.
Equipment
▲ CRITICAL The equipment and apparatus presented here are for informational purposes. For example, several manufacturers offer variants of the dose calibrator presented in this list that would be suitable for measurement of the final product radioactivity concentration. The cation-exchange and reverse-phase cartridges that are presented were available at the time of writing and were effective in our method development and evaluations presented here.
Bond Elut SCX Cartridges, 100 mg, particle size 40 μm (Agilent, cat. no. 12102013)
Accument basic pH meter (Fisher, cat. no. 13-636-AB15PB)
CRC-25PET Dose Calibrator (Capintec, cat. no. 5130-3215)
Sep-Pak C18 Plus Short Cartridge, 360 mg sorbent per cartridge, 55–105 μm particle size (Waters, cat. no. WAT020515; this cartridge is commonly not required, and it is only relevant if a final purification is needed; Table 1)
Ethanol 96% (e.g., EMD Millipore/Merck Millipore, cat. no. 1.00971)
Reaction vial (e.g., Wheaton V vial 10 ml, diameter 24 mm, cat. no. W015285), alternatively an evacuated vial 10 ml is also applicable (e.g., Mallinckrodt, Evacuated Vials for use with UltraTechnekow 10 ml, cat. no. N784A0)
Heating block (e.g., Biostep, metal block thermostat, cat. no. GT53-S0001, metal block, d = 25 mm (reaction vial dependent), cat. no. F3509)
Sterile syringe filter: Millex-GV Syringe Filter Unit, 0.22 μm, PVDF, 33 mm (EMD Millipore/Merck Millipore, cat. no. SLGV033RS), or sterile syringe filter, ultra-low protein binding, Cathivex-GV 25 mm, 0.22 μm, PVDF (EMD Millipore/Merck Millipore, cat. no. SLGV0250S)
Luer-Lok connector for the SCX cartridge (neoLab (http://www.myneolab.de), Connector, LL female/olive 4.0–5.0 mm, cat. no. 21889)
Reagent Setup
0.1 M HCl 0.1 M HCl is used for the elution of the 68Ge/68Ga generator (Eckert & Ziegler). Dilute 8.3 ml of 37% HCl with water (Ultrapure) to 1,000 ml. Can be stored at room temperature (21 °C); stable for 1 year.
0.05 M HCl 0.05 M HCl is used for the elution of the 68Ge/68Ga generator (ITM Isotope Technologies Munich AG). Dilute 4.2 ml of 37% HCl with water (Ultrapure) to 1,000 ml. Can be stored at room temperature (21 °C); stable for 1 year.
0.6 M HCl 0.6 M HCl is used for the elution of the 68Ge/68Ga generator (iThemba 68Ge/68Ga generator, IDB Holland bv). Dilute 50 ml of 37% HCl with water (Ultrapure) to 1,000 ml. Can be stored at room temperature (21 °C); stable for 1 year.
5.5 M HCl solution 5.5 M HCl solution is used for the preconditioning of the SCX cartridge. Mix 23 ml of 37% HCl in 27 ml of water (Ultrapure). Can be stored at room temperature (21 °C); stable for 1 year.
5 M NaCl/HCl solution 5 M NaCl/HCl solution is used for the elution of 68Ga from the SCX cartridge. Mix 7.3 g of NaCl and 625 μl of 5.5 M HCl in 25 ml of water (Ultrapure). Can be stored at room temperature (21 °C); stable for 1 year.
1.4% ascorbic acid solution 1.4% ascorbic acid solution is used to reduce radiolysis in this protocol. The ascorbic acid solution should be freshly prepared.
1 M sodium acetate buffer, pH 4.5 1 M sodium acetate buffer, pH 4.5, is used for the labeling of DOTA-like conjugated peptides in this protocol. Mix 4.1 g of sodium acetate in 50 ml of water (Ultrapure) and 1 ml of concentrated HCl (37%), adjusted with acetic acid (100%) to a pH of 4.5. Can be stored at room temperature (21 °C); stable for 1 year.
Reaction solution Reaction solution is used for the labeling of the DOTA-like conjugated peptide. Mix 25–35 nmol of the DOTA-like conjugated peptide (e.g., 40–50 μg of the DOTATOC or DOTATATE) and 0.35 ml of 1 M sodium acetate buffer (pH = 4.5) in water (e.g., 0.3–2.5 ml). To reduce radiolysis of the radiolabeled peptide, the reaction mixture should also contain a radical scavenger such as 0.35 ml of 1.4% ascorbic acid solution. The reaction solution should be freshly prepared.
Phosphate buffer Phosphate buffer for the final neutralization is used in this protocol. Mix 3.05 g of disodium monohydrogen phosphate dodecahydrate (Ph. Eur.) and 0.462 g of sodium dihydrogen phosphate dihydrate (Ph. Eur.) in 20 ml of water for injection. Can be stored at room temperature (21 °C); stable for 1 year.
Preconditioning of the SCX cartridge Preconditioning of the SCX cartridge used for trapping of 68Ga of the 68Ge/68Ga generator eluate: if necessary, cut the SCX cartridge. Plug in the Luer-lok barb fitting to the SCX cartridge or the cutoff cartridge, as shown in Figure 2. Rinse the SCX cartridge with 1 ml of 5.5 M HCl and subsequently with 10 ml of water for injection.
Figure 2.
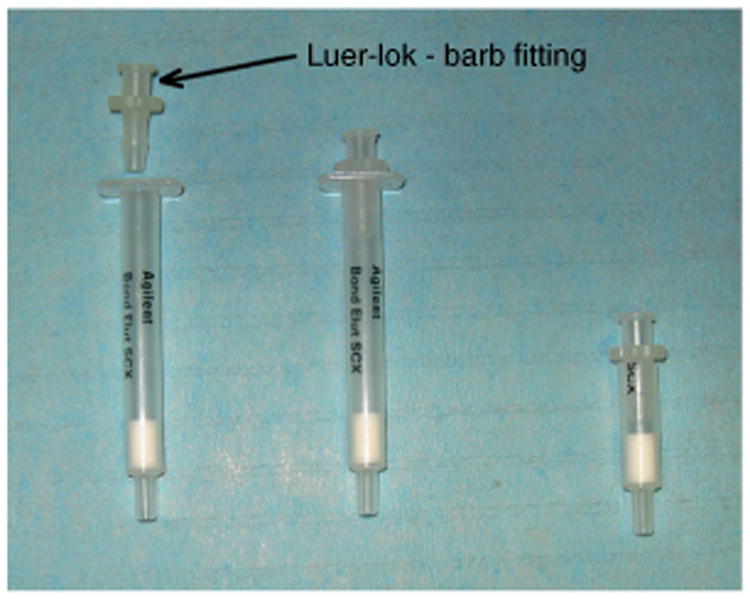
Preconditioning of the SCX cartridge, SCX cartridge with Luer-lock barb fitting.
Equipment Setup
Generators This procedure will work with a variety of different generators. We describe the specifications and elution conditions for three generators that we have experience with. ! CAUTION 68Ga emits both positrons and gamma rays. It is imperative that employees using these procedures observe the guidelines set forth by their institution and the Nuclear Regulatory Commission, and that they observe ALARA (as low as reasonably achievable) protocols to minimize radiation exposure. When handling any radioactive material, proper protective equipment, shielding, body and ring dosimetry badges, and a survey meter are required.
Eckert & Ziegler This 68Ge/68Ga generator can be ordered from Eckert & Ziegler Radiopharma Segment Headquarters (for sales offices world-wide please contact info.eurotope@ezag.de). As dictated by the manufacturer, this generator is eluted with 6 ml of 0.1 M HCl. The generator eluate can be transferred directly through the SCX cartridge. ! CAUTION 68Ga emits both positrons and gamma rays; refer to the precautionary advice above.
ITM Isotope Technologies Munich AG This 68Ge/68Ga generator (itG 68Ge/68Ga generator) can be ordered from ITM Isotope Technologies Munich AG (for sales offices world-wide please contact sales@itg-garching.de). As dictated by the manufacturer, the generator is eluted with 0.05 M HCl. The generator eluate can be transferred directly through the SCX cartridge. ! CAUTION 68Ga emits both positrons and gamma rays; refer to the precautionary advice above.
IDB Holland bv This 68Ge/68Ga generator (iThemba 68Ge/68Ga generator) can be ordered from IDB Holland bv (for sales offices world-wide please visit http://www.idb-holland.com/gallium-68-generator/, www.sales@idb-holland.com). As dictated by the manufacturer, the generator is eluted with 0.6 M HCl. ! CAUTION 68Ga emits both positrons and gamma rays; refer to the precautionary advice above.
▲ CRITICAL The generator eluate must be diluted with five times the volume with water before transferring the eluate through the SCX cartridge.
Procedure
Labeling and quality control of DOTA-like peptides with 68Ga ● TIMING 12–16 min
! CAUTION The adsorbed 68Ga emits both positrons and gamma rays. It is imperative that employees using these procedures observe the guidelines set forth by their institution and the Nuclear Regulatory Commission, and that they observe ALARA protocols to minimize radiation exposure. When you are handling any radioactive material, proper protective equipment, shielding, body and ring dosimetry badges, and a survey meter should be used appropriately according to these guidelines.
▲ CRITICAL Check all the reagents, tips, caps and reaction vessels and the 68Ge/68Ga generator eluate in parallel for the presence of iron contamination (Box 1).
1| Elute the68Ge/68Ga generator with 6 ml of aqueous HCl. The concentration of HCl for eluting the generator depends on the type of generator (see ‘Generators’ in Equipment Setup section and Reagent Setup). A flow rate of ∼2 ml per min elutes 68Ga from the generator.
2| If the concentration of HCl used is 0.6 M (iThemba 68Ge/68Ga generator), dilute the 68Ge/68Ga generator eluate with 30 ml of water.
3| Transfer the eluate through the SCX cartridge. If the concentration of HCl for eluting the generator is 0.1 M or lower (depending on the used generator), the eluate of the generator can be transferred directly through the SCX cartridge by connecting the outlet of the 68Ge/68Ga generator with the SCX cartridge.
4| Detach the SCX cartridge and dry it with a stream of air.
5| Elute the 68Ga, which is trapped onto the SCX, with 0.5 ml of 5 M NaCl/HCl solution into the reaction vial, which contains the reaction solution; the final pH in the reaction vial should be pH 3–4. Incubate the reaction mixture at 85–95 °C for 8–12 min.
6| (Optional) Determine the optimal reaction time by analyzing 1- to 2-μl aliquots of the reaction mixture by radio-iTLC or radio-HPLC, as described in Box 2.
▲ CRITICAL STEP We recommend that this initial experiment be done so as to achieve the required radiochemical purity (% incorporated 68Ga) for final product release.
▲ CRITICAL STEP For radio-iTLC, a 1- to 2-μl aliquot of the reaction mixture can be directly applied to the iTLC plate.
7| Determine the efficiency of the reaction. To do this, remove a 1- to 2-μl aliquot of the reaction mixture and analyze it by radio-iTLC or radio-HPLC, as described in Box 2.
? TROUBLESHOOTING
8| At the end of the reaction period—after you are satisfied that the reaction has gone to completion or you have completed the steps in the Troubleshooting table—add 2 ml of sterile sodium phosphate buffer to the reaction mixture to adjust the pH of the solution to 6–7.
? TROUBLESHOOTING
9| Pass the reaction mixture through a sterile filter, as mentioned in the equipment list.
If the reaction mixture reaches a radiochemical purity (expressed as % of incorporation of activity in the DOTA-like peptide) that meets the stated release criterion (typically >95%) and the solution has been sterile filtered, the final product may be diluted further for dose administration to any desired volume for animal or patient studies, respectively.
■ PAUSE POINT
? TROUBLESHOOTING
10| The stability of the final product at room temperature should be investigated in a separate labeling experiment by radio-HPLC. If a radical scavenger is used, the labeled peptide can be usually applied within 4 h. For a clinical routine production of 68Ga-labeled radiopharmaceuticals, it has been shown that a time distance of a minimum of 2 h after the first elution is sufficient for starting a second radiolabeling reaction. As described in the ANTICIPATED RESULTS, the decay of 68Ge of the generator delivers enough new 68Ga for the second synthesis.
? TROUBLESHOOTING
Troubleshooting advice can be found in table 2.
Table 2.
Troubleshooting table.
| step(s) | problem | solution |
|---|---|---|
| Steps 7 and 8 | Unchelated 68Ga is discovered in the solution | Take a sample and measure its pH. The final pH during the reaction should be between 3 and 4. If the pH is <3, add sodium acetate buffer (Reagent Setup) to the reaction mixture stepwise in 100-ml aliquots until the pH is between 3 and 4. If the pH is higher than 4, adjust the pH with diluted HCl or use a lower amount of sodium acetate buffer for the next labeling so that the pH of the reaction mixture is between 3 and 4 After re-adjusting the pH of the reaction mixture, incubate the reaction solution for an additional 7 min and analyze an aliquot by radio-iTLC and/or radio-HPLC. |
| Unchelated 68Ga is still present in the solution after control of the pH of the reaction mixture | Incubate the reaction solution for an additional 5 min and analyze another aliquot by radio-iTLC and/or radio-HPLC | |
| Unchelated 68Ga is still present in the solution after the additional incubation | Add 5–7 nmol DOTA-like conjugated peptide (if specific activity is not a main focus) and incubate for an additional 5 min. Analyze another aliquot by radio-iTLC and/or radio-HPLC | |
| Steps 7–9 | Unchelated 68Ga is still present in the solution after incubation with additional peptide | Apply the reaction mixture to an activated SPE (e.g., Sep-Pak) cartridgea. Wash the cartridge with 5 ml of water (for injection) and elute dropwise the 68Ga-labeled peptide with 0.5 ml of a 1:1 ethanol:saline solution |
| Steps 7 and 8 | Unchelated 68Ga is present in the solution and you suspect that this is due to iron contamination of the reaction mixture | Check all used reagents for iron contamination (including the generator eluate and other components (e.g., reaction vial)) using a colorimetric test (Box 1) or an atomic absorption spectrometer. Replace the contaminated reagent or component. If iron is detectable on the preconditioned SCX cartridge, rinse the SCX cartridges before the labeling with 1 ml of HCl (5.5 M), wait 2 min, and rinse again with 1 ml of HCl (5.5 M) and then with 10 ml of water. If iron is detectable in the reaction vial, rinse the vial before the labeling with 1 ml of HCl (5.5 M) and then with 10–20 ml of water |
| Steps 7–10 | Multiple radioactive peaks are observed from a sample that is known to be analytically pure and free of isomers | Radiolysis may be occurring. Add ∼5 mg of a radical scavenger to the original reaction buffer and start the labeling procedure again. Possible radical scavengers include ascorbic acid, ethanol, gentisic acid and methionine |
Sep-Pak purification of the labeled peptide: If a Sep-Pak C-18 cartridge is required to remove unreacted Ga3+, it should be activated by preconditioning with ethanol. For this purpose, rinse the cartridge with 5 ml of ethanol and then with 5 ml of water (‘for injection’ quality). Transfer the reaction mixture through the C-18 cartridge and rinse the cartridge again with 2 ml of water (‘for injection’ quality). After elution of the 68Ga-labeled peptide with ethanol, it is essential to either remove the organic solvent from the resulting solution or to dilute the solution with isotonic saline solution before using the labeled compound as a radiopharmaceutical for animal studies or patient administration. The solvent can be evaporated by allowing a gentle stream of inert gas (helium, argon or nitrogen) to pass over the solution. To avoid adherence of the labeled peptide onto the inner surface of the reaction vial, the solution should not be concentrated to dryness.
● Timing
Steps 1–10, labeling and quality control of DOTA-like peptides with 68Ga: 12–16 min
Box 1, determination of iron: ∼6 min
Box 2, measurement of radiolabeling efficiency: iTLC (option A), ∼6 min; HPLC (option B), 10–15 min
Anticipated Results
Our method for the labeling of DOTA-like conjugated peptides with 68Ga has been published in earlier publications13,14. This labeling procedure was routinely used in >1,000 synthesis runs, for >3,000 patient scans and in several nuclear medicine facilities. If the labeling is carried out in the absence of heavy-metal contaminations (especially iron and copper), the probability of success using this protocol is high. Typically, the labeling efficiency of the 68Ga-labeled DOTA-like conjugated peptides should be >95% within 12 min at 90 °C. By using the above-mentioned iTLC method, Rf values of ∼0.8–1.0 are obtained for the labeled peptide, whereas any unchelated 68Ga (colloidal) remains at the start point of the TLC plate or migrates with a retardation factor of ∼Rf = 0–0.1 (Fig. 3). In the case of a radiopharmaceutical production of a 68Ga-labeled peptide (e.g., for patient doses), the iTLC method may need to be changed to adhere to current local pharmaceutical regulations.
Figure 3.
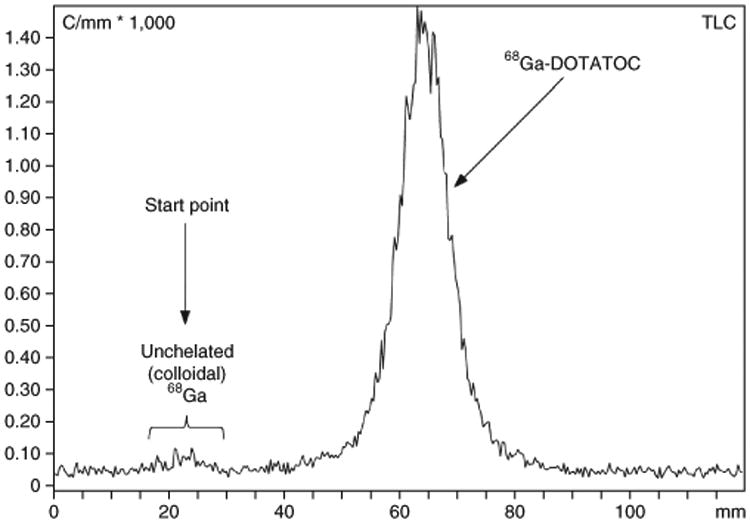
An example of a radio-iTLC chromatogram of [68Ga]-DOTATOC using the iTLC method described in Box 2 (mobile phase consisting of 1 M ammonium acetate/methanol 1:1 vol/vol). The 68Ga-labeled peptide has an approximate retardation factor of 0.8–1.0.
Depending on the hydrophobicity or hydrophilicity of the peptide, the HPLC column and the gradient applied, the retention times for different 68Ga-labeled peptides will vary. A typical HPLC chromatogram of the 68Ga-labeled peptide 68Ga-DOTATOC is shown in Figure 4. By using the above-mentioned HPLC system and HPLC method, the 68Ga-labeled peptide has an approximate retention time of 9 ± 3 min, and it can be determined by the radiodetector of the HPLC. Any nonchelated 68Ga should be detected in the first 1–3 min of the run at a flow rate of 1.2 ml/min (void volume).
Figure 4.
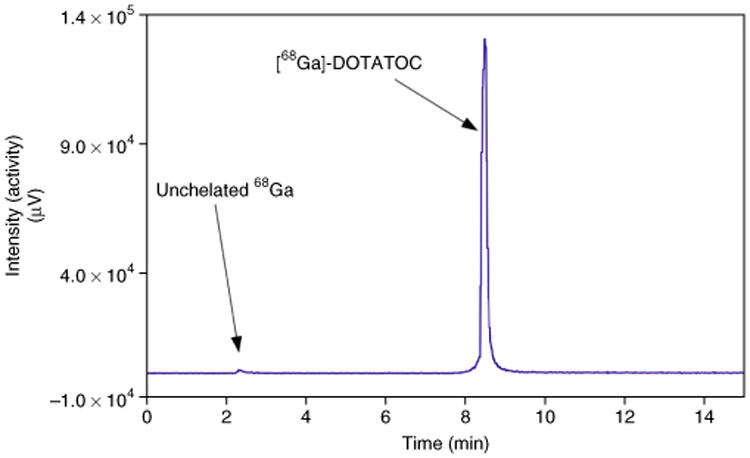
An example of a HPLC chromatogram of [68Ga]-DOTATOC using the HPLC system previously described, a mobile phase consisting of 0.1% TFA in 5% acetonitrile/water (solution A) and 0.01% TFA in 95% acetonitrile/water (solution B) and a gradient from 0–2 min 100% A, 2–15 min to 100% B (flow rate: 1.2 ml/min). The 68Ga-labeled peptide has an approximate retention time of 9 ± 3 min (refs. 13,14).
Elution behavior of the 68Ge/68Ga generator
After elution of the 68Ge/68Ga generator, new 68Ga will be formed by decay of the parent radionuclide 68Ge. The growth of 68Ga subsequent to elution follows first-order kinetics described by well-known radioactive daughter growth equations. Thus, elution of 68Ga after 68 min results in an approximate activity that is 50% of the total 68Ge present in the column. It follows then that, after 136 min (two half-lifes of 68Ga), the expected elution of 68Ga is 75% of total 68Ge present, which is a useful activity of 68Ga to start a new synthesis run. After ∼10 h (10 half lives), >99% of the activity of 68Ga will have grown into equilibrium and may be eluted (Fig. 5).
Figure 5.
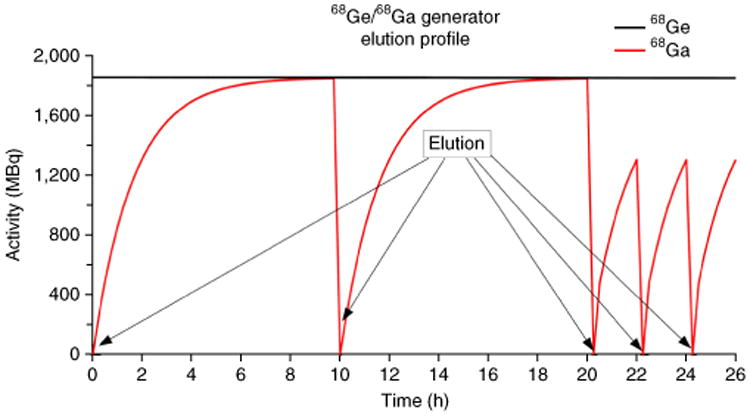
Elution profile of the 68Ge/68Ga generator and formation of 68Ga. 10 h after the former elution, there is an equilibrium of formation of 68Ga and decay to 68Zn, and the maximum of the elutable activity of 68Ga is reached. By two half-lives after the former elution, 75% of the maximum 68Ga activity is already elutable from the generator.
An example of the progress of 68Ga activity after elution of the generator and start of an automated pharmaceutical synthesis run for the labeling of, e.g., DOTATOC, is shown in Figure 6. The synthesis is completed after 14 min, and an additional 6 min are required for the quality control (radio-iTLC, pH). Overall, the final radiopharmaceutical product is ready to be delivered to the clinics within 20 min from the start of the synthesis (including time for elution of the generator).
Figure 6.
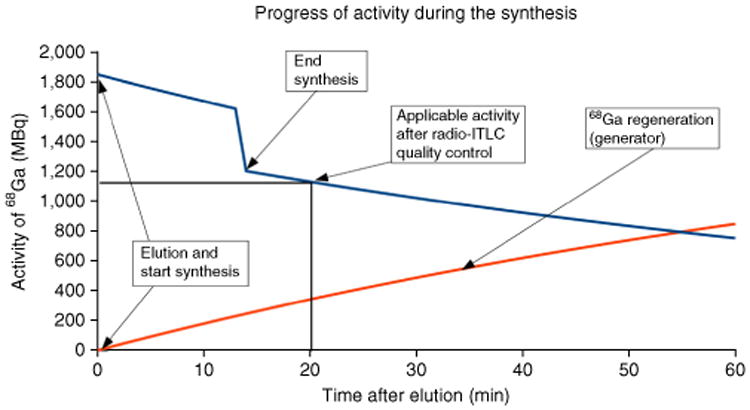
Progress of 68Ga activity during one automated synthesis run for the routine pharmaceutical production of 68Ga-DOTATOC—synthesis time: 14 min, time until application: 20 min (vertical black line), start activity: 1.85 GBq, activity after synthesis: 1.20 GBq, applicable activity for patients: 1.13 GBq (horizontal black line). Blue line: progress of eluted activity of 68Ga that is used for the automated synthesis run. Red line: regeneration of 68Ga activity in the 68Ge/68Ga generator after elution. The loss of activity at the end of the synthesis results from the loss of final product due to adsorption processes during the sterile filtration and in the synthesis module and also because of the taken samples for the quality control.
For routine use of 68Ga-labeled peptides in clinical practice, a sequential production of 68Ga-labeled radiopharmaceuticals is often required. Figure 7 shows the expected activity in sequentially performed synthesis runs. While the second synthesis starts 2 h after the first elution, the start activity is ∼25% lower compared with the first daily synthesis run (Fig. 7). Importantly, radioactive decay of 68Ga produces measureable quantities of stable isotope 68Zn. The concentration of zinc in the generator eluate increases as a function of time after elution4, as shown in Figure 8a. High concentrations of zinc ions may interfere with 68Ga labeling reactions; therefore, the time between the last elution of the generator and the start of a radiolabeling experiment should not be longer than 48 h. Although the procedure that we describe here eliminates most cations (such as Zn), significant quantities of Zn indeed build continuously between generator elutions. Thus, it is prudent to minimize the potential for interference by frequent flushes of the generator to remove stable Zn and any other impurities that may be associated with the solid matrix generator materials. After 3 d post-prior elution of the 68Ga/68Ga generator, the calculated ratio of 68Zn versus 68Ga corresponds to 43 (Fig. 8b). The influence of zinc, iron, copper and other metal ions on labeling efficiency is described in detail in the literature21,22. The colorimetric test with thioacetic acid as standard procedure for the determination of critical concentrations of heavy metals, as described in the European Pharmacopoeia23, is a very useful tool for the routine quality control of the generator eluate. In some countries, the determination of the concentration of iron and zinc in the generator eluate is requested. The test kits, as mentioned in Reagents, are based on the formation of colored metal complexes with high extinction coefficients, and they allow the easy determination of iron and zinc in a range between 0.1 and 5 μg/ml.
Figure 7.
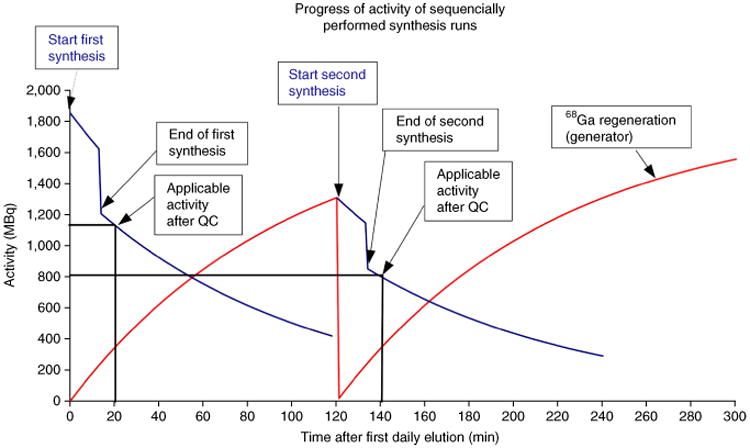
Progress of activity of sequentially performed synthesis runs for the routine pharmaceutical production of 68Ga-DOTATOC—first start activity: 1.85 GBq, activity after first synthesis: 1.20 GBq, first applicable activity (20 min, vertical black line): 1.13 GBq (horizontal black line), second start activity: 1.31 GBq, activity after second synthesis: 0.85 GBq, second applicable activity (140 min, vertical black line): 0.80 GBq (horizontal black line). Blue line: progress of eluted activity on 68Ga that is used for the automated synthesis run. Red line: regeneration of 68Ga activity in the 68Ge/68Ga generator after elution.
Figure 8.
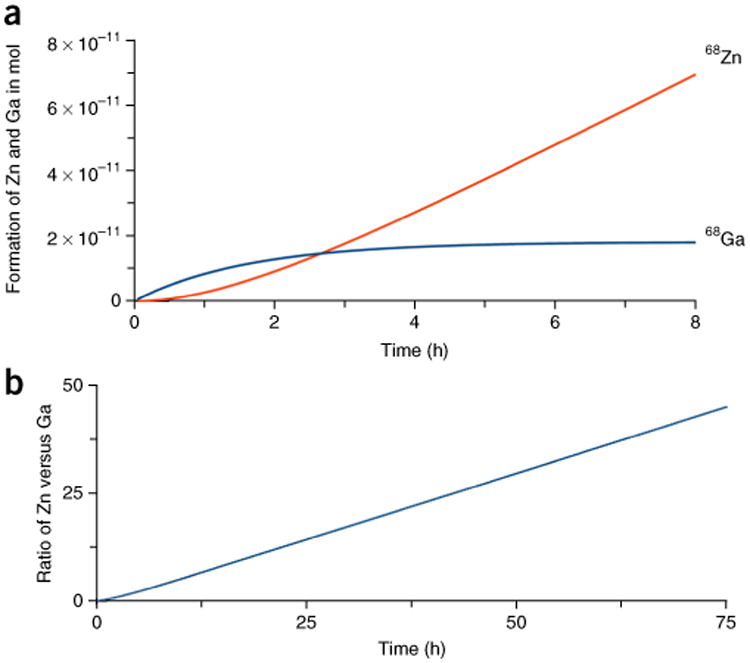
Decay of 68Ga and formation of 68Zn. (a) Calculated formation of 68Ga and 68Zn as f (time) in hours after elution of a 50 mCi (1.85 GBq) 68Ge/68Ga generator. The specific activity of 68Ga corresponds to 9.81 × 10−15 mol/MBq. (b) Calculated molar ratio of 68Zn versus 68Ga as f (time) in hours after post-prior elution of 50 mCi (1.85 GBq) 68Ga of a 68Ge/68Ga generator. In theory, 3 d after post-prior elution the molar ratio of [68Zn]/[68Ga] = 43.
Footnotes
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
Author Contributions: D.M. conceived and developed the original NaCl-based method; W.A.P.B. and I.K. revalidated the procedures and optimized the conditions; M.G. suggested suitable cation-exchange resigns and the NaCl elution from SCX cartriges; M.B. and A.O. evaluated the method from medical point of view; and I.T. and M.K.S. tested the procedure on different generators. All authors wrote and edited the manuscript.
Competing Financial Interests: The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Menda Y, et al. Repeatability of gallium-68 DOTATOC positron emission tomographic imaging in neuroendocrine Tumors. Pancreas. 2013;42:937–943. doi: 10.1097/MPA.0b013e318287ce21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Atomic Energy Agency; 2010. Production of Long Lived Parent Radionuclides for Generators, 68Ge, 92Sr, 90Sr and 188W. http://www-pub.iaea.org/MTCD/publications/PDF/Pub1436_web.pdf. [Google Scholar]
- 3.Velikyan I. Prospective of 68Ga-radiopharmaceutical development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Blois E, et al. Characteristics of SnO2-based 68Ge/68Ga generator and aspects of radiolabelling DOTA-peptides. Appl Radiat Isot. 2011;69:308–315. doi: 10.1016/j.apradiso.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Meyer GJ, Mäcke H, Schuhmacher J, Knapp WH, Hofmann M. 68Ga labelled DOTA-derivatised peptide ligands. Eur J Nucl Med Mol Imaging. 2004;31:1097–1104. doi: 10.1007/s00259-004-1486-0. [DOI] [PubMed] [Google Scholar]
- 6.Eder M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: A new PET tracer for imaging of prostate cancer. Pharmaceuticals. 2014;7:779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhernosekov KP, et al. Processing of generator produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 8.Roesch F. Post-processing via cation exchange cartridges: versatile options. In: Baum RP, Roesch F, editors. Theranostics, Gallium-68 and Other Radionuclides Recent Results in Cancer Research. Vol. 194. Springer-Verlag; 2012. pp. 33–42. [DOI] [PubMed] [Google Scholar]
- 9.Eppard E, Wuttke M, Nicodemus PL, Rösch F. Ethanol-based post-processing of generator-derived 68Ga toward kit-type preparation of 68Ga-radiopharmaceuticals. J Nucl Med. 2014;55:1023–1028. doi: 10.2967/jnumed.113.133041. [DOI] [PubMed] [Google Scholar]
- 10.Seemann J, Eppard E, Waldron BP, Ross TL, Roesch F. Cation exchange-based post-processing of 68Ga-eluate: a comparison of three solvent systems for labelling of DOTATOC, NO2AP(BP) and DATA(m.) Appl Radiat Isot. 2015;98:54–59. doi: 10.1016/j.apradiso.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Müller D, Klette I, Baum RP. The combined cationic-anionic purifcation of the 68Ge/68Ga generator eluate for the labeling of fragile peptides. World J Nucl Med. 2011;10:77–78. [Google Scholar]
- 12.Loktionova NS, et al. Improved column-based radiochemical processing of the generator produced 68Ga. Appl Radiat Isot. 2011;69:942–946. doi: 10.1016/j.apradiso.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Mueller D, et al. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem. 2012;23:1712–1717. doi: 10.1021/bc300103t. [DOI] [PubMed] [Google Scholar]
- 14.Schultz MK, Mueller D, Baum RP, Leonard Watkins G, Breeman WAP. A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl Radiat Isot. 2013;76:46–54. doi: 10.1016/j.apradiso.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maina T, et al. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur J Nucl. 2016;43:964–973. doi: 10.1007/s00259-015-3232-1. [DOI] [PubMed] [Google Scholar]
- 16.Baum RP, et al. First-in-human study demonstrating tumor-angiogenesis by PET/CT imaging with Ga-68-NODAGA-THERANOST, a high-affinity peptidomimetic for alpha(v)beta(3) integrin receptor targeting. Cancer Biother Radiopharm. 2015;30:152–159. doi: 10.1089/cbr.2014.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao YJ, Sheng XR, Pan XM. The effects of NaCl concentration and pH on the stability of hyperthermophilic protein Ssh10b. BMC Biochem. 2007;8:28. doi: 10.1186/1471-2091-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominy BN, Perl D, Schmid FX, Brooks CL. The effects of ionic strength on protein stability: the cold shock protein family. J Mol Biol. 2002;319:541–554. doi: 10.1016/S0022-2836(02)00259-0. [DOI] [PubMed] [Google Scholar]
- 20.Lindman S, et al. Salting the charged surface: pH and salt dependence of protein G B1 stability. Biophys J. 2006;90:2911–2921. doi: 10.1529/biophysj.105.071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Šimeček J, Hermann P, Wester HJ, Notni J. How is 68Ga labeling of macrocyclic chelators influenced by metal ion contaminants in 68Ge/68Ga generator eluates? ChemMedChem. 2013;8:95–103. doi: 10.1002/cmdc.201200471. [DOI] [PubMed] [Google Scholar]
- 22.Chakravarty R, Chakraborty S, Dash A, Pillai MR. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl Med Biol. 2013;40:197–205. doi: 10.1016/j.nucmedbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 23.European Pharmacopoeia. 8th. Council of Europe; 2010. 2.4.8 Heavy Metals; pp. 128–131. [Google Scholar]


