Abstract
Background
Prenatal exposure to organophosphate insecticides may be associated with autism spectrum disorders and related behaviors. This association may be modified by single nucleotide polymorphisms in the paraoxonase (PON1) enzyme.
Objective
We examined the relationship of prenatal organophosphate insecticide biomarkers with reciprocal social, repetitive, and stereotypic behaviors in 8-year old children, and modification of this relationship by child PON1 polymorphisms.
Methods
Among 224 pregnant women, we quantified concentrations of six nonspecific dialkyl phosphate (DAP) metabolites of organophosphate insecticides in two urine samples collected at ~16 and ~26 weeks gestation. When children were eight years old, we administered the Social Responsiveness Scale (SRS), a continuous measure of various dimensions of interpersonal behavior, communication, and repetitive/stereotypic behaviors. We estimated the association between a 10-fold increase in the sum of six DAP concentrations (ΣDAP) and SRS scores. We examined whether child PON1192 and PON1−108 genotypes modified this association.
Results
After covariate adjustment, ΣDAP concentrations were not associated with SRS scores [β = −1.2; 95% confidence interval (CI): −4.0, 1.6]. Among children with the PON1−108TT genotype, ΣDAP concentrations were associated with 2.5-point higher (95% CI: −4.9, 9.8) SRS scores; however, the association was not different from the 1.8-point decrease (95% CI: −5.8, 2.2) among children with PON1−108CT/CC genotypes (ΣDAP × PON1−108 p-value = 0.54). The association between ΣDAP concentrations and SRS scores was not modified by PON1192 (ΣDAP × PON1192 p-value = 0.89).
Conclusions
In this cohort, prenatal urinary DAP concentrations were not associated with children’s social behaviors; these associations were not modified by child PON1 genotype.
Keywords: Children, insecticides, autism spectrum disorders, prenatal, neurodevelopment
Introduction
Autism spectrum disorder (ASD), a condition characterized by impaired interpersonal behavior or communication and repetitive or stereotypic behaviors, affects approximately 1% of children in the United States (American Psychiatric Association 2013; Centers for Disease Control and Prevention (CDC) 2013). While the autistic phenotype develops and manifests in early childhood, the abnormal neural circuitry behind ASDs most likely develops in utero (London et al. 2007). Gestational exposures play an important role in ASD risk (Mendelsohn et al. 2008; Chomiak et al. 2013), but few genetic or environmental risk factors have been found to contribute definitively to ASD risk (Kalkbrenner et al. 2014).
An in utero environmental exposure that may increase the risk of ASDs is the class of insecticides known as organophosphates (OP). Although the use of OP insecticides has declined by 45% in the United States since 1980, 75 million pounds of OP insecticides were used in agricultural and residential settings during 2001 (U.S. EPA 2004). Once ingested, about 75% of OP insecticides are metabolized into dialkyl phosphates (DAPs) by the enzyme paraoxonase 1 (PON1). DAPs do not inhibit acetylcholinesterase, have longer biological half-lives than the parent insecticide, and are excreted in the urine (Davies et al. 1997; Franklin et al., 1981). Thus, urinary DAP concentrations are suitable biomarkers for OP pesticide exposures in epidemiological studies. One limitation of using DAPs as OP pesticide exposure biomarkers is that the metabolites can be present in food due to environmental degradation of the parent insecticides.15 Thus, DAPs may not specifically reflect exposure to the parent insecticides.
Because these neurotoxic insecticides can cross the placenta, fetal neurodevelopment may be impacted by the mother’s exposure during pregnancy (Eskenazi et al. 2007). In addition, genetic variations in the efficiency of PON1 may modify the association between OP insecticide exposure and neurodevelopment (Eskenazi et al. 2010; Engel et al. 2011). Despite evidence from animal studies demonstrating that in utero OP insecticide exposure affects neurodevelopment (Levin et al. 2010; Oliveri et al. 2015), few epidemiological studies have examined the relationship between prenatal OP insecticide exposure and ASD diagnosis or autistic behaviors (Eskenazi et al. 2007; Furlong et al. 2014; Shelton et al. 2014). We are not aware of any studies that have investigated whether child PON1 polymorphisms modify the association between OP insecticide exposure and ASD.
To address this research gap, we used a prospective pregnancy and birth cohort of 224 mothers and their children to examine the association between maternal urinary OP insecticide metabolite concentrations during pregnancy and social behaviors linked with ASD at 8 years of age, and determine if this association was modified by child PON1 polymorphisms.
Methods
Study participants
We used data collected from the Health Outcomes and Measures of the Environment (HOME) Study, a prospective birth cohort from the greater Cincinnati, Ohio, metropolitan area designed to investigate the relationship between low-level environmental chemical exposures and children’s growth and development. Details about eligibility, subject recruitment, data collection, and follow-up for the HOME Study are described elsewhere (Braun et al. 2016). Briefly, we identified and contacted pregnant women from nine prenatal clinics associated with three hospitals in the Cincinnati area from March 2003 to January 2006. Eligibility criteria at enrollment included: a) age ≥ 18 years, b) 16 ± 3 weeks of pregnancy, c) residence in a home built before 1978, d) no history of HIV infection, and e) no chemotherapy or radiation treatments nor medications taken for thyroid disorders or seizure. We obtained written informed consent from all women for themselves and their children after the study protocols had been explained. The institutional review boards (IRBs) of Cincinnati Children’s Hospital Medical Center (CCHMC) and all involved delivery hospitals approved this study. The Centers for Disease Control and Prevention (CDC) IRB relied on the determinations made by the CCHMC IRB.
Of 1,263 eligible women, 468 enrolled in the study, and 67 women dropped out of the study before delivery. The remaining 401 women gave birth to 389 live singleton infants, 9 sets of twins, and 3 still-born infants. Of the 389 women with singletons, 228 completed follow-up when their children were 8 years old (range = 7.5–10 years) and 224 (57%) of these women and their children had complete exposure and covariate data.
Measurement of insecticide exposure
Maternal urine samples were shipped to the CDC for analysis. We quantified six OP insecticide metabolite concentrations in urine: dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP) using a modification of the analytical method of Bravo et al. (2004) that employs gas chromatography-tandem mass spectrometry with isotope dilution calibration. These dialkyl phosphate metabolites (DAPs) are common metabolites of about 75% of the OP insecticides used in the United States.
Quality control (QC) materials, prepared at the CDC from spiked pooled urine, were analyzed with standards, blanks, and study samples. The QC concentrations were evaluated using standard statistical probability rules (Caudill et al. 2008). The limits of detection (LODs) varied depending on the metabolite and ranged from 0.2 μg/L for DMTP to 0.6 μg/L for DMP. We used the reported value for nonzero concentrations below the LOD, and we imputed DAP concentrations that were reported as zero by choosing a random number between zero and the lowest nonzero value for that metabolite.
We converted the metabolite concentrations to molar concentrations (nmol/L) and summed them to obtain overall concentrations of diethyl alkyl phosphates (Σ DEs: DEP, DETP, and DEDTP), dimethyl alkyl phosphates (ΣDMs: DMP, DMTP, DMDTP), and a sum of all six of the DAPs (ΣDAP). To control for individual variation in urine dilution, we measured urinary creatinine concentrations and calculated creatinine-standardized ΣDAP concentrations by dividing metabolite concentrations by creatinine concentration (Larsen 1972). Because ΣDAP concentrations varied widely between 16- and 26-weeks gestation, we averaged the log10-transformed creatinine-adjusted ΣDAP concentrations across the two samples.
Reciprocal social, repetitive, and stereotypic behaviors
Mothers completed the Social Responsiveness Scale (SRS) (Constantino 2005) in our study clinic when their children were 8 years of age. The SRS is a valid and reliable measure of interpersonal behaviors, communication, and repetitive or stereotypic behaviors (Bölte et al. 2008; Constantino 2005). An advantage to the SRS is that it assesses autistic behaviors along a continuum, rather than a binary yes/no diagnosis, using 65 Likert-scale questions that are summed and transformed into a total T-score (with mean ± SD, 50 ± 10 in the normative sample). Higher scores indicate more autistic behaviors. T-scores ≥ 60 are considered to be indicative of clinically significant deficiencies in reciprocal social behavior, and T-scores ≥ 75 are consistent with a clinical diagnosis of ASDs. However, the SRS cannot be used as the sole instrument to diagnose children with ASD. Instead, it provides a useful tool with which to examine ASD behaviors along a continuum.
Confounding variables
We adjusted for potential confounding factors that may be associated with both OP insecticide exposure and autistic behaviors based on biological plausibility and prior knowledge. We obtained maternal sociodemographic and perinatal factors, including maternal age at delivery, race, marital status, education, parity, insurance status, household income, prenatal vitamin use, maternal serum cotinine concentration, and children’s sex using structured interviews and chart reviews conducted by trained research staff. We measured maternal depressive symptoms during the second trimester with the Beck Depression Inventory-II (Beck et al. 1996). The frequency of fresh fruit and vegetable consumption during pregnancy was measured using a standardized questionnaire administered by trained research staff.
PON genotyping data
DNA was extracted from frozen cord blood using the 5PRIME PerfectPure DNA blood kit (5PRIME, Gaithersburg, MD, USA). We used the Applied Biosystems predesigned TaqMan assays (Applied Biosystems, Carlsbad, CA, USA) for rs705379 (−108C/T) and rs662 (192Q/R) with 15 ng of genomic DNA. TaqMan results were read on an ABI 7900HT real time PCR system. PON1108 and PON1192 genotypes were available for 92% (n = 206) and 92% (n = 205) of children, respectively.
Statistical analysis
We began our analysis by describing maternal urinary ΣDAP concentrations and SRS scores by covariates. Then, we calculated the intraclass correlation coefficient (ICC) of urinary ΣDAP concentration between 16 and 26 weeks gestation by dividing variance of the repeated measurements between women by the sum of the variance of the repeated measurements between and within the women in order to quantify the percent of total variation that is due to differences in ΣDAP concentrations between the women.
Next, we assessed the form of the exposure-response relationship using locally weighted regression (LOESS) plots of log-transformed concentrations and SRS scores. With lack of significant evidence of nonlinearity in ΣDAP-SRS association, a parsimonious regression model with a single linear term of log10-transformed ΣDAP concentration as the predictor and SRS as the outcome variable was used for all analyses. Then, we estimated the unadjusted association for a 10-fold increase in maternal urinary ΣDAP concentrations and SRS scores using linear regression. We then adjusted for each potential confounder, individually and as a complete set, to quantify the magnitude and direction of confounding.
Finally, we examined whether the association between maternal urinary ΣDAP concentrations and SRS scores were modified by child PON1 polymorphisms (CT/CC vs. TT in PON1−108; QQ/QR vs. RR in PON1192) using covariate-adjusted regression models with a product interaction term between maternal urinary ΣDAP concentrations and PON1 polymorphisms to produce polymorphism-specific estimates. We evaluated effect measure modification using the p-value of the interaction term between ΣDAP concentration and PON1 polymorphism to determine if the association between ΣDAP concentration and SRS score was significantly different between participants with different polymorphisms. Because of our small sample size in strata of PON1 polymorphisms, we considered the ΣDAP-SRS association to be modified by PON1 polymorphisms when the p-value for the product interaction term was <0.20.
Sensitivity analyses
We performed sensitivity analyses to test the robustness of our results. First, we assessed whether creatinine adjustment altered our results. We used ΣDAP concentrations as our exposure variable without creatinine adjustment. In addition, we examined whether maternal race or child sex modified the relationship between maternal urinary ΣDAP concentrations and children’s SRS scores since previous research suggests that race/ethnicity may modify these associations (Furlong et al. 2014). Additionally, because variability of ΣDAP concentrations within each woman was so great, we analyzed the relationship between ΣDAP concentrations and children’s SRS scores separately for creatinine-adjusted 16-week concentrations and creatinine-adjusted 26-week concentrations using a covariate-adjusted regression model, as above with the average of the two ΣDAP concentration samples. This was done to confirm that using a covariate-adjusted regression with the average of the two values would yield comparable results. Finally, separate 16-week and 26-week covariate-adjusted regression models with a product interaction term between maternal urinary ΣDAP concentrations and child PON1−108 polymorphisms were computed to identify any possible genetic sensitivity that is dependent on the timing of exposure.
Results
The median of the average maternal urinary ΣDAP metabolite concentrations was 59.9 nmol/L, and the median of creatinine-adjusted maternal urinary ΣDAP metabolite concentrations was 73.5 nmol/g creatinine. Women who were non-Hispanic white, older, married, had higher household income, more education, private health insurance, fewer depression symptoms, consumed more fruits and vegetables, and no prior deliveries, had higher median creatinine-adjusted urinary ΣDAP concentrations than other women (Table 1). Women and children who completed the 8-year follow-up visit were similar to the original cohort in terms of sociodemographic, perinatal, and infant factors (results not shown; see Braun et al., 2016). In addition, ΣDAP concentrations during pregnancy were similar among women who did and did not complete follow-up (median: 60 vs. 62 nmol/L, respectively).
Table 1.
Maternal urinary ΣDAP concentrations (nmol/L), creatinine-standardized ΣDAP concentrations (nmol/g creatinine), and children’s SRS scores measured at 8 years of age by covariates (HOME study, Cincinnati, OH).a
| Covariate | N (%) | Median ΣDAP (25th, 75th Percentile) | Median Creatinine-Standardized ΣDAP Means (25th, 75th Percentile) | Mean SRS Scores (Standard Deviation) | N (%) with SRS score ≥ 60 |
|---|---|---|---|---|---|
| Overall | 224 (100) | 60 (31, 127) | 74 (33, 152) | 51 (11) | 44 (20) |
| Maternal Race | |||||
| White | 136 (61) | 53 (25, 101) | 88 (43, 194) | 48 (9) | 14 (10) |
| Black | 75 (33) | 75 (39, 188) | 47 (19, 95) | 56 (12) | 27 (36) |
| Other | 13 (6) | 104 (56, 144) | 80 (56, 134) | 50 (12) | 3 (13) |
| Maternal Age (years) | |||||
| 18–25 | 59 (26) | 77 (36, 141) | 62 (34, 98) | 54 (11) | 16 (27) |
| 25–35 | 131 (59) | 56 (27, 106) | 72 (30, 182) | 50 (10) | 23 (18) |
| 35 or older | 34 (15) | 65 (23, 181) | 117 (48, 213) | 49 (14) | 5 (15) |
| Maternal Education | |||||
| High school degree or less | 56 (25) | 60 (27, 149) | 49 (14, 90) | 56 (12) | 19 (34) |
| Some College | 63 (28) | 59 (31, 99) | 52 (26, 95) | 52 (9) | 15 (24) |
| Bachelor’s degree or beyond | 105 (47) | 62 (33, 137) | 111 (51, 263) | 47 (10) | 10 (10) |
| Marital Status | |||||
| Married | 140 (62) | 59 (27, 127) | 87 (40, 208) | 48 (10) | 16 (11) |
| Unmarried | 84 (38) | 67 (31, 124) | 52 (19, 97) | 55 (11) | 28 (33) |
| Household Income (per year) | |||||
| $20,000 or less | 58 (26) | 59 (28, 131) | 38 (16, 95) | 56 (11) | 21 (36) |
| $20,000–$40,000 | 34 (15) | 84 (35, 146) | 70 (38, 163) | 53 (11) | 9 (26) |
| $40,000–$80,000 | 75 (33) | 54 (23, 103) | 75 (36, 172) | 49 (10) | 10 (13) |
| $80,000 or higher | 57 (25) | 78 (42, 130) | 94 (50, 218) | 47 (9) | 4 (7) |
| Insurance Status | |||||
| Private | 157 (70) | 60 (31, 124) | 88 (43, 207) | 49 (10) | 21 (13) |
| Public or none | 67 (30) | 58 (30, 132) | 44 (17, 86) | 55 (12) | 23 (34) |
| Maternal Depressive Symptoms | |||||
| Minimal | 175 (78) | 62 (31, 127) | 83 (35, 182) | 49 (10) | 27 (15) |
| Mild | 29 (13) | 60 (20, 141) | 45 (20, 95) | 56 (12) | 9 (31) |
| Moderate or severe | 20 (9) | 48 (29, 99) | 52 (26, 83) | 58 (12) | 8 (40) |
| Parity | |||||
| 0 | 104 (46) | 65 (32, 147) | 95 (46, 215) | 50 (11) | 17 (16) |
| 1 | 66 (30) | 60 (34, 110) | 62 (26, 123) | 50 (11) | 12 (18) |
| 2 or higher | 54 (24) | 55 (25, 114) | 45 (20, 75) | 53 (11) | 15 (28) |
| Prenatal Vitamins | |||||
| Never | 27 (12) | 60 (38, 131) | 52 (29, 91) | 56 (11) | 9 (33) |
| Every day or weekly | 197 (88) | 60 (29, 124) | 74 (33, 178) | 50 (11) | 35 (18) |
| Fresh Fruit/Vegetable Consumption | |||||
| < 1–3x/Month | 28 | 43 (19, 122) | 40 (24, 94) | 54 (10) | 8 (29) |
| 1–6x/Week | 117 | 56 (27, 104) | 55 (27, 110) | 51 (11) | 25 (21) |
| ≥ Daily | 79 | 95 (41, 214) | 121 (67, 285) | 49 (11) | 11 (14) |
| Child Sex | |||||
| Female | 127 (57) | 58 (27, 117) | 72 (31, 146) | 51 (11) | 25 (20) |
| Male | 97 (43) | 64 (32, 137) | 74 (37, 166) | 51 (11) | 19 (20) |
| Tobacco use | |||||
| Nonsmoker | 69 (31) | 55 (32, 137) | 107 (52, 263) | 47 (10) | 6 (9) |
| Secondhand Smoke | 128 (57) | 65 (32, 131) | 65 (30, 122) | 52 (11) | 31 (24) |
| Smoker | 27 (12) | 38 (21, 102) | 35 (15, 78) | 55 (10) | 7 (26) |
Average of ΣDAP concentrations at 16 and 26 weeks gestation
The average SRS score was 51 (standard deviation [SD] = 11). Higher (worse) SRS scores were observed among children of women who were non-Hispanic black, younger at delivery, less educated, unmarried, earning less income, covered by public or no health insurance, more depressed, and not regularly taking prenatal vitamins (Table 1). Forty-four (20%) children had an SRS score ≥ 60, and six (3%) children had an SRS score ≥ 75.
There was substantial within-person variability in the urinary ΣDAP concentration measurements taken 10 weeks apart. Nearly all of the variation was within-women (ICC = 0.07). The median difference in a woman’s two urinary measurements was 70.3 nmol/L, and the differences ranged from 0.06 nmol/L to as high as 3,199 nmol/L (Figure 1).
Fig 1.
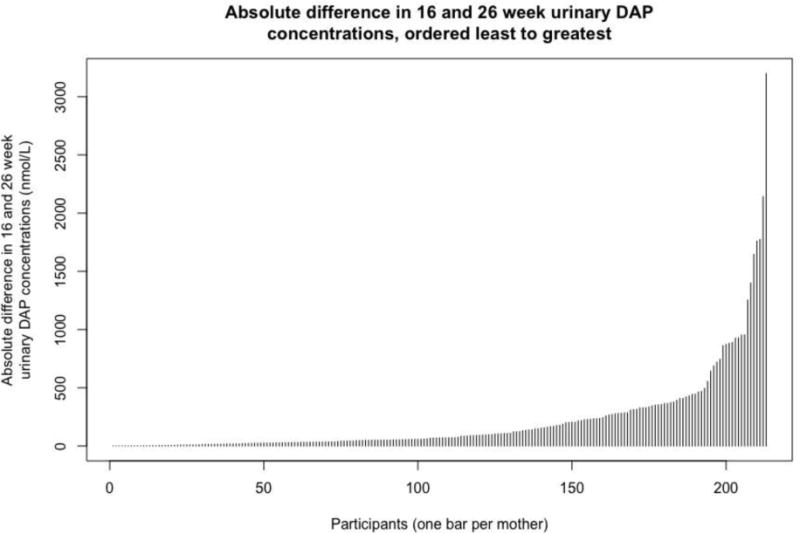
Absolute difference in each participant’s urinary ΣDAP concentration measured at 16 weeks gestation and 26 weeks gestation. Each bar represents the absolute difference in ΣDAP concentration measured at 16 weeks gestation and 26 weeks gestation for one participant (n = 224). The bars are ordered from least difference to greatest difference, with the participant with the smallest absolute difference located at the far left and the participant with the largest absolute difference located at the far right.
In our unadjusted model, each 10-fold increase in maternal urinary ΣDAP concentration was associated with a 3.4-point decrease in SRS score (95% CI: −6.1, −0.7). Adjustment for individual covariates, especially maternal education and household income, attenuated this estimate towards the null. Our final model adjusting for all covariates showed a null association between maternal urinary ΣDAP concentrations and children’s SRS scores (β = −1.2; 95% CI: −4.0, 1.6) (Figure 2). We obtained similar results when examining maternal urinary DM (β = −0.8; 95% CI: −3.3, 1.6) and DE concentrations (β = −0.8; 95% CI: −2.9, 1.2).
Fig 2.
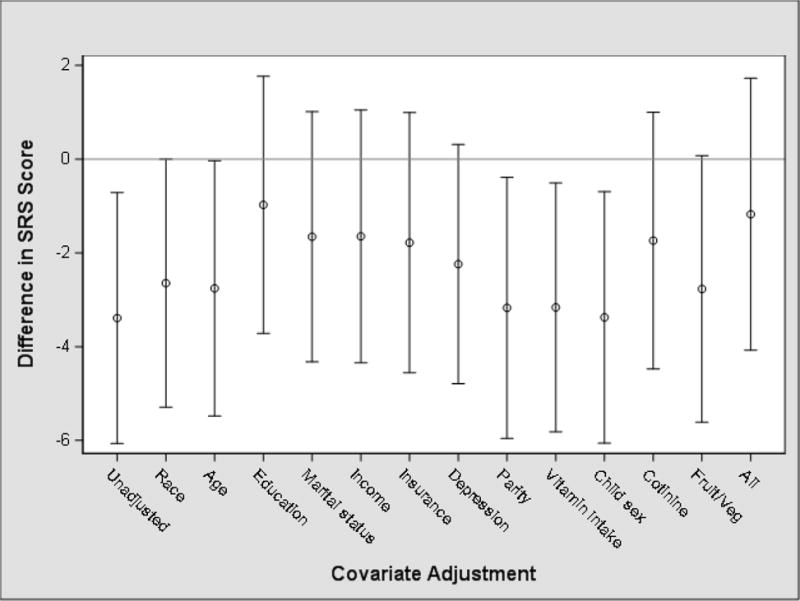
Unadjusted and adjusted mean difference (95% confidence interval) in children’s SRS scores at 8 years of age with a 10-fold increase in maternal urinary ΣDAP concentrations during pregnancy (n = 224). Each point estimate is the association of prenatal urinary DAP concentrations with child SRS scores from separate models without any covariate adjustment (Unadjusted), adjustment for each covariate, and fully adjusted for all the listed covariates (All).
The covariate-adjusted association between ΣDAP concentrations and SRS scores was not modified by child PON1 genotype (Figures 3 and 4, Supplemental Table 1). We found that the ΣDAP-SRS association was positive among children with the −108TT genotype (β = 2.5; 95% CI: −4.9, 9.8), but this association was not significantly different than children with the − 108CC or −108CT genotype (β = −1.8; 95% CI: −6.2, 2.1) (ΣDAP × PON1−108 effect measure modification p-value = 0.54). The ΣDAP-SRS association did not differ significantly by child PON1192 genotype (ΣDAP × PON1192 effect measure modification p-value = 0.89) (Figure 4).
Fig 3.
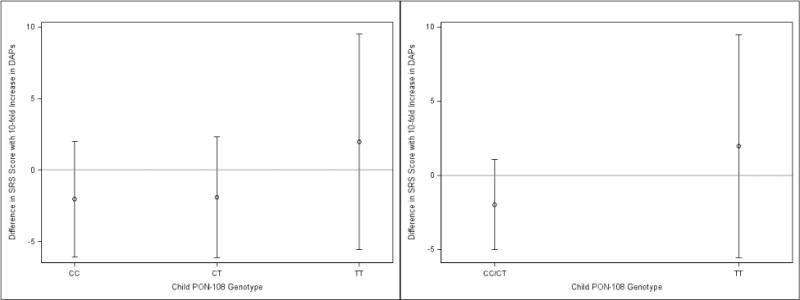
Adjusted mean change in SRS Scores per 10-fold Increase in maternal ΣDAPs: Stratified by child PON1−108.a,b,c, a- Adjusted for maternal race, age, education, marital status, household income, health insurance, maternal depression, parity, prenatal vitamins, child sex, maternal serum cotinine concentrations, and frequency of fresh fruit and vegetable consumption during pregnancy. b-Effect measure modification p-values for three and two category PON1−108 equal to 0.61 and 0.33, respectively, c-N’s for CC, CT, and TT equal to 96, 76, and 34, respectively.
Fig 4.
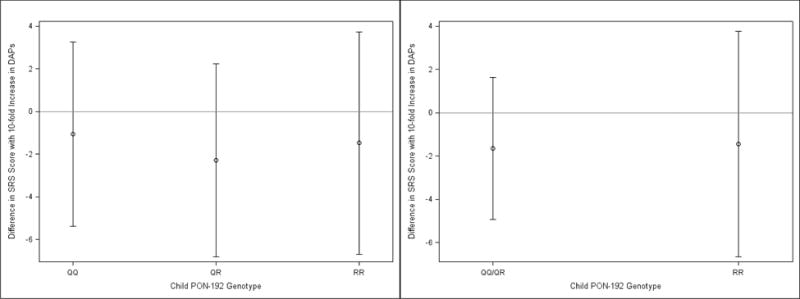
Adjusted mean change in SRS Scores per 10-fold Increase in maternal ΣDAPs: Stratified by child PON1192.a,b,c, a- Adjusted for maternal race, age, education, marital status, household income, health insurance, maternal depression, parity, prenatal vitamins, child sex, maternal serum cotinine concentrations, and frequency of fresh fruit and vegetable consumption during pregnancy. b-Effect measure modification p-values for three and two category PON1192 equal to 0.92 and 0.94, respectively. c-N’s for CC, CT, and TT equal to 75, 76, and 54, respectively.
Sensitivity analyses
We noted no notable differences in our results adjusted for log10-transformed creatinine concentrations instead of creatinine-standardizing ΣDAP concentrations. The ΣDAP-SRS association was not modified by child sex (effect measure modification p-values ≥ 0.48) or maternal race (effect measure modification p-values ≥ 0.48) (Supplemental Tables 2 and 3). The results from the 16-week ΣDAP concentrations model (β = −0.8; 95% CI: −2.9, 1.3) and from the 26-week ΣDAP concentrations model (β = 0.0; 95% CI: −2.3, 2.4) were comparable to the results from the averaged ΣDAP concentrations model. Finally, there were no notable differences between the results of the separate 16-week and 26-week covariate-adjusted regression models with a product interaction term between maternal urinary ΣDAP concentrations and child PON1−108 polymorphisms (effect measure modification p-values ≥ 0.59) and the results from the averaged ΣDAP concentrations model.
Discussion
We did not observe an association between prenatal urinary ΣDAP concentrations and children’s interpersonal behavior, communication, and repetitive and stereotypic behaviors in our cohort after adjustment for covariates. Moreover, we did not find evidence that child PON1 genotype modified the association between prenatal urinary ΣDAP concentrations and SRS scores, despite stronger positive associations between maternal ΣDAP concentrations and SRS scores among children with the potentially more sensitive PON1−108TT genotype compared to those with the PON1−108CC/CT genotypes.
Among studies most similar in design to ours, Furlong et al. (2014) found no overall association between urinary ΣDAP concentrations in the third trimester of pregnancy and SRS scores at 7–9 years of age in a multi-ethnic urban cohort in New York City. However, they did note an association of prenatal OP exposure with deficits in social functioning among blacks and among boys. In contrast, a prospective cohort study of mainly Latino farm-workers in California found that higher prenatal and postnatal urinary ΣDAP concentrations were associated with an approximately 2-fold increase in risk of pervasive developmental disorder (PDD) (prenatal odds ratio (OR) = 2.3; postnatal OR = 1.7) (Eskenazi et al. 2007). In addition, a population-based case-control study in California found that proximity to fields sprayed with OP insecticides during pregnancy was associated with a 60% increased risk for ASD diagnosis (Shelton et al. 2014). Finally, other studies from the same cohorts mentioned above have found impairments on a variety of neurodevelopmental measures with increasing prenatal urinary ΣDAP concentrations (Engel et al. 2011; Eskenazi et al. 2010; Rauh et al. 2011).
Discrepancies in the results of studies examining prenatal OP insecticide exposure and children’s autistic behaviors or risk of ASD diagnosis could be due to differences in OP insecticide exposure where the populations under study live. Two of the studies took place in California (Shelton et al. 2014; Eskenazi et al. 2007), where agricultural use of insecticides is abundant, and another took place in New York City (Engel et al. 2011), where residential use of OP insecticides was common at the time. Maternal urinary ΣDAP concentrations in the prospective cohort of Latino farm-workers in California were higher than concentrations among women in the New York City cohort. Concentrations among participants in the HOME Study (median = 60 nmol/L), who live in urban and suburban environments, were lower than those in participants in the California (geometric mean = 112 nmol/L) and New York City studies (geometric mean = 74 nmol/L) (Engel et al. 2016). In addition, discrepancies across studies may exist because DAPs are non-specific and it is likely that the types of OP insecticides used in the HOME Study are different than those used in the California and New York City cohorts (Harley et al. 2015, Yolton et al. 2013). Therefore, exposure metrics across studies may not be completely comparable. Finally, because of the non-specific nature of DAPs, it is possible that urinary DAP concentrations reflect exposure to DAPs in the diet and not the parent insecticide residue. Indeed, we observed that the frequency of fresh fruit and vegetable intake was positively associated with urinary ΣDAP concentrations among HOME Study women, but we were unable to determine if this was due to exposure to the parent insecticides or their metabolites. Given that HOME Study women were not living in areas with high agricultural or residential pesticide use, we speculate that the relative contribution of diet to DAP concentrations varies across studies and is likely greater among HOME Study women compared to previous studies examining the neurotoxicity of OP pesticides (Engel et al., 2016).
Finally, the results of these studies could also be due to different methods used to evaluate ASD or autistic behaviors. In Shelton et al.’s case-control study, the cases were children who had clinical diagnoses of ASD, as confirmed through multiple tests. In the cohort studies, including ours, autistic and social behaviors were assessed using questionnaires and considered as a continuum rather than an all-or-none diagnosis.
Our null associations between urinary ΣDAP concentrations and SRS scores could be due to several factors. First, urinary ΣDAP concentrations exhibited high within-person variability, as evidenced by the relatively low ICC between the repeated maternal urinary ΣDAP concentrations. This potential exposure misclassification may have limited our ability to detect an association and if non-differential, our effect estimates would be attenuated towards the null. While we were able to use two urinary ΣDAP concentrations to classify prenatal OP insecticide exposure, it is likely that there was still additional exposure misclassification and more than two urinary ΣDAP concentrations are necessary to accurately assess exposure. Second, maternal urinary ΣDAP metabolite concentration may not accurately reflect a woman’s exposure to OP insecticides. While OP insecticides are metabolized into DAP in the body, the DAPs measured in urine may not originate solely from OP insecticide exposure because DAPs can exist in the environment and be consequently ingested by humans (Lu et al. 2005). The result is that our exposure measure reflects both those DAPs from OP insecticides as well as DAPs ingested from foods (e.g., fruits or vegetables) consumed by a participant. Furthermore, it is not the case that the entire amount of parent insecticide ingested is excreted as measurable metabolites in urine. A dosing study in rats that were orally administered the OP insecticide malathion reported that only about 9–10% of the insecticide given was ultimately excreted in urine as DMs (DMPs, DMTPs, DMDTPs) (Chen et al. 2013). This may have resulted in additional misclassification of a woman’s true OP insecticide exposure during pregnancy, as urinary DAPs measured may only represent a fraction of the exposure to the parent insecticide. Finally, we do not believe that selection bias played a role in biasing our results given that those participants who completed follow-up were similar to the original cohort in terms of sociodemographic, perinatal, and infant characteristics, and had baseline urinary DAP concentrations similar to those who did not complete follow-up.
Our assessment of children’s autistic behaviors using SRS scores has several advantages over clinical diagnosis of ASD. First, it is a valid and reliable measure of autistic phenotype in children that can be administered to parents without special training or intensive observation (Constantino 2005), thus, facilitating its use in prospective cohort studies. Second, the SRS evaluates the ASD phenotype on a continuous spectrum rather than a dichotomous outcome (i.e., diagnosis yes or no). The strengths of measuring continuous traits include increasing statistical power in analysis, reducing outcome misclassification, and accounting for children with mild symptoms who may not otherwise meet diagnostic criteria (Sagiv et al. 2015). Although the SRS has its advantages, a main limitation is that higher scores are not necessarily specific to ASD, as deficits in social reciprocity may also be found in conditions such as ADHD, language problems, maladaptive behavior, social anxiety, and mood disorders (Constantino et al. 2003; Hus et al. 2013; Pine et al. 2008; Reiersen et al. 2007). The imperfect specificity of this test may mean that our outcome measure captures behaviors associated with these other conditions rather than social and behavioral deficiencies specific to autism spectrum disorder.
In our evaluation of the association between urinary ΣDAP concentrations and SRS scores, we noted an appreciable difference between our unadjusted model and our model fully adjusted for all covariates (Figure 2). The fully-adjusted model showed no association between ΣDAP concentration and SRS score but the unadjusted model showed a negative association between the two, implying the presence of negative confounding (Mehio-Sibai et al. 2005). We observed that confounding by many of the covariates related to socioeconomic status and OP pesticide exposure (i.e., fruit and vegetable intake) explained the unexpected protective association. We did not observe additional negative confounding from fruit and vegetable intake after adjusting for socioeconomic factors. For example, high income was associated with higher urinary ΣDAP concentrations and was also associated with better SRS scores. While we expected those with higher SES to have better SRS scores because better health outcomes often accompany higher SES, we also expected insecticide exposure to be higher in women with lower SES because these women may live in less desirable areas where OP insecticides are more likely to be applied. Instead, we observed that women with high SES had higher urinary ΣDAP concentrations, which may be the result of measuring ΣDAP metabolites that came from fresh produce that women with high SES are more likely to consume (Donauer et al., 2016; Yolton et al. 2013).
When stratifying by child PON1 type, we found suggestive evidence that the relationship between ΣDAP concentrations and SRS scores was stronger among children with the PON1−108TT genotype, but this effect measure modification was not significant even by liberal standards (i.e., p-value < 0.20). Our finding of a positive association between maternal urinary ΣDAP and child SRS scores in children with PON1−108TT is consistent with prior literature showing increased susceptibility to OP insecticides among children with −108TT genotype (Eskenazi et al. 2010) and the known biology of the PON1 gene because the PON1−108C has been found to have greater catalytic activity than the PON1−108T polymorphism. Therefore, those with PON1−108T may have greater exposure to neurotoxic OP insecticides and hence a higher risk of neurodevelopmental disorders. We did not note significant modification of the ΣDAP-SRS association in child PON1192, possibly because both 192Q and 192R have enhanced ability to break down different OP insecticides. For instance, the 192Q genotype hydrolyzes diazinon faster, whereas the 192R genotype hydrolyzes from parathion faster (Richter et al. 2009). Hence, neither 192Q nor 192R may be better than the other at cumulative OP insecticide detoxification. It is critical to note that our statistical power to examine effect modification is limited because of our small sample size for children without wild-type genotypes.
In this cohort, we did not observe an association between maternal urinary ΣDAP concentrations during pregnancy and children’s SRS scores at 8 years of age. While there was some evidence of a positive association between ΣDAP concentrations and SRS scores among children with PON1−108TT, this association did not significantly differ from the association among children with PON1−108TC/CC genotypes. Replication in other studies with larger sample sizes or pooling similar existing studies can provide more precise results that would be valuable additions to this body of literature.
Supplementary Material
Highlights.
No association between maternal DAP levels and autistic behaviors in children
DAP level-SRS association did not differ significantly by child PON1 polymorphism
Urinary DAP levels varied greatly across 2nd and 3rd trimesters of pregnancy
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R00 ES020346, R01 ES024381, R01 ES020349, PO1 ES11261, R01 ES015517, and R01 ES014575).
We acknowledge the technical assistance of M.A. Montesano, P. Olive, and T. Bernert in measuring the urinary concentrations of DAP metabolites and creatinine, and serum cotinine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. Fifth. American Psychiatric Publishing; 2013. Autism Spectrum Disorder, 299.00 (F84.0) [Google Scholar]
- Beck Aaron T, Steer Robert A, Brown Gregory K. Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. p. b9. [Google Scholar]
- Bölte Sven, Poustka Fritz, Constantino John N. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS) Autism Research. 2008;1.6:354–363. doi: 10.1002/aur.49. [DOI] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) Study. Int J Epidemiol. 2016:1–10. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Roberto, et al. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. Journal of Exposure Science and Environmental Epidemiology. 2004;14.3:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home Inventory Administration Manual. Little Rock, AK: Print Design; 2003. [Google Scholar]
- Caudill Samuel P, Schleicher Rosemary L, Pirkle James L. Multi-rule quality control for the age-related eye disease study. Statistics in Medicine. 2008;27.20:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- Chen L, et al. Absorption and excretion of organophosphorous insecticide biomarkers of malathion in the rat: implications for overestimation bias and exposure misclassification from environmental biomonitoring. Regulatory Toxicology and Pharmacology. 2013;65.3:287–293. doi: 10.1016/j.yrtph.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Chomiak Taylor, Turner Nathanael, Hu Bin. What we have learned about autism spectrum disorder from valproic acid. Pathology Research International. 2013 doi: 10.1155/2013/712758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale Western Psychological Services: Los Angeles. 2005 [Google Scholar]
- Constantino John N, Hudziak James J, Todd Richard D. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42.4:458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Davies JE, Peterson JC. Surveillance of occupational, accidental, and incidental exposure to organophosphate pesticides using urine alkyl phosphate and phenolic metabolite measurements. Ann NY Acad Sci. 1997;837:257–268.e. doi: 10.1111/j.1749-6632.1997.tb56879.x. [DOI] [PubMed] [Google Scholar]
- Donauer S, Altaye M, Xu Y, et al. An Observational Study to Evaluate Associations Between Low-Level Gestational Exposure to Organophosphate Pesticides and Cognition During Early Childhood. American Journal of Epidemiology. 2016;184:410–8. doi: 10.1093/aje/kwv447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Stephanie M, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environmental Health Perspectives. 2011;119.8:1182. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Stephanie M, et al. Prenatal organophosphorus pesticide exposure and child neurodevelopment at 24 months: an analysis of four birth cohorts. Environmental Health Perspectives. 2016;124.6:822. doi: 10.1289/ehp.1409474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi Brenda, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental Health Perspectives. 2007:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi Brenda, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environmental Health Perspectives. 2010;118.12:1775. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CA, Fenske RA, Greenhalgh R, Mathieu L, Denley HV, Leffingwell JT, et al. Correlation of urinary pesticide metabolite excretion with estimated dermal contact in the course of occupational exposure to Guthion. J Toxicol Environ Health. 1981;7(5):715–731. doi: 10.1080/15287398109530014. [DOI] [PubMed] [Google Scholar]
- Furlong Melissa A, et al. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environment International. 2014;70:125–131. doi: 10.1016/j.envint.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, et al. Prenatal Exposure to Organophosphorous Pesticides and Fetal Growth: Pooled Results from Four Longitudinal Birth Cohort Studies. Environmental Health Perspectives. 2015 doi: 10.1289/ehp.1409362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus Vanessa, et al. Factors influencing scores on the social responsiveness scale. Journal of Child Psychology and Psychiatry. 2013;54.2:216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner Amy E, et al. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current problems in pediatric and adolescent health care. 2014;44.10:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lausen Knud. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clinica Chimica Acta. 1972;38.2:475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Levin Edward D, et al. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behavioural Brain Research. 2010;208.2:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London Eric. The role of the neurobiologist in redefining the diagnosis of autism. Brain Pathology. 2007;17.4:408–411. doi: 10.1111/j.1750-3639.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, et al. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. Journal of Toxicology and Environmental Health, Part A. 2005;68.3:209–227. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- Mehio-Sibai Abla, et al. A positive or a negative confounding variable? A simple teaching aid for clinicians and students. Annals of Epidemiology. 2005;15.6:421–423. doi: 10.1016/j.annepidem.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Mendelsohn Nancy J, Bradley Schaefer G. Seminars in Pediatric Neurology. 1. Vol. 15. WB Saunders; 2008. Genetic evaluation of autism. [DOI] [PubMed] [Google Scholar]
- Oliveri AN, Bailey JM, Levin Edward D. Developmental exposure to organophosphate flame retardants causes behavioral effects in larval and adult zebrafish. Neurotoxicology and Teratology. 2015;52:220–227. doi: 10.1016/j.ntt.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine Daniel S, et al. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47.6:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh Virginia, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental Health Perspectives. 2011:201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen Angela M, et al. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48.5:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Richter Rebecca J, Jarvik Gail P, Furlong Clement E. Paraoxonase 1 (ON1) status and substrate hydrolysis. Toxicology and Applied Pharmacology. 2009;235.1:1–9. doi: 10.1016/j.taap.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv Sharon K, Kalkbrenner Amy E, Bellinger David C. Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environmental Health. 2015;14.1:8. doi: 10.1186/1476-069X-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton Janie F, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental Health Perspectives. 2014;122.10:1103. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Environmental Protection Agency (U.S. EPA). Office of Prevention Pesticides and Toxic Substances. Pesticide industry sales and usage — 2000 and 2001 market estimates. Washington (DC): U.S. EPA; May, 2004. [Google Scholar]
- Yolton Kimberly, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environmental Health. 2013;12.1:1. doi: 10.1186/1476-069X-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


