Abstract
Objectives To determine how soon after delivery the risk of post-pregnancy hypertension increases in women with hypertensive disorders of pregnancy and how the risk evolves over time.
Design Nationwide register based cohort study.
Setting Denmark.
Populations 482 972 primiparous women with a first live birth or stillbirth between 1995 and 2012 (cumulative incidence analyses), and 1 025 118 women with at least one live birth or stillbirth between 1978 and 2012 (Cox regression analyses).
Main outcome measures 10 year cumulative incidences of post-pregnancy hypertension requiring treatment with prescription drugs, and hazard ratios estimated using Cox regression.
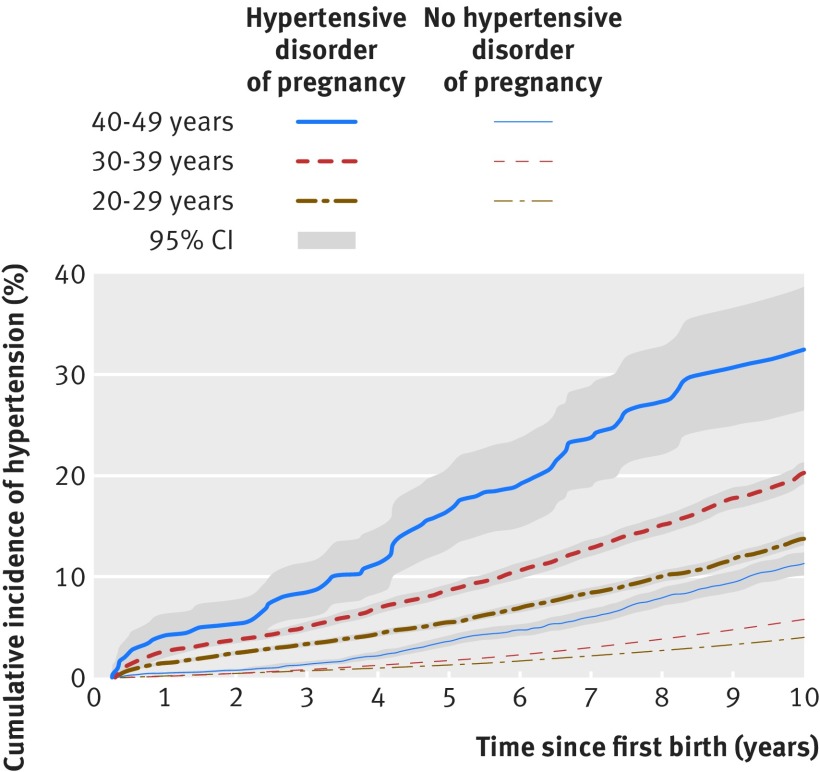
Results Of women with a hypertensive disorder of pregnancy in a first pregnancy in their 20s, 14% developed hypertension in the first decade post partum, compared with 4% of women with normotensive first pregnancies in their 20s. The corresponding percentages for women with a first pregnancy in their 40s were 32% and 11%, respectively. In the year after delivery, women with a hypertensive disorder of pregnancy had 12-fold to 25-fold higher rates of hypertension than did women with a normotensive pregnancy. Rates in women with a hypertensive disorder of pregnancy were threefold to 10-fold higher 1-10 years post partum and remained twice as high even 20 or more years later.
Conclusions The risk of hypertension associated with hypertensive disorders of pregnancy is high immediately after an affected pregnancy and persists for more than 20 years. Up to one third of women with a hypertensive disorder of pregnancy may develop hypertension within a decade of an affected pregnancy, indicating that cardiovascular disease prevention in these women should include blood pressure monitoring initiated soon after pregnancy.
Introduction
Hypertensive disorders of pregnancy (pre-eclampsia; eclampsia; haemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome; and gestational hypertension) affect up to 10% of pregnancies.1 2 Women with a hypertensive disorder of pregnancy have increased risks of post-pregnancy hypertension, ischaemic heart disease, and stroke,3 4 5 which has prompted changes to guidelines by the American Heart Association and the European Society of Cardiology to include hypertensive disorders of pregnancy as risk factors for cardiovascular disease in women.6 7 However, clinical awareness of the link between hypertensive disorders of pregnancy and cardiovascular disease is incomplete,8 9 10 which may result in delayed diagnosis and jeopardise the health of these women. Furthermore, it is unclear how soon after an affected pregnancy screening for hypertension and other markers of cardiovascular disease should be initiated.2 6 11 Recent work suggests that the immediate postpartum years are important, with one study reporting a fivefold increase in hypertension rates in the first five years after a pre-eclamptic pregnancy,12 and another finding that 25-45% of women with a hypertensive disorder of pregnancy developed hypertension within five years of delivery.13 However, precisely when the increased risk of hypertension appears after a pregnancy affected by a hypertensive disorder of pregnancy and how the risk changes with time since pregnancy remain unclear. Understanding patterns of hypertension risk after a hypertensive disorder of pregnancy would enable clinicians to plan appropriate post-pregnancy follow-up and diagnose hypertension as early as possible.
In a cohort of more than one million women delivering in Denmark in 1978-2012, we examined the timing and trajectory of post-pregnancy hypertension risk on a fine temporal scale, in women with and without a history of hypertensive disorders of pregnancy. Specifically, we estimated cumulative incidences of post-pregnancy hypertension over the first 10 years post partum and compared rates of post-pregnancy hypertension in women with and without a hypertensive disorder of pregnancy, by time since most recent pregnancy.
Methods
Data sources
The Danish civil registration system continuously updates personal and vital status information through the unique personal identification number assigned to all Danish residents.14 Contacts with the healthcare system and filled prescriptions are registered using the personal identification number, enabling register based studies with little loss to follow-up. The national patient register contains information on hospital discharge diagnoses assigned since 1977 and outpatient diagnoses assigned since 1995.15 The medical birth register contains information on live births and stillbirths since 1973, with gestational age at delivery from 1978.16 The national prescription register contains data on prescriptions filled since 1994.17
Study cohorts
In this study we used two cohorts: one for the estimation of cumulative incidence of post-pregnancy hypertension and the other cohort for the estimation of hazard ratios for post-pregnancy hypertension.
Cumulative incidences of post-pregnancy hypertension—using the medical birth register, we identified all women with a first pregnancy lasting 20 or more weeks and ending in live birth or stillbirth between 1995 and 2012. We excluded women with any cardiac or circulatory system disorder (international classification of diseases, eighth revision (ICD-8), codes 390-458, or 10th revision (ICD-10) codes I00.0-I99.9) registered in the national patient register before their first delivery, and women with known or potential pregestational hypertension.
Hazard ratios for post-pregnancy hypertension—using the medical birth register, we identified all women who had at least one pregnancy that lasted 20 or more weeks and ended in live birth or stillbirth between 1978 and 2012, and who were living in Denmark at some point during the follow-up period, 1995-2012. We excluded women with any cardiac or circulatory system disorder registered in the national patient register before their first delivery or before 1995, whichever came later, and women with known or potential pregestational hypertension.
Hypertensive disorders of pregnancy (exposure)
We considered women to have a hypertensive disorder of pregnancy in a given pregnancy if they had a diagnosis of gestational hypertension, pre-eclampsia, eclampsia, or HELLP syndrome registered in the national patient register any time between one month before delivery and seven days post partum. To be considered exposed to a hypertensive disorder of pregnancy in our study, women with diagnoses registered outside this time window also had to have at least one diagnosis registered within the window. (We adopted this restriction to try to ensure that diagnoses of hypertensive disorders of pregnancy reflected true cases; we judged that assigning a hypertensive disorder of pregnancy designation would be questionable if a woman’s only hypertensive disorder of pregnancy diagnoses were registered outside this time window, particularly if a diagnosis was assigned many weeks before delivery and never alluded to again.) Since by definition a hypertensive disorder of pregnancy involves incident hypertension in a pregnant woman with onset after 20 weeks’ gestation, we considered only diagnoses registered after this point. As registered in the national patient register, gestational hypertension is defined as hypertension without accompanying proteinuria (ICD-8 code 637.00, ICD-10 code O13.9 or O16.9), whereas in moderate pre-eclampsia, mild or moderate hypertension is accompanied by proteinuria (ICD-8 codes 637.03, 637.09, or 637.99, ICD-10 code O14.0 or O14.9). Severe pre-eclampsia fulfills the criteria for moderate pre-eclampsia, with the addition of one or more of severe hypertension, severe proteinuria, signs of organ failure (including the HELLP syndrome), or generalised seizures (ICD-8 codes 637.04, 637.19, 762.19, 762.29, or 762.39, ICD-10 codes O14.1, O14.2, or O15.0-15.9). If a woman was registered with more than one hypertensive disorder of pregnancy in a single pregnancy, we classified her as having the most severe disorder registered. In an additional analysis, we used an alternative categorisation for hypertensive disorders of pregnancy whereby we classified the condition as early onset for deliveries at less than 34 weeks’ gestation, intermediate onset for deliveries at 34-36 weeks, and term for deliveries at 37 or more weeks, regardless of the severity of the registered hypertensive disorder of pregnancy.
Hypertension (outcome)
We considered a woman to have new onset post-pregnancy hypertension from the time she filled a second prescription for antihypertensive drugs (Anatomic Therapeutic Chemical codes C02-03 or C07-09 registered in the national prescription register) within a six month period. When defining hypertension we ignored antihypertensive drug use that was potentially related to treatment for hypertensive disorders of pregnancy (use from 20 weeks before delivery to three months post partum).
Exclusion of women with known or potential pregestational hypertension
In the cumulative incidence analyses we considered women with antihypertensive drug use before their first pregnancy or up to 20 weeks’ gestation in that first pregnancy to have pregestational hypertension and excluded them from the cohort.
In the relative risk analyses we excluded women with pregnancies before 1994 (ie, predating the national prescription register) who filled prescriptions for antihypertensive drugs in 1994, as we did not know whether hypertension was pregestational or post-gestational in women using drugs at the start of follow-up. Similarly, we excluded women with a first pregnancy in 1994 if they filled prescriptions for antihypertensive drugs before 20 weeks’ gestation or 3-12 months after delivery (ie, beyond the period when such treatment could be associated with pregnancy related hypertension) since we did not know if this use began before or after pregnancy. We also excluded women with a first pregnancy after 1995 and antihypertensive drug use before 20 weeks’ gestation in that pregnancy.
Covariates
Age, maternal birth year, parity, diabetes, smoking, and body mass index were considered a priori to be potential confounders of an association between hypertensive disorders of pregnancy and later hypertension. Information on age and maternal birth year (civil registration system), parity (medical birth register), and diabetes (types 1 and 2, national patient register, ICD-8 codes 249.00-249.09 or 250.00-250.09, ICD-10 codes E10.0-E11.9) was available for the entire study period. Information on first trimester smoking status and prepregnancy body mass index (medical birth register) was available from 1991 and 2004 onwards, respectively.
Statistical analyses
To calculate 10 year cumulative incidences of hypertension, we followed women from three months after their first delivery until the first of: hypertension, 10 years after the first delivery, death, emigration, “missing” in the civil registration system, or 31 December 2012. For women with at least two pregnancies, we also conducted similar analyses for the decade after the second delivery.
For the estimation of relative risks of post-pregnancy hypertension we followed women from 1 January 1995 or three months after their first delivery in the study period, whichever came later, until the first of: hypertension, death, emigration, “missing” in the civil registration system, or 31 December 2012. Using Cox regression with maternal age as the underlying time, we estimated hazard ratios for post-pregnancy hypertension by history of hypertensive disorders of pregnancy. Firstly, we estimated hazard ratios by number of years since a woman’s most recent pregnancy, to examine the immediate effect of a hypertensive disorder of pregnancy on subsequent risk of hypertension. In these analyses, hypertensive disorder of pregnancy status was a time dependent variable reflecting a woman’s experience in the most recent pregnancy and was updated for each subsequent pregnancy. Secondly, we estimated hazard ratios by most severe hypertensive disorder of pregnancy to date, stratified by attained age. History of hypertensive disorders of pregnancy was a “cumulative” time dependent variable, reflecting the most severe hypertensive disorder of pregnancy (if any) the woman had experienced to date. A woman’s status could switch from, for example, no hypertensive disorder of pregnancy to pre-eclampsia if the pre-eclamptic pregnancy came later, but she could not revert to having no history of a hypertensive disorder of pregnancy if an unaffected pregnancy followed an affected one. Finally, in women with at least two pregnancies who were hypertension-free between pregnancies, we estimated hazard ratios comparing rates of hypertension in women with a hypertensive disorder of pregnancy in the first pregnancy, in the second pregnancy, or in both pregnancies, with rates in women with two normotensive pregnancies, by number of years since the second pregnancy. For women with more than two pregnancies, follow-up in these analyses ended at their third pregnancy.
In all Cox regression analyses, we stratified the baseline hazards by parity (1, 2, ≥3 live births and/or stillbirths) and maternal birth year (five year intervals) to ensure that we compared rates in women of the same age and parity; including maternal birth year helped to account for possible time trends in hypertensive disorders of pregnancy and hypertension treatment. Analyses estimating hazard ratios by most severe hypertensive disorder of pregnancy to date were also adjusted for time since most recent pregnancy (<1 year, 1 year intervals from 1-9 years, 10-14 years, 15-19 years, and ≥20 years). In sensitivity analyses in the full cohort, we additionally adjusted for diabetes as a time dependent variable (such that we considered women without diabetes at baseline who developed diabetes during follow-up to be without diabetes until their diagnosis, after which they contributed person time to the diabetes group). In subcohorts of women with available information, we further adjusted for smoking and prepregnancy body mass index. Smoking status (smoker or non-smoker) and prepregnancy body mass index (<18.5, 18.5-24, 25-29, 30-34, or ≥35) were considered as time independent variables based on information from the woman’s first pregnancy in or after 1991 and 2004, respectively. We evaluated violation of the proportional hazards assumption by plotting Martingale residuals against attained age.18
All analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Cary, NC).
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants.
Results
10. year cumulative incidences of post-pregnancy hypertension
We identified 482 972 women with no cardiac or circulatory system disorders or hypertension registered before their first delivery and whose first delivery occurred in or after 1995. Of these women, 23 235 (4.8%) had a hypertensive disorder of pregnancy in their first pregnancy, and 16 611 developed hypertension during follow-up. Women with a normotensive first pregnancy in their 20s, 30s, or 40s had cumulative incidences of hypertension of 4.0%, 5.7%, and 11.3%, respectively, in the decade after delivery (fig 1, supplementary table 1). The corresponding incidences for women whose first pregnancy was complicated by a hypertensive disorder of pregnancy were 13.7%, 20.3%, and 32.4%, respectively. A similar pattern was observed in the decade after a second pregnancy in women with two pregnancies, where either pregnancy could have been complicated by hypertensive disorders of pregnancy (see supplementary table 1).
Fig 1 Ten year cumulative incidences of hypertension by years since first pregnancy in women with and without a hypertensive disorder of pregnancy, by age at first delivery, Denmark, 1995-2012. Follow-up began in 1995 or three months post partum, whichever came later
Relative risks of post-pregnancy hypertension
After excluding women with cardiovascular disease (n=47 142) or pregestational hypertension (n=20 249), 1 025 118 women had at least one eligible pregnancy between 1978 and 2012. Table 1 presents baseline characteristics of the women in this cohort by history of a hypertensive disorder of pregnancy in the pregnancy immediately preceding study entry. In this cohort, 59 319 (5.8%) women had one or more pregnancies complicated by hypertensive disorders of pregnancy between 1978 and 2012; 183 423 developed hypertension during follow-up (1995-2012).
Table 1.
Baseline characteristics of the study cohort used to estimate relative risks of post-pregnancy hypertension, at study entry, Denmark, 1995-2012*. Values are numbers (percentages) unless stated otherwise
| Characteristics | Normotensive | Hypertensive disorder of pregnancy in most recent pregnancy† | ||
|---|---|---|---|---|
| Gestational hypertension | Moderate pre-eclampsia | Severe pre-eclampsia | ||
| Age (years): | ||||
| <20 | 14 138 (1.4) | 56 (0.6) | 314 (1.3) | 134 (1.8) |
| 20-24 | 108 333 (11.0) | 810 (9.3) | 3006 (12.5) | 1051 (14.0) |
| 25-29 | 279 569 (28.4) | 2554 (29.4) | 7590 (31.6) | 2567 (34.2) |
| 30-34 | 258 279 (26.2) | 2277 (26.2) | 6089 (25.3) | 2131 (28.4) |
| 35-39 | 166 099 (16.9) | 1485 (17.1) | 3555 (14.8) | 1016 (13.5) |
| 40-44 | 102 678 (10.4) | 888 (10.2) | 2155 (9.0) | 427 (5.7) |
| 45-49 | 44 445 (4.5) | 467 (5.4) | 1003 (4.2) | 141 (1.9) |
| ≥50 | 11 324 (1.2) | 156 (1.8) | 345 (1.4) | 36 (0.5) |
| Total | 984 865 | 8693 | 24 057 | 7503 |
| Parity: | ||||
| 1 | 698 753 (70.9) | 7087 (81.5) | 19 955 (82.9) | 6700 (89.3) |
| 2 | 220 210 (22.4) | 1248 (14.4) | 3281 (13.6) | 634 (8.4) |
| ≥3 | 65 902 (6.7) | 358 (4.1) | 821 (3.4) | 169 (2.3) |
| Total | 984 865 | 8693 | 24 057 | 7503 |
| Diabetes (type 1 or 2): | ||||
| Yes | 4667 (0.5) | 111 (1.3) | 385 (1.6) | 129 (1.7) |
| No | 980 198 (99.5) | 8582 (98.7) | 23 672 (98.4) | 7374 (98.3) |
| Total | 984 865 | 8693 | 24 057 | 7503 |
| First trimester smoking‡: | ||||
| Yes | 162 644 (23.8) | 1029 (15.5) | 3314 (18.2) | 847 (13.3) |
| No | 520 173 (76.2) | 5592 (84.5) | 14 879 (81.8) | 5544 (86.8) |
| Total | 682 817 | 6621 | 18 193 | 6391 |
| Prepregnancy body mass index§: | ||||
| <18.5 | 14 134 (4.5) | 69 (1.8) | 153 (2.1) | 105 (3.5) |
| 18.5-24 | 203 234 (64.8) | 1757 (46.4) | 3608 (48.4) | 1716 (57.0) |
| 25-29 | 63 288 (20.2) | 1005 (26.5) | 1983 (26.6) | 687 (22.8) |
| 30-34 | 22 345 (7.1) | 551 (14.6) | 1030 (13.8) | 310 (10.3) |
| ≥35 | 10 649 (3.4) | 405 (10.7) | 686 (9.2) | 194 (6.4) |
| Total | 313 650 | 3787 | 7460 | 3012 |
*Based on full cohort of women with one or more pregnancies between 1978 and 2012, unless indicated otherwise (see ‡ and §).
†History of hypertensive disorder of pregnancy in most recent pregnancy before start of follow-up. Not all women with a pregnancy complicated by a hypertensive disorder of pregnancy had the disorder in the pregnancy immediately preceding the start of follow-up; history of a hypertensive disorder of pregnancy in the most recent pregnancy was a time dependent variable that was reset with every pregnancy during follow-up. Therefore, although 40 253 women had a history of a hypertensive disorder of pregnancy in the most recent pregnancy at the start of follow-up (as shown in this table), 59 319 had a pregnancy affected by a hypertensive disorder of pregnancy between 1978 and 2012 (as noted in the Results).
‡First trimester smoking status in woman’s first pregnancy resulting in live birth or stillbirth in 1991-2012.
§Prepregnancy body mass index in woman’s first pregnancy resulting in live birth or stillbirth in 2004-12.
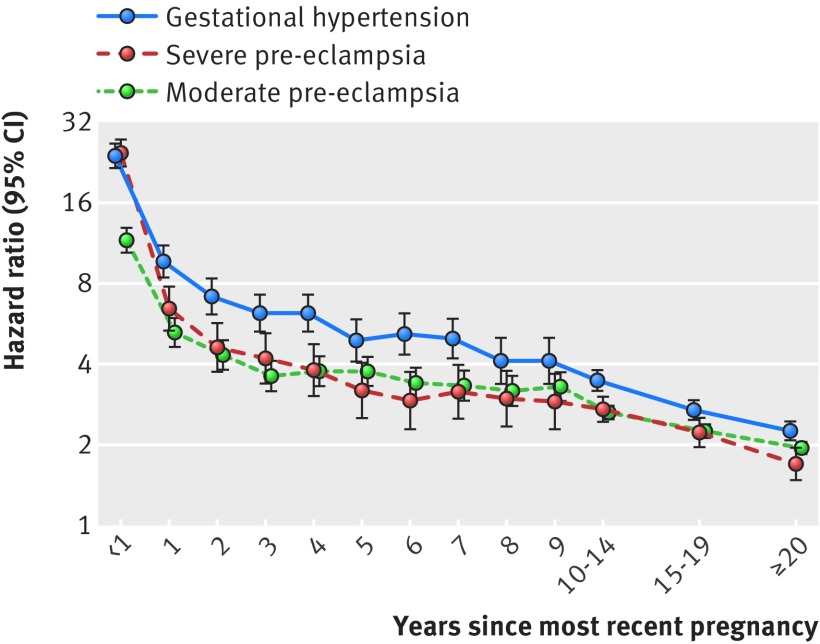
Hypertensive disorder of pregnancy in most recent pregnancy—in the year after a woman’s latest delivery, rates of post-pregnancy hypertension were 12-fold to 25-fold higher in women with a hypertensive disorder of pregnancy than in women with a normotensive pregnancy (fig 2, supplementary table 2). One to five years post partum, rates were fourfold to 10-fold higher in women with a hypertensive disorder of pregnancy in the latest pregnancy. Hazard ratios were lower thereafter, but even more than 20 years post partum, women with a hypertensive disorder of pregnancy in their most recent pregnancy had twice the rate of hypertension as women whose most recent pregnancy was normotensive. With the exception of the first year post partum, hazard ratios for gestational hypertension were statistically significantly higher than hazard ratios for pre-eclampsia, regardless of the time that had elapsed since pregnancy (fig 2, supplementary table 2). Observed association magnitudes did not differ statistically significantly by severity of pre-eclampsia. Additional adjustment for diabetes, smoking, and body mass index did not meaningfully change the results (see supplementary tables 3-5). In the first years after delivery, there was a tendency towards higher rates of hypertension in women with early onset hypertensive disorders of pregnancy, compared with women with intermediate onset or term hypertensive disorders of pregnancy, but overall, hazard ratios did not vary statistically significantly by timing of onset of the hypertensive disorder of pregnancy (see supplementary figure 1 and supplementary table 6).
Fig 2 Hazard ratios for hypertension by severity of hypertensive disorder of pregnancy (if any) in the most recent pregnancy and time since most recent pregnancy, among women with at least one live birth or stillbirth in Denmark, 1978-2012. Hazard ratios compare rates of hypertension among women with severe pre-eclampsia (orange), moderate pre-eclampsia (green), and gestational hypertension (black) in the latest pregnancy with rates of hypertension in women whose most recent pregnancy was normotensive. Follow-up began in 1995 or three months post partum, whichever came later. Hazard ratios are adjusted for maternal age, maternal birth year, and parity (see also supplementary figure 1 for corresponding figure with hypertensive disorders of pregnancy classified by timing of delivery)
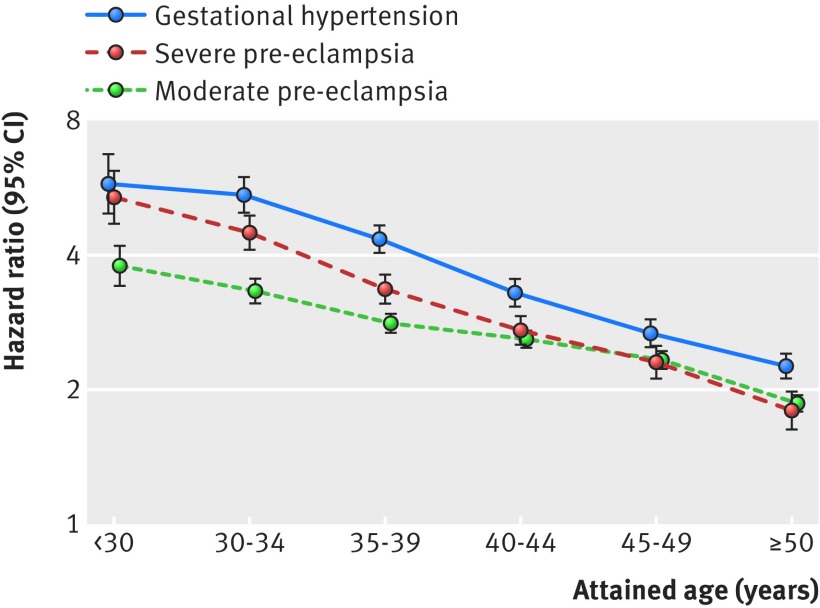
History of hypertensive disorders of pregnancy—rates of post-pregnancy hypertension were twofold to sixfold higher in women with a history of hypertensive disorders of pregnancy than in women the same age with only normotensive pregnancies, with the strongest associations in younger women (fig 3, supplementary table 7). For all but women aged less than 30 years, a history of gestational hypertension was more strongly associated with post-pregnancy hypertension than a history of pre-eclampsia, regardless of the severity of the pre-eclampsia.
Fig 3 Hazard ratios for hypertension by history of most severe hypertensive disorder of pregnancy and attained maternal age, among women with at least one live birth or stillbirth in Denmark, 1978-2012. Hazard ratios compare rates of hypertension among women with severe pre-eclampsia (orange), moderate pre-eclampsia (green), and gestational hypertension (black) as their most severe hypertensive disorder of pregnancy (cumulative history over all pregnancies) with rates of hypertension in women whose pregnancies were all normotensive. Follow-up began in 1995 or three months postpartum, whichever came later. Hazard ratios are adjusted for maternal age, maternal birth year, parity, and time since most recent pregnancy
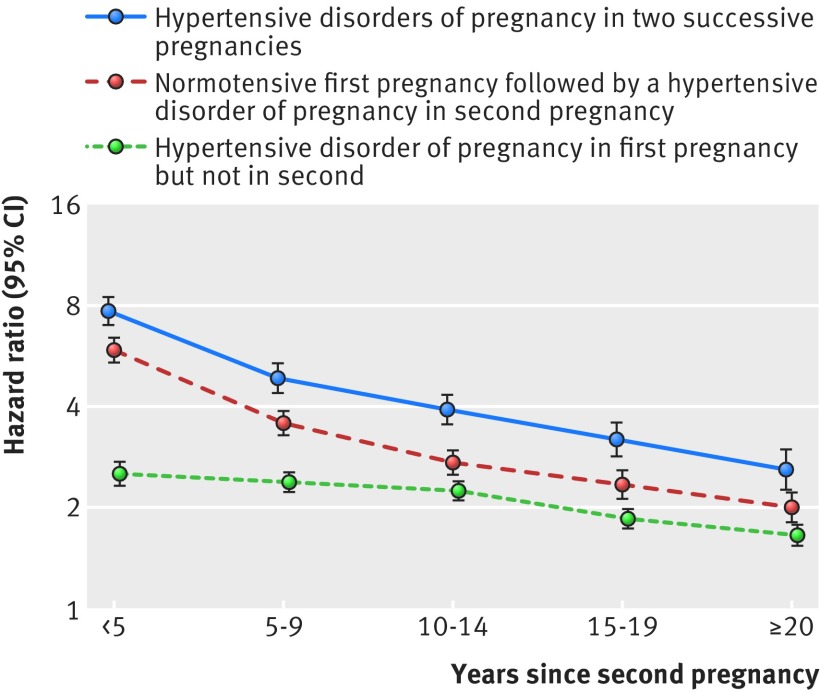
Sequence of hypertensive disorders of pregnancy—for women with at least two pregnancies, having a hypertensive disorder of pregnancy in the first pregnancy but not in the second was associated with a doubling of the rate of post-pregnancy hypertension, compared with having two normotensive pregnancies, regardless of the time elapsed since the second pregnancy (fig 4, supplementary table 8). The associations were even stronger in women with a normotensive first pregnancy and a hypertensive disorder of pregnancy in the second pregnancy, and greatest (hazard ratio range 2.6-7.8) in women with hypertensive disorders of pregnancy in both pregnancies.
Fig 4 Hazard ratios for hypertension by hypertensive disorder of pregnancy sequence in two first pregnancies by number of years since the second pregnancy, among women with at least two live births or stillbirths in Denmark, 1978-2012. Hazard ratios compare rates of hypertension among women with a hypertensive disorder of pregnancy in first pregnancy but not in the second (green), a normotensive first pregnancy followed by a hypertensive disorder of pregnancy in second pregnancy (red), and hypertensive disorders of pregnancy in two successive pregnancies (purple), with rates in women with two successive normotensive pregnancies. Follow-up began in 1995 or 3 months after the second delivery, whichever came later. Hazard ratios are adjusted for maternal age, maternal birth year, and parity
Sensitivity analyses
Table 2 shows the distribution of antihypertensive drug use in the first year post partum among women with a first delivery in or after 1995. Some hypertension identified during this year might reflect undetected pregestational hypertension. Consequently, we examined the effect of excluding women who developed hypertension 3-12 months post partum on our cumulative incidences and hazard ratios. Follow-up began one year post partum, and in the analyses we included only women with no hypertension 3-12 months after delivery. Beginning follow-up one year post partum in women free of hypertension at that time slightly reduced the cumulative incidences for women with hypertensive disorders of pregnancy (see supplementary table 1). In the analyses of cumulative history of hypertensive disorders of pregnancy, hazard ratio magnitudes in women aged less than 35 years decreased (see supplementary table 9), whereas hazard ratios for older women changed little, if at all. Excluding women with early post-pregnancy hypertension from the analyses of hypertensive disorder of pregnancy sequence reduced the magnitudes of hazard ratios estimated for elapsed times of five years or less since the second pregnancy (see supplementary table 10).
Table 2.
Antihypertensive drug use in the year after first delivery in women without previous antihypertensive drug use, by history of hypertensive disorders of pregnancy in Denmark, 1995-2012*
| Hypertensive disorder of pregnancy in first pregnancy | Total No of women | Antihypertensive drug use in year after delivery | |||
|---|---|---|---|---|---|
| None | <3 months post partum | <3 months and 3-12 months post partum | 3-12 months post partum only | ||
| Yes | 23 826 | 21 216 | 1915 | 513 | 182 |
| No | 461 290 | 458 995 | 1335 | 276 | 684 |
*485 116 women had their first delivery in or after 1995; these women are included in the table, which also includes information on use of antihypertensive drugs from delivery until one year post partum. Of these women, 2144 did not enter follow-up (which began three months after delivery) because they were censored before this point due to, for example, development of heart disease. Consequently, in the text we state that the estimates of cumulative incidence are based on a slight smaller number of women (n=482 972).
Discussion
In a large nationwide cohort, 14-32% of women with a hypertensive disorder of pregnancy in their first pregnancy developed hypertension in the decade after delivery, compared with 4-11% of women with normotensive first pregnancies. Rates of post-pregnancy hypertension in women with a hypertensive disorder of pregnancy in the most recent pregnancy were 12-fold to 25-fold higher in the first year post partum and up to 10-fold higher in the decade after delivery, than in women without a hypertensive disorder of pregnancy. Hypertension rates among women with a previous hypertensive disorder of pregnancy remained doubled more than 20 years later.
Our large cohort allowed us to examine the timing and trajectory of post-pregnancy hypertension risk associated with hypertensive disorders of pregnancy in short, discrete time intervals. Including all parous women in Denmark in the cohort minimised the possibility of selection bias, and using prospectively collected register data eliminated the possibility of recall bias. Validation of diagnoses for hypertensive disorders of pregnancy in the national patient register has shown that although their sensitivity is variable (such that some misclassification of pregnancies complicated by hypertensive disorders of pregnancy as normotensive almost certainly occurred), their specificity is excellent (>99%),19 and the potential impact of misclassification of hypertensive disorders of pregnancy diagnoses on the observed associations is therefore likely negligible. Although guidelines for the diagnosis of hypertensive disorders of pregnancy have changed in the past 35 years, most notably in the past decade, the blood pressure thresholds used to define gestational hypertension and pre-eclampsia, and the assumption that pre-eclampsia typically includes proteinuria, have been fairly consistent. Furthermore, major changes in definition put forward by the American College of Obstetrics and Gynecology,2 the Royal College of Obstetricians and Gynaecologists,11 and the International Society for the Study of Hypertension in Pregnancy,20 which allow for conditions other than proteinuria (eg, fetal growth restriction) to define pre-eclampsia, were published late in the study period or after the study period ended. Registration of filled prescriptions in the national prescription register occurs automatically through the personal identification number, which should ensure that registration of all dispensed drugs in Denmark is correct and virtually complete.17 However, women using antihypertensive drugs for other indications will have been misclassified as having hypertension. Hypertension rates based on drug use also underestimate the true rates in the study population, since not all hypertension is detected, and not all women with a diagnosis are treated.21 However, since clinical awareness of the link between hypertensive disorders of pregnancy and later cardiovascular disease remains incomplete,9 10 22 the probability of having a diagnosis of hypertension was unlikely to differ for women with and without a history of hypertensive disorders of pregnancy. Hypertension diagnosed within one year post partum was undoubtedly also a mixture of undetected prepregnancy hypertension and new onset hypertension. From a clinical perspective, however, whether hypertension detected post partum predated the pregnancy or is a sequela of hypertensive disorders of pregnancy, is of minor importance; either way, women with a previous hypertensive disorder of pregnancy have much higher rates of hypertension than their peers, and clinical contact with these women therefore represents an important opportunity for detection. Adjustment for diabetes and smoking did not change the observed associations, suggesting that these variables could not explain our findings. Although adjustment for body mass index also did not substantially change our results, these analyses were underpowered and we cannot exclude the possibility that body mass index might play a role in the association between hypertensive disorders of pregnancy and post-pregnancy hypertension. We also cannot discount the possibility that unmeasured risk factors for hypertension (eg, alcohol and salt intake, stress) might have confounded the observed associations, but this is unlikely, since to do so these factors would also have to be associated with hypertensive disorders of pregnancy.
Most studies assessing hypertension risk after a hypertensive disorder of pregnancy have reported risks averaged over follow-up periods ranging from a few years to decades (eg,3 4 5 13). Apart from one study examining hazard ratios for hypertension in five year intervals after delivery,12 studies have not focused on hypertension risk specifically by time since a pregnancy complicated by a hypertensive disorder of pregnancy —that is, on the timing of hypertension onset and how the risk of hypertension changes over time. Our work indicates that the immediate postpartum years are important: the increased risks of hypertension associated with hypertensive disorders of pregnancy already exist shortly after an affected pregnancy. Furthermore, for women aged more than 30 years, gestational hypertension was more strongly associated with post-pregnancy hypertension than was pre-eclampsia, which is consistent with findings from some,23 24 but not all,4 13 25 previous studies and is thought to reflect aetiological differences between gestational hypertension and pre-eclampsia.26 27 Hypertension during and after pre-eclampsia might be a marker of wider multiorgan system disruption, whereas the mechanisms underlying gestational hypertension may be more narrowly focused. An excess of undetected prepregnancy hypertension in women with gestational hypertension could also explain higher rates of hypertension immediately after an affected pregnancy, but differential detection of prepregnancy hypertension depending on later type of hypertensive disorder of pregnancy seems implausible. Furthermore, such an excess would not explain why risks remained larger for gestational hypertension than for pre-eclampsia decades after delivery.
Women with hypertensive disorders of pregnancy had much higher 10 year risks of post-pregnancy hypertension than did women of the same age with normotensive pregnancies. The substantial absolute risks of hypertension in the decade after a hypertensive disorder of pregnancy indicate that the processes linking hypertensive disorders of pregnancy and hypertension are already operational during or shortly after pregnancy. Whether the risk of hypertension (and later cardiovascular disease) in women with hypertensive disorders of pregnancy is due to common predisposing factors or to a downstream effect of pathophysiological processes specific to hypertensive disorders of pregnancy, is currently the focus of debate.28 29 30 Either hypothesis could explain the finding that women with hypertensive disorders of pregnancy in two pregnancies had higher risks of hypertension than women with one affected pregnancy. However, the finding that, among women with a hypertensive disorder of pregnancy in only one of two pregnancies, a hypertensive disorder of pregnancy in the second pregnancy was more strongly associated with later hypertension than a hypertensive disorder of pregnancy in the first pregnancy, argues against the importance of processes initiated by hypertensive disorders of pregnancy. If post-partum hypertension was the direct consequence of the hypertensive disorder of pregnancy itself, one would expect the effect of a single affected pregnancy to be the same regardless of sequence, after taking into account time since most recent pregnancy.
The study results suggest that the as yet poorly understood processes leading to hypertension related to hypertensive disorders of pregnancy begin while the affected women are still relatively young. At the very least, initiation of regular blood pressure assessments soon after a pregnancy complicated by a hypertensive disorder of pregnancy is essential for prompt identification of hypertension in these women. Because the number of women potentially at risk of hypertension related to hypertensive disorders of pregnancy is large, and routine follow-up could conceivably last years or even decades, an algorithm to identify those at greatest risk (the subgroup most likely to benefit from screening) is urgently needed; identification of biomarkers that predict which women will develop hypertension after hypertensive disorders of pregnancy would be even more useful. Furthermore, the degree to which early identification and timely treatment of hypertension in women with a history of hypertensive disorders of pregnancy can reduce their burden of cardiovascular disease, is also unknown and needs to be clarified. In the quest to minimise the lifetime impact of hypertensive disorders of pregnancy on women’s post-pregnancy health, we now need data, particularly from randomised clinical trials, to support clinical decision making and policy on clinical follow-up for women with a history of hypertensive disorders of pregnancy.
What is already known on this topic
Women with a history of hypertensive disorders of pregnancy have a twofold to fourfold increased post-pregnancy risk of developing essential hypertension, an important risk factor for cardiovascular disease
It is unclear how soon after delivery the risk of hypertension increases in women with hypertensive disorders of pregnancy
It is also unclear how the risk changes with time since pregnancy, and therefore, there is no evidence on which to base recommendations for clinical follow-up of these women
What this study adds
Women had substantially increased risks of post-pregnancy hypertension in the decade after a hypertensive disorder of pregnancy (14-32% after a first pregnancy, depending on age at delivery)
Cardiovascular disease prevention in women with hypertensive disorders of pregnancy should include blood pressure monitoring initiated soon after pregnancy
Web extra.
Extra material supplied by authors
Supplementary material: supplementary tables 1-10 and supplementary figure
Contributors: IB conceived the study, contributed to the study design, classified register data, interpreted study results, and drafted the paper. SB designed the study; planned the statistical analyses; obtained, linked, and analysed the data; interpreted the study results; and revised the paper. MM, JAL, and HB contributed to the study design, interpreted the study results, and revised the paper. JW designed the study, planned the statistical analyses and oversaw their conduct, interpreted the study results, and revised the paper. BT conceived the study, interpreted the study results, and revised the paper. HAB designed the study, planned the statistical analyses, obtained the data, interpreted the study results, and drafted and revised the paper. She is guarantor of the paper. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This study was funded by the Danish Council for Independent Research and the Danish Heart Association. Neither had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The researchers acted independently from the study sponsors in all aspects of this study.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: IB and SB were supported by a grant from the Danish Council for Independent Research; IB also received grant support from the Danish Heart Association; no other support for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Studies based solely on data from the Danish national registers do not require approval from the Danish research bioethics committees, as study participants are never contacted, and consent is not required for the use of register information. The study’s use of register data was covered by the approval extended by the Danish Data Protection Agency to all register based studies conducted by Statens Serum Institut (approval No 2015-57-0102).
Data sharing: This study is based on Danish national register data. These data do not belong to the authors but to the Danish Ministry of Health, and the authors are not permitted to share them, except in aggregate (as, for example, in a publication). However, interested parties can obtain the data on which the study was based by submitting a research protocol to the Danish Data Protection Agency (Datatilsynet) and then, once Data Protection Agency permission has been received, applying to the Ministry of Health’s Research Service (Forskerservice) at forskerservice@ssi.dk.
Transparency: The guarantor (HAB) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1-7. 10.1016/j.ejogrb.2013.05.005 pmid:23746796. [DOI] [PubMed] [Google Scholar]
- 2. American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122-31.pmid:24150027. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974 10.1136/bmj.39335.385301.BE pmid:17975258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944-51. 10.1161/HYPERTENSIONAHA.109.130765 pmid:19433776. [DOI] [PubMed] [Google Scholar]
- 5.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1-19. 10.1007/s10654-013-9762-6 pmid:23397514. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L, Benjamin EJ, Berra K, et al. American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404-23. 10.1016/j.jacc.2011.02.005 pmid:21388771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piepoli MF, Hoes AW, Agewall S, et al. Authors/Task Force Members. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur Heart J 2016;37:2315-81. 10.1093/eurheartj/ehw106 pmid:27222591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by womenʼs health care providers of long-term cardiovascular disease risk after preeclampsia. Obstet Gynecol 2015;125:1287-92. 10.1097/AOG.0000000000000856 pmid:26000498. [DOI] [PubMed] [Google Scholar]
- 9.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy 2012;31:50-8. 10.3109/10641955.2010.544955 pmid:21332326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidrich M-B, Wenzel D, von Kaisenberg CS, Schippert C, von Versen-Höynck FM. Preeclampsia and long-term risk of cardiovascular disease: what do obstetrician-gynecologists know?BMC Pregnancy Childbirth 2013;13:61 10.1186/1471-2393-13-61 pmid:23497157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Collaborating Centre for Women’s and Children’s Health. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy.RCOG Press, 2011. [PubMed] [Google Scholar]
- 12.Engeland A, Bjørge T, Klungsøyr K, Skjærven R, Skurtveit S, Furu K. Preeclampsia in pregnancy and later use of antihypertensive drugs. Eur J Epidemiol 2015;30:501-8. 10.1007/s10654-015-0018-5 pmid:25784365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veerbeek JHW, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015;65:600-6. 10.1161/HYPERTENSIONAHA.114.04850 pmid:25561694. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541-9. 10.1007/s10654-014-9930-3 pmid:24965263. [DOI] [PubMed] [Google Scholar]
- 15.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(Suppl):30-3. 10.1177/1403494811401482 pmid:21775347. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull 1998;45:320-3.pmid:9675544. [PubMed] [Google Scholar]
- 17.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health 2011;39(Suppl):38-41. 10.1177/1403494810394717 pmid:21775349. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika 1993;80:557-72 10.1093/biomet/80.3.557. [DOI] [Google Scholar]
- 19.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol 2007;166:117-24. 10.1093/aje/kwm139 pmid:17556761. [DOI] [PubMed] [Google Scholar]
- 20.Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97-104.pmid:26104417. [DOI] [PubMed] [Google Scholar]
- 21.Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 2013;3:e003423 10.1136/bmjopen-2013-003423 pmid:23996822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald SE, Walker M, Ramshaw H, Godwin M, Chen XK, Smith G. Hypertensive disorders of pregnancy and long-term risk of hypertension: what do Ontario prenatal care providers know, and what do they communicate?J Obstet Gynaecol Can 2007;29:705-10. 10.1016/S1701-2163(16)32601-9 pmid:17825134. [DOI] [PubMed] [Google Scholar]
- 23.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009;114:961-70. 10.1097/AOG.0b013e3181bb0dfc pmid:20168095. [DOI] [PubMed] [Google Scholar]
- 24.Nisell H, Lintu H, Lunell NO, Möllerström G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol 1995;102:876-81. 10.1111/j.1471-0528.1995.tb10874.x pmid:8534622. [DOI] [PubMed] [Google Scholar]
- 25.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 2003;326:845 10.1136/bmj.326.7394.845 pmid:12702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egeland GM, Klungsøyr K, Øyen N, Tell GS, Næss Ø, Skjærven R. Preconception cardiovascular risk factor differences between gestational hypertension and preeclampsia: Cohort Norway Study. Hypertension 2016;67:1173-80. 10.1161/HYPERTENSIONAHA.116.07099 pmid:27113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010;122:478-87. 10.1161/CIRCULATIONAHA.109.895458 pmid:20644016. [DOI] [PubMed] [Google Scholar]
- 28.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents?Circulation 2010;122:579-84. 10.1161/CIRCULATIONAHA.110.943407 pmid:20660802. [DOI] [PubMed] [Google Scholar]
- 29.Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health?Epidemiol Rev 2014;36:57-70. 10.1093/epirev/mxt006 pmid:24025350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staff AC, Redman CWG, Williams D, et al. Global Pregnancy Collaboration (CoLab). Pregnancy and long-term maternal cardiovascular health: progress through harmonization of research cohorts and biobanks. Hypertension 2016;67:251-60.pmid:26667417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: supplementary tables 1-10 and supplementary figure