Abstract
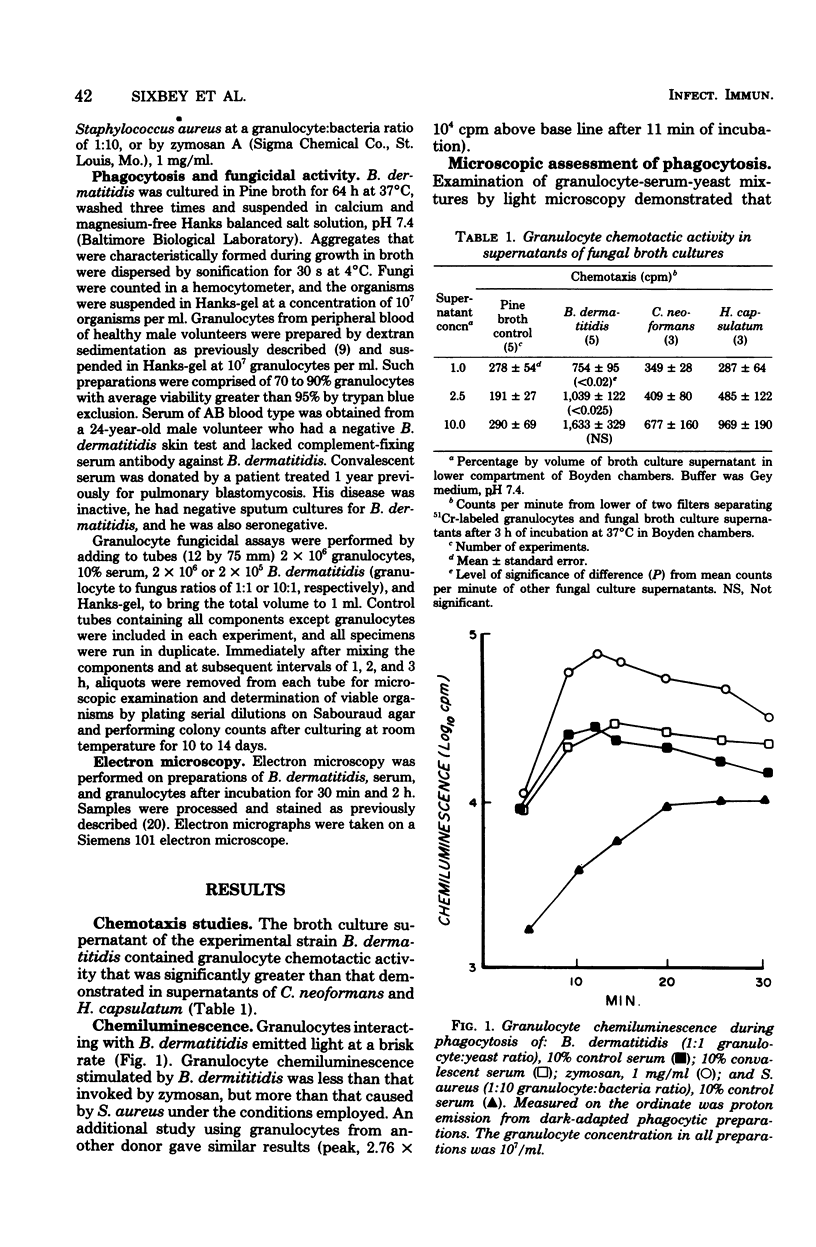
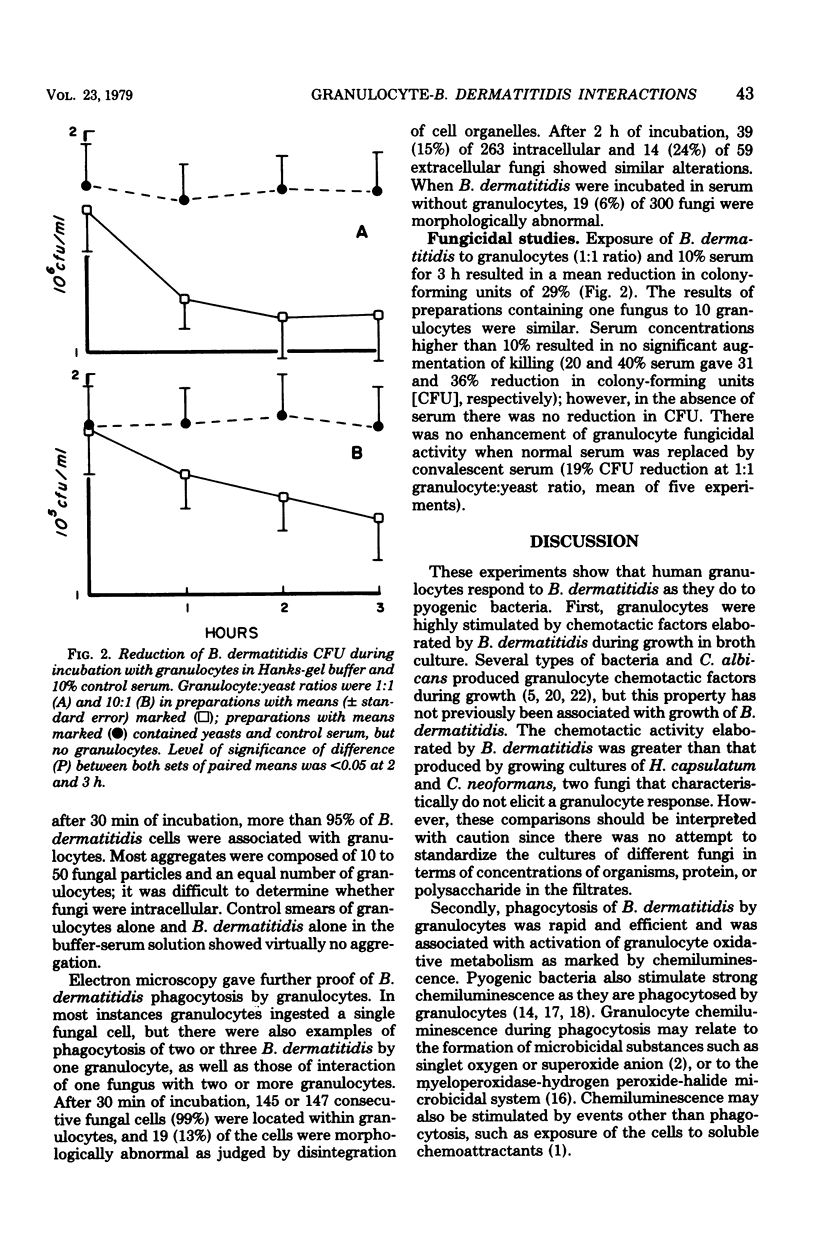
We studied interactions in vitro between human granulocytes and the yeast-like form of Blastomyces dermatitidis, because granulocytes are prominent in the host response to systemic blastomycosis. In Boyden chamber assays, broth culture filtrates of B. dermatitidis contained levels of granulocyte chemotactic activity that were significantly higher than those present in similar culture filtrates of Histoplasma capsulatum and Cryptococcus neoformans, two fungi that characteristically do not elicit granulocytes in infected tissues. Microscopic study, including electron microscopy, demonstrated that granulocytes phagocytosed B. dermatitidis promptly and efficiently. Moreover, granulocytes emitted light (chemiluminescence) at a brisk rate during phagocytosis of B. dermatitidis, indicating activation of intracellular metabolic pathways. However, fungicidal assay showed that granulocytes (1:1 cell-yeast ratio, 10% serum) killed only 29% of the B. dermatitidis inoculum during 3 h of incubation. Taken together, these findings suggest that there is disparity between phagocytosis and intracellular killing of B. dermatitidis by human granulocytes, perhaps because of resistance of this fungus to granulocyte microbicidal mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred C. D., Hill H. R. Effect of chemoattractants on chemiluminescence. Infect Immun. 1978 Mar;19(3):833–838. doi: 10.1128/iai.19.3.833-838.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. S., Pappagianis D. Inhibition by lysozyme of growth of the spherule phase of Coccidioides immitis in vitro. Infect Immun. 1974 Sep;10(3):616–623. doi: 10.1128/iai.10.3.616-623.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E. Chemotactic factor produced by Candida albicans. Infect Immun. 1977 Dec;18(3):568–573. doi: 10.1128/iai.18.3.568-573.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L. Growth of Histoplasma capsulatum. VI. Maintenance of the mycelial phase. Appl Microbiol. 1970 Mar;19(3):413–420. doi: 10.1128/am.19.3.413-420.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (first of three parts). N Engl J Med. 1974 Mar 28;290(13):717–723. doi: 10.1056/NEJM197403282901306. [DOI] [PubMed] [Google Scholar]
- Sun C. N., White H. J., Towbin E. J. Histochemistry and electron microscopy of the renal papilla in a genetic strain of rats with diabetes insipidus. Nephron. 1972;9(5):308–317. doi: 10.1159/000180162. [DOI] [PubMed] [Google Scholar]
- WANG C. J., SCHWARZ J. Phagocytosis of yeast cells in vitro. Am J Pathol. 1959 Jul-Aug;35(4):901–907. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]



