Abstract
We report a fatal case of post-partum streptococcal toxic shock syndrome in a patient who was previously healthy and had presented to the emergency department with an extensive blistering ecchymotic lesions over her right buttock and thigh associated with severe pain. The pregnancy had been uncomplicated, and the mode of delivery had been spontaneous vaginal delivery with an episiotomy. She was found to have septicemic shock requiring high inotropic support. Subsequently, she was treated for necrotizing fasciitis, complicated by septicemic shock and multiple organ failures. A consensus was reached for extensive wound debridement to remove the source of infection; however, this approach was abandoned due to the patient’s hemodynamic instability and the extremely high risks of surgery. Both the high vaginal swab and blister fluid culture revealed Group A beta hemolytic streptococcus infection. Intravenous carbapenem in combination with clindamycin was given. Other strategies attempted for streptococcal toxic removal included continuous veno-venous hemofiltration and administration of intravenous immunoglobulin. Unfortunately, the patient’s condition worsened, and she succumbed to death on day 7 of hospitalization.
Keywords: Streptococcal toxic shock syndrome, Necrotizing fasciitis, Toxic epidermal necrolysis
Introduction
Streptococcal toxic shock syndrome is an invasive streptococcal skin and soft tissue infection caused by toxins released by Streptococcus pyogenes, also known as group A beta hemolytic streptococcus. This aggressive form of invasive streptococcal infection is associated with the release of streptococcal pyrogenic exotoxins, which are a group of superantigens. By binding simultaneously to a major histocompatibility complex class II molecule and specific V-β region of T-cell receptors, these superantigens are able to stimulate the proliferation and activation of T-cells, leading to a massive increase in the secretion of proinflammatory cytokines and resulting in profound shock and organ failure [1]. The streptococcal pyrogenic antigens include bacteriophage-encoded SpeA, SPeC, and protein cysteine SpeB, as well as mitogenic factor [MF, SpeF], and streptococcal superantigen [SSA], which were identified only recently [2]. Invasive streptococcal infection is associated with poor outcomes, and the mortality rate can be as high as 80% [3]. Among the factors that are associated with poor outcomes in patients who subsequently develop necrotizing fasciitis or myonecrosis are the non-specific initial presentation, which leads to a delayed diagnosis, the aggressive nature of the infection (which is frequently underestimated), the delay in starting empirical- and organism-specific antimicrobials, and the delay in removing the source of the infection through surgery [4].
Case report
The patient was a previously healthy, 25-year-old housewife who had given birth to a 3.1-kg baby girl in the 37th week of her first pregnancy at a private hospital. The pregnancy had been uncomplicated and the mode of delivery had been a spontaneous vaginal delivery with an episiotomy. Post-delivery, the patient had complained of severe pain, mainly in the region of her buttocks. She had developed shortness of breath of sudden onset at 03:00 h the next day that was associated with worsening pain over her right thigh, which prompted her to seek treatment at the emergency department of a district hospital. Upon arrival, she was drowsy and tachypneic with an oxygen saturation of 56% on room air, which required her to receive a high-flow oxygen supply. She also had tachycardia with a pulse was 121 bpm. She was afebrile at 36 °C. Her blood pressure was unrecordable initially and, despite resuscitation with 30 mL/kg of normal saline, she was persistently hypotensive with a blood pressure of 56/30 mmHg. A subsequent noradrenaline infusion was started, and her blood pressure steadied. A stat dose of IV heparin 5000 units was given after a presumptive diagnosis of pulmonary embolism and a IV amoxicillin-clavulanate stat dose was given for septicemic shock as a possible differential diagnosis due to persistent hypotension.
She was then transferred to a tertiary hospital with an intensive care facility for further workup and management. Upon arrival, she was electively intubated for severe metabolic and lactic acidosis with worsening respiratory distress. A total of 120 mL/kg (6 L) of crystalloid was given. The patient was persistently hypotensive post-intubation despite fluid resuscitation, and another 3 inotropes (adrenaline, vasopressin and dobutamine) were sequentially added and titrated. A physical examination revealed a grossly swollen right thigh with extensive blistering ecchymotic patches over her right thigh extending to her right buttock. The diagnosis at that time was necrotizing fasciitis of the right thigh with septicemic shock complicated by acute kidney injury, rhabdomyolysis, coagulopathy with thrombocytopenia and ischemic hepatitis. She was started empirically on IV meropenem in combination with IV clindamycin and IV vancomycin after appropriate cultures were taken from blood and blister fluid. A high vaginal swab for culture and sensitivity was also taken. She was then transferred to an intensive care unit for further management. Orthopedic and surgical opinions were sought for removal of the source of the infection. An extensive wound debridement was initially planned after optimising her condition. A CT pulmonary angiography was also done and revealed an incidental finding of a small pulmonary embolism. A bedside echocardiography showed good contractility and the heart chambers were not grossly dilated to suggest a massive pulmonary embolism or cardiomyopathy that can cause hemodynamic instability. Intravenous immunoglobulin was given on the same day for toxin neutralization and applied for 5 days. Continuous veno-venous hemofiltration (CVVH) was started due to severe lactic acidosis, with lactate ranging from 9.8 to 18 mmol/L and a grossly elevated creatinine kinase of 29783 U/L, as well as for toxin removal.
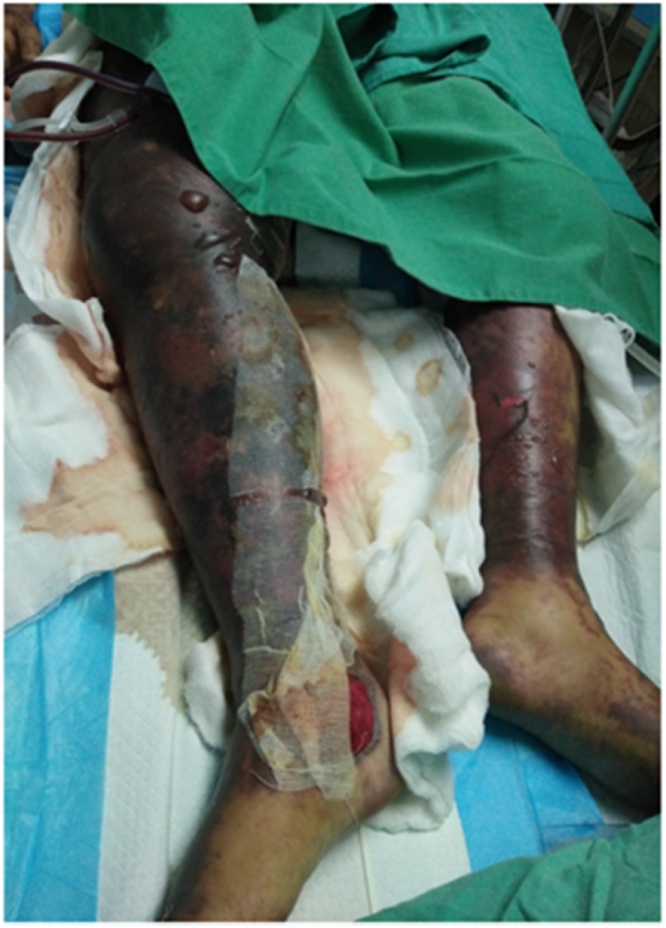
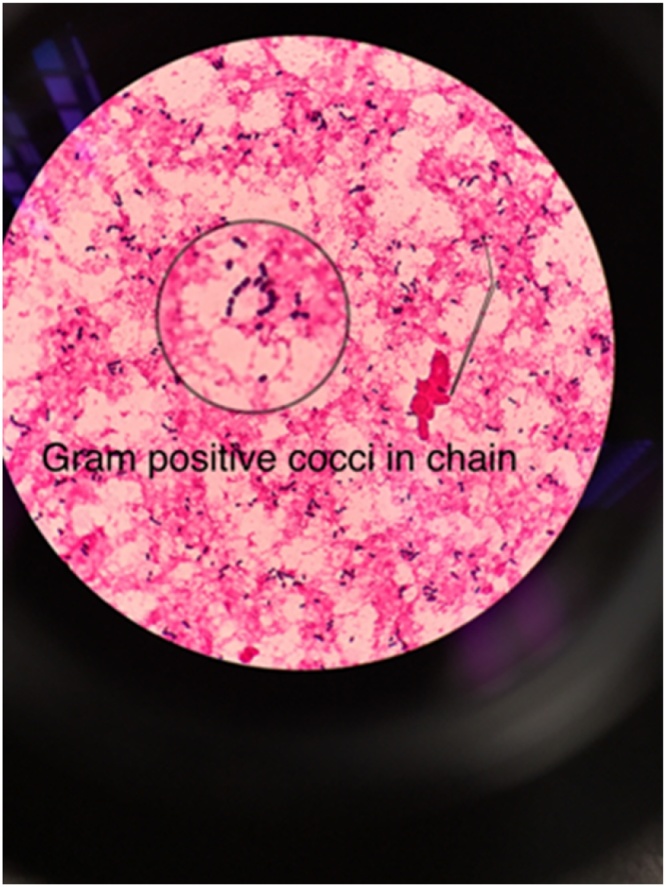
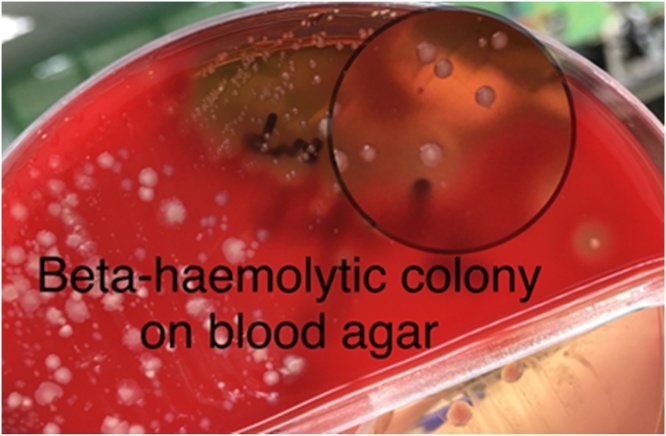
The condition of the patient remained critical for the next 3 days. She required 4 inotropes and CVVH was continued for persistent lactic acidosis as well as high creatinine kinase. The skin lesion spread over her bilateral upper and lower limbs developed a bluish discoloration and produced blistering of the bilateral lower limbs that extended to her right lower abdomen (Fig. 1). A similar lesion was also noted on her right upper limb that extended to her right elbow. Both high vaginal swab and blister fluid culture revealed Gram positive cocci in chains on staining (Fig. 2) and grew S. pyogenes (Fig. 3). The multiple blood cultures taken at our centre had a negative yield, but the blood culture taken at the district hospital prior to the transfer was positive for group A beta hemolytic streptococcus. A multidisciplinary discussion was held on day 6 of admission with the involvement of orthopedics, internal medicine, obstetrics and gynecology, the microbiology and radiology team, and the diagnosis of group A streptococcal toxic shock syndrome with necrotizing fasciitis was reached. The IV clindamycin was restarted for toxin suppression. A high dose of IV crystalline penicillin G was given ever four hours for better time-dependent killing of streptococci. CVVH was continued for toxin removal. Surgical intervention was not feasible and a skin biopsy was not performed.
Fig. 1.
Blistering of the bilateral lower limbs.
Fig. 2.
Gram positive cocci in chains on gram staining.
Fig. 3.
Streptococcus pyogenes colony on blood agar.
She succumbed to death on day 7 of admission, with the cause of death determined to be septic shock with tissue necrosis and toxic shock syndrome secondary to S. pyogenes.
Discussion
Streptococcal toxic shock syndrome, as defined by the Working Group on Severe Streptococcal Infections, is diagnosed when there is an isolation of group A Streptococci from a sterile site with systolic blood pressure ≤ 90 mmHg plus 2 or more of these signs; tissue necrosis, desquamation skin, acute respiratory distress syndrome (ARDS), liver impairment, coagulopathy (platelets ≤ 100,000/mm3) and renal impairment (creatinine ≥2 mg/dL) [5]. The streptococci enter a normally sterile site through the vagina, pharynx, mucosa and skin in more than half of the cases of streptococcal toxic shock syndrome [6]. Risk factors for an increased risk of invasive streptococcal infection include young or old age (i.e., neonates and older adults), surgical procedures, diabetes, both penetrating and non-penetrating trauma, concurrent viral infection of the skin and mucosal linings, and the use of non-steroidal anti-inflammatory medication [6]. This particular patient was healthy with no known medical illness that would predispose her to invasive streptococcal infection and the pregnancy had been uncomplicated. The group A streptococci isolated from the vaginal swab could have been a colonizer of the genital tract, and the spontaneous vaginal delivery and episiotomy would have provided a portal of entry to the pelvis and, subsequently, the lower limbs, resulting in a devastating invasive infection. The use of non-steroidal anti-inflammatory medication might mask the early signs and symptoms of streptococcal infection, which may prevent early presentation and could result in a more severe infection.
Approximately 0.03–1% of women of reproductive age have asymptomatic vaginal carriage of group A streptococci [7]. Risk factors for increased frequency of isolation of group A streptococci from vaginal swab cultures include vulvovaginitis, puerperal sepsis and prior or concurrent pharyngitis [8]. The most common serotype of Streptococcus pyogenes isolated in human female urogenital tract infection, puerperal sepsis and neonatal infections are S. pyogenes serotype M28 [9]. Serotypes of group A streptococci that are associated with puerperal infections, particularly M28 isolates, carry a 37.4-kb genomic island designated Region of difference 2 (RD2), which encodes 7 putative proteins and potential virulence factors that are shared with group B streptococci [10].
A breach of the body’s mucosal barrier results in the entry of streptococci into deep tissues and the bloodstream. In some cases, the organism is able to penetrate intact mucosal membranes and transient bacteremia must occur for streptococci to be able to cause streptococcal toxic shock syndrome [11]. In this case, all 4 blood culture specimens taken in our center failed to yield group A streptococcus but was positive at the referring facility. A combination of empirical antibiotics started in the district hospital [12] and the body immune system clearance mechanism either from pre-existing type-specific immunity or non-specific clearance by the reticuloendothelial system might be responsible for the failure to detect streptococcal bacteremia in this patient [11].
Stevens et al. described streptococcal toxic shock syndrome as having 3 phases [6]. The first phase is characterized by an influenza-like prodrome with fever chills, myalgia, nausea, vomiting and diarrhea that precedes the drop in blood pressure by 24–48 h. In patients who subsequently have necrotizing fasciitis and myonecrosis, the most common complaint is pain that progressively increases in severity. This particular complaint is usually the symptom that prompts the patient to seek medical attention. The patient discussed here had a typical initial presentation of this invasive streptococcal infection, which was non-specific, and this vague presentation could be the reason for dismissal by a general practitioner, despite the need for deep venous thrombosis to be ruled out in postpartum patients. The second phase of streptococcal toxic shock syndrome typically presents with persistent fever, tachycardia and tachypnea and, among patients who also develop necrotizing fasciitis and myonecrosis, an increasing severe pain at the site of infection [13]. The final phase of streptococcal toxic shock syndrome manifests with the sudden onset of shock and organ failure in addition to the signs and symptoms mentioned above. Most often, clinical evidence of necrotizing fasciitis is a late finding that often occurs after the development of hypotension. The progress of shock and multiple organ failure can be very rapid and lead to death within 24–48 h of hospitalization [6]. An elevated level of serum creatinine that is more than twice the normal level would suggest early manifestation of renal impartment in the second stage before the development of profound organ failure and hypotension. Individuals with necrotizing fasciitis and myonecrosis would have markedly elevated creatinine phosphokinase. The full blood picture in this patient revealed left-shift neutrophilia that included myelocytes and metamyelocytes. Some toxic granulations and neutrophil vacuolation also suggest severe infection. The thrombocytopania in this patient could have been an early sign of disseminated intravascular coagulopathy that led to a massive gastrointestinal bleed resulting in a further drop in the platelet count [6].
Source control and removal via prompt and aggressive surgical exploration, as well as debridement, are of paramount importance for managing deep-seated streptococcal infection, and failure to remove the devitalised tissue will lead to the persistence of shock and organ failure [11]. If tissue perfusion and a mean arterial pressure >65 mmHg cannot be maintained despite fluid challenge with several liters of crystalloid fluid, then invasive monitoring is required to achieve the goal of a mean arterial pressure >65 mmHg. Monitoring of the hematocrit is important in persistently hypotensive patients despite achieving the above mentioned goal because haemolysins produced by group A streptococci can cause a dramatic drop in the circulating red cell mass. The use of vasopressors, such as high-dose dopamine, adrenaline and phenylephrine, in patients with refractory hypotension, especially those with concurrent disseminated intravascular coagulopathy, could lead to symmetrical gangrene of all 4 limbs [10]. Caution must be taken with the use of excessive vasopressors. Dialysis and hemoperfusion may be employed to non-specifically remove the circulating toxins.
Antimicrobial therapy with initial broad-spectrum empirical coverage must be given promptly; once an invasive streptococcal infection is confirmed, high-dose penicillin combined with clindamycin should be given. A high dose of penicillin with a maximum frequency of doses every 4 h would be required to provide good time-dependent killing of the streptococci. All group A streptococci remain sensitive to penicillin; however, in a severe, deep-seated infection where a large number of streptococci are present in the stationary phase of growth, penicillin would be less effective because penicillin-binding proteins are not expressed. Clindamycin belongs to a class of lincosamide antibiotics that act by suppressing protein synthesis and the production of exotoxin. Clindamycin has a longer half-life than penicillin and there is an indifferent interaction between penicillin and clindamycin [11].
The successful use of intravenous immunoglobulin (IVIG) for exotoxin neutralisation in treating streptococcal toxic shock syndrome has been described in several reports, with a decreased of mortality rate compared with matched historical controls, but double-blinded clinical trials have so far failed to show any significant differences [14], [15]. In cases treated with IVIG, early administration and more than one dose of IVIG should be given because different batches of IVIG have variable neutralizing activity against streptococcal exotoxins [16].
Conclusion
In conclusion, post-partum streptococcal toxic shock syndrome (STSS) is a rare, but well recognized, highly fatal complication of an infection by a toxigenic strain of S. pyogenes. Thus, high index of suspicion is needed among clinicians for early recognitions, and early aggressive management of the patient could improve patient outcomes.
Conflict of interest
There is no conflict of interest declared.
Contributor Information
Wei Chuan Chua, Email: weichuanchua@gmail.com.
Saedah Ali, Email: saedahali@gmail.com.
Sanihah Che Omar, Email: sanihah_che@usm.my.
Wan Mohd Nazaruddin Wan Hassan, Email: nazarudin@usm.my.
S. Praveena Seevaunnantum, Email: praveena@usm.my.
Rhendra Hardy Mohd Zaini, Email: rhendra@gmail.com.
Mohd Hasyizan Hassan, Email: miezan82@yahoo.com.
Alwi Muhd Besari, Email: dralwi@usm.my.
Zaidah Abd Rahman, Email: drzaidah@usm.my.
Zeti Norfidiyati Salmuna Ayub, Email: zetinorfidiyati@gmail.com.
Sabrina Abd Ghani, Email: sab_rina@usm.my.
Normalinda Yaacob, Email: drnorma_yaacob@usm.my.
Wan Rosilawati Wan Rosli, Email: rosirosi@usm.my.
References
- 1.Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248(May (4956)):705–712. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 2.Bisno A.L., Brito M.O., Collins C.M. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3(April (4)):191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- 3.Davies H.D., McGeer A., Schwartz B., Green K., Cann D., Simor A.E. Low DE. invasive group a streptococcal infections in Ontario, Canada. N Engl J Med. 1996;335(August (8)):547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian K.N., Lam K.S. Malignant necrotising streptococcal myositis: a rare and fatal condition. J Bone Joint. 2003;85(March (2)):277–278. doi: 10.1302/0301-620x.85b2.12970. [DOI] [PubMed] [Google Scholar]
- 5.Breiman R.F., Davis J.P., Facklam R.R., Gray B.M., Hoge C.W., Kaplan E.L. Defining the group A streptococcal toxic shock syndrome: rationale and consensus definition. JAMA. 1993;269(January (3)):390–391. [PubMed] [Google Scholar]
- 6.Stevens D.L., Tanner M.H., Winship J., Swarts R., Ries K.M., Schlievert P.M. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Eng J Med. 1989;321(July (1)):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 7.Jaquiery A., Stylianopoulos A., Hogg G., Vulvovaginitis Grover S. clinical features, aetiology, and microbiology of the genital tract. Arc Dis Childhood. 1999;81(July (1)):64–67. doi: 10.1136/adc.81.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anteby E.Y., Yagel S., Hanoch J., Shapiro M., Moses A.E. Puerperal and intrapartum group A streptococcal infection. Infect Dis Obstet. Gynecol. 1999;7(6):276–282. doi: 10.1002/(SICI)1098-0997(1999)7:6<276::AID-IDOG5>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson B.K., Norgren M., McGregor K., Spratt B.G., Normark B.H. Group A streptococcal infections in Sweden: a comparative study of invasive and noninvasive infections and analysis of dominant T28 emm28 isolates. Clin Infect Dis. 2003;37(November (9)):1189–1193. doi: 10.1086/379013. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S., Green N.M., Sitkiewicz I., LeFebvre R.B., Musser J.M. Identification and characterization of an antigen I/II family protein produced by group A Streptococcus. Infect Immun. 2006;74(July (7)):4200–4213. doi: 10.1128/IAI.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant A.E., Stevens D.L. Streptococcus pyogenes. In: Bennett J.E., Dolin R., Blaser M.J., editors. 8th ed. vol. 2. Elsevier, Saunders; Philadelphia, PA: 2015. (Mandell, Douglas, and Bennett's principles and practice of infectious diseases). Chapter 199. [Google Scholar]
- 12.Weinstein M.P., Reller L.B., Murphy J.R., Lichtenstein K.A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983;5(January (1)):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Stevens D.L. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995;1(July (3)):69. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry W., Hudgins L., Donta S.T., Pesanti E.L. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(June (24)):3315–3316. [PubMed] [Google Scholar]
- 15.Beaulieu A.A. Intravenous immunoglobulin for group a streptococcal toxic shock syndrome: a reassessment of efficacy. 46th Annual Meeting; Oct 26, Idsa; 2008. [Google Scholar]
- 16.Norrby-Teglund A., Kaul R., Low D.E., McGeer A., Andersson J., Andersson U. Evidence for the presence of streptococcal-superantigen-neutralizing antibodies in normal polyspecific immunoglobulin G. Infect Immun. 1996;64(December (120)):5395–5398. doi: 10.1128/iai.64.12.5395-5398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]