Abstract
Exercise intolerance with myalgia, muscle stiffness, and recurrent rhabdomyolysis due to mutations in the DMD gene can mimic metabolic myopathies leading to delayed or inaccurate diagnoses. In this retrospective chart review, we report 3 unrelated boys with exertional myalgia, muscle stiffness, myoglobinuria, and normal neurological examination due to an identical point mutation in the DMD gene: a hemizygous T-to-C change in exon 15 (c.1724T>C) resulting in an amino acid substitution of leucine to proline at codon 575. Two of the 3 boys had normal dystrophin immunostaining and Western blot analysis in muscle. This missense mutation has been reported twice before, with at least 1 patient exhibiting rhabdomyolysis. Our report, however, is the first to describe in detail the clinical findings associated with this specific mutation. Further studies and clinical reports are needed to better understand the pathogenicity of the mutation.
Keywords: BMD, dystrophin, DMD, exercise intolerance, rhabdomyolysis
Dystrophinopathies are caused by mutations in the DMD gene that lead to abnormal dystrophin function. Disease severity ranges from milder phenotypes, including patients with asymptomatic elevations in serum creatine kinase (CK) levels1–4 and patients with muscle cramps and recurrent myoglobinuria, to the more severe phenotypes of DMD-associated dilated cardiomyopathy and the progressive muscular dystrophies of Duchenne (DMD) and Becker (BMD) types.
The DMD gene is located on chromosome Xp21 and consists of 79 exons. It is divided into four domains: N-terminal, rod, cysteine-rich, and carboxy-terminal. Large deletions involving these domains have been identified in approximately 65% of DMD and 85% of BMD patients.5,6 Duplications and point mutations within the gene are found in 25–30% of DMD patients and 10–20% of BMD patients. The phenotype is best correlated with the degree of expression of functional dystrophin,7 which is largely determined by the degree of preservation of the reading frame of the spliced message obtained from the deleted allele.8,9 Preservation of the reading frame leads to a truncated dystrophin protein with decreased quantity or reduced immunostaining on muscle biopsy and produces the milder BMD phenotype. Disruption of the reading frame typically leads to severely reduced or absent dystrophin and the severe phenotype of DMD.10
With the ready availability of molecular genetic testing, manifestations of muscle cramps with elevated CK and myoglobinuria due to mutations in the DMD gene are being increasingly recognized. Exon deletions throughout the DMD gene, particularly mutations involving the proximal third of the rod domain, have been described in association with this milder pseudometabolic phenotype.11–13 These deletions have all been associated with abnormal dystrophin quantity and/or quality on muscle immunohistochemistry or Western blot analysis.14–21 Herein we report 3 unrelated boys who presented with exertional myalgia, rhabdomyolysis, and myoglobinuria without fixed muscle weakness or calf hypertrophy due to an identical point mutation in the DMD gene. We report in detail the clinical features associated with this specific missense mutation and highlight the importance of considering DMD mutations in such cases.
CASE REPORT
Case 1
Patient 1 is a 12-year-old boy who has experienced episodes of exercise-induced myalgia and muscle stiffness in the lower extremities since 5 years of age. Symptoms resolve following a period of rest for about 20 minutes, at which point he can resume his activities at a lower level of intensity. He has experienced myoglobinuria on three occasions after prolonged, strenuous exercise. His previous medical history is unremarkable, and developmental milestones were all age-appropriate. His parents, 15-year-old brother, and 8-year-old sister are all healthy. There is no family history of similar complaints, muscle weakness, or cardiac abnormalities.
On examination, his weight was 42 kg (50–75th percentile), height was 140 cm (10th percentile), and head circumference was 56 cm (50–98th percentile). A complete physical and neurological examination was normal. There was no muscle hypertrophy, and he did not have a Gower sign.
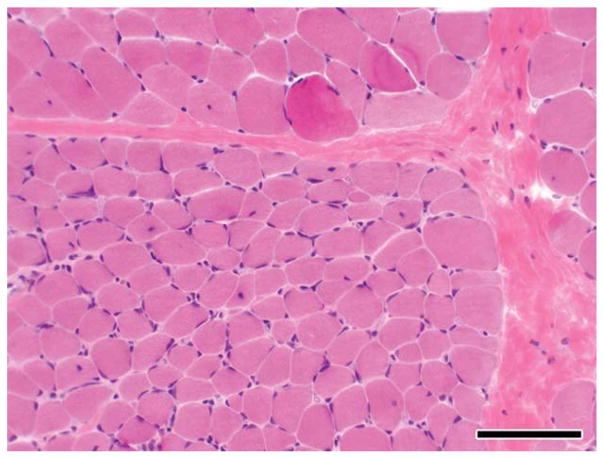
Serum CK levels were persistently elevated, ranging from 4000 to >10,000 U/L (normal <250 U/L), both at rest and after exercise. A muscle biopsy at 8 years of age demonstrated myopathic changes, including interstitial fibrosis, muscle fiber size variability, and fiber atrophy (Fig. 1). Electron microscopy revealed normal mitochondria and glycogen content. Immunohistochemical analysis demonstrated normal dystrophin staining for antibodies against the carboxy-terminus and rod domain as well as normal dysferlin, merosin, and a-sarcoglycan staining. Dystrophin Western blot analysis confirmed normal dystrophin quantity and molecular size.
FIGURE 1.
Hematoxylin and eosin (H&E) staining of the muscle biopsy from patient 1 demonstrating fiber size variation, internal nuclei, and increased perimysial fibrosis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Case 2
Patient 2 is a 17-year-old boy with recurrent episodes of exertional muscle pain and stiffness primarily involving the calves and proximal upper extremities. The symptoms were first noted at 5 years of age. They typically last for 1–2 hours following soccer or basketball practice, and are relieved by a warm bath or rest. He has experienced episodes of myoglobinuria after prolonged physical exertion. Previous medical history and developmental milestones were normal. His parents and 11-year-old brother are asymptomatic. There is no family history of similar symptoms, muscle disease, or cardiac abnormalities.
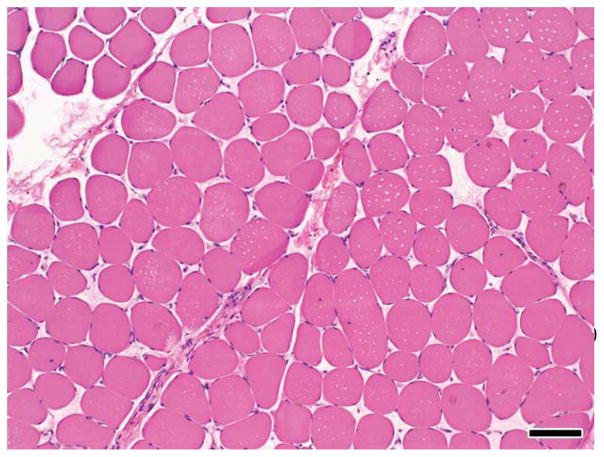
On examination, his height was 177 cm (50th percentile), weight was 59 kg (30th percentile), and head circumference was 56 cm (50th percentile). Neurological examination was normal. There was no muscle hypertrophy, and he did not have a Gower sign. He has had persistently elevated serum CK levels, ranging from 1000 to 8000 U/L at rest and up to 16,500 U/L after exercise. Electromyography at 14 years of age demonstrated findings consistent with a non-inflammatory myopathy. Muscle biopsy 1 year later revealed focal areas of muscle fiber necrosis and regeneration with mild endomysial fibrosis (Fig. 2). Electron microscopy revealed normal mitochondria and muscle glycogen content. Immunohistochemical analysis demonstrated normal dystrophin staining for antibodies against the carboxy-terminus and rod domain as well as normal dysferlin, merosin, and a-sarcoglycan staining. Western blot analysis confirmed normal dystrophin quantity (50–100%) and size.
FIGURE 2.
H&E staining of the muscle biopsy from patient 2 demonstrating fiber size variation, fiber necrosis, internal nuclei, and increased perimysial fibrosis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Further Laboratory Evaluation. An extensive laboratory evaluation was pursued on both patients and was normal. This included a complete blood count with differential, serum electrolytes, serum uric acid, serum lactic acid, liver and renal function tests, plasma carnitine levels, plasma acylcarnitine profile, enzymatic activity of phosphorylase A, phosphorylase B kinase, myoadenylate deaminase, phosphoglycerate kinase, phosphoglycerate mutase, lactate dehydrogenase, carnitine palmitoyl transferase (CPT) II, urine organic acids, in vitro fatty acid oxidation studies (fibroblasts), mitochondrial electron transport chain activity of complexes I–IV (fibroblasts), electrocardiogram, and echocardiogram. DNA testing for CPT II was normal in both patients. DNA testing for glycogen storage disease type II, type V, and myoadenylate deaminase deficiency performed on patient 1 was normal. Patient 2 had normal DNA testing for glycogen storage disease type 0 and glycogen storage disease type V.
DMD Molecular Genetic Testing. The persistently elevated CK levels and negative metabolic workup in both patients led us to test for mutations in the DMD gene. Initial deletion and duplication testing was normal, but full gene sequencing (patient 1: Athena Diagnostics, Worcester, Massachusetts; patient 2: Baylor College of Medicine, Houston, Texas) identified a hemizygous T-to-C change in exon 15 of the DMD gene (c.1724T>C), resulting in an amino acid substitution of leucine to proline at codon 575 (p.Leu575Pro) within the proximal rod domain in both patients. Sequencing of the DMD gene in the asymptomatic brother of each patient was normal, and targeted analysis in the asymptomatic mother of patient 2 identified a similar mutation. She had no male siblings and has not had a CK level measured. The mother of patient 1 has not undergone DNA testing or CK measurement. No other male members in this family were available for testing. Haplotype comparison of the 2 patients was not done.
Recently, we have identified the same mutation in a 9-year-old boy with exercise-induced cramps and fatigue. Symptoms began at 3 years of age. He has had no documented episodes of myoglobinuria. Examination was normal, except for 4þ/5 strength in the right biceps and a highly arched palate. There was no muscle hypertrophy, and he did not have a Gower sign. His serum CK levels have ranged between 2611 and 14,127 U/L. Metabolic testing for fatty acid oxidation disorders was normal. As the clinical phenotype matched with our other 2 cases, no further evaluation was pursued. The clinicopathological features of our 3 patients are compared in Table 1.
Table 1.
Clinicopathological features of our patients.
| Patient 1 | Patient 2 | Patient 3* | |
|---|---|---|---|
| Gender | Male | Male | Male |
| Age | 12 years | 17 years | 9 years |
| Age symptoms started | 5 years | 5 years | 3 years |
| Symptoms | Exercise-related myalgia, cramps, and muscle stiffness | Exercise-related myalgia, cramps, and muscle stiffness | Exercise-related muscle cramps and fatigue |
| Myoglobinuria | 3 episodes | >1 episode | No |
| Developmental delay | No | No | No |
| Family history | Negative | Negative | Negative |
| Neurological exam | Normal | Normal | Strength:4þ/5 in right biceps |
| Calf hypertrophy | No | No | No |
| Gower sign | Negative | Negative | Negative |
| Creatine kinase | Elevated (15ã 40×) | Elevated (15ã 65×) | Elevated (10ã 55×) |
| Muscle biopsy | Moderate fiber size variability, increased internal nuclei, type I fiber atrophy, rare fibers with peripheral basophilic cap, no fiber necrosis | Muscle fiber necrosis and regeneration with mild endomysial fibrosis, increase in internal nuclei, normal muscle fiber architecture | Not done |
| Immunohistochemistry | Normal dystrophin staining | Normal dystrophin staining | Not done |
| Immunoblot | Normal dystrophin quantity and molecular weight | Normal dystrophin quantity and molecular weight | Not done |
| Gene sequencing | c.1724T>C in exon 15 | c.1724T>C in exon 15 | c.1724T>C in exon 15 |
Patient 3 was recently identified with this similar missense mutation and has not had further workup.
DISCUSSION
The dystrophinopathies are characterized by a wide spectrum of clinical severity, with the severe end of the spectrum including diffuse progressive muscle disease characteristic of the DMD and BMD phenotypes. DMD and BMD are characterized by proximal muscle weakness, calf pseudohypertrophy, elevated serum CK levels, and dystrophic features on muscle histopathology. DMD is relentlessly progressive and manifests in early childhood, with affected boys typically becoming wheelchair-bound by age 12 years. Cardiomyopathy develops in virtually all affected individuals by 18 years of age and few survive beyond the third decade. BMD is typically later in onset and clinically milder than DMD. Most patients continue to walk after age 15 years and survive well into adult-hood.22 Although DMD is clinically homogeneous, the severity of BMD may be widely variable, even within families.23 Muscle analysis reveals qualitative and quantitative abnormalities of dystrophin in both DMD and BMD. Quantitative abnormalities more often result in DMD, with dystrophin levels ranging from 0–5%, whereas BMD patients often have qualitative abnormalities.24,25
The mildest clinical presentations of dystrophinopathy include asymptomatic elevation of serum CK1–4 and isolated quadriceps myopathy.12,26–29 Asymptomatic elevation of serum CK was reported to be associated with deletions involving exons 32–44, 48–53, and a missense mutation in exon 21. These patients had either normal or abnormal dystrophin analysis. Isolated quadriceps myopathy is characterized by atrophy of the quadriceps muscles and elevated CK levels. Biopsy reveals myopathic changes, and dystrophin is abnormal qualitatively, quantitatively, or both. Deletion of exons 45–48 in the DMD gene have been reported in patients with isolated quadriceps myopathy.
Numerous cases of dystrophinopathy-related post-exercise myalgia and cramps, as well as post-exercise muscle stiffness and exertional rhabdomyolysis, have been attributed to DMD deletions. For the clinician, these symptoms are suggestive of an underlying metabolic condition, such as a glycogen storage disorder, fatty acid oxidation disorder, or mitochondrial cytopathy. Although an evaluation for these disorders is warranted, the inclusion of DMD gene analysis is often not considered in the absence of fixed muscle weakness or hypertrophy. Increasing reports of such pseudometabolic presentations due to DMD mutations emphasize the need to consider DMD molecular genetic analysis in these patients.
To date, several case reports describing this pseudometabolic presentation due to DMD mutations have documented exon deletions in the rod domain of the DMD gene. A number of males with this phenotype due to deletions involving proximal rod domain have been reported. Abnormal dystrophin size or quantity was evident on immunostain and immunoblot.13,15,17 Other reports do not contain sufficient information to infer that there was an abnormality of dystrophin associated with proximal rod domain deletions.30 A carrier female with a deletion of exons 10–23 was reported to have exertional muscle pain as her only symptom, and immunohistochemical examination of muscle with antibodies against the N-terminal domain showed a mosaic pattern suggestive of a dystrophinopathy carrier state.14 A missense mutation in the proximal rod domain has also been described in an 8-year-old boy who had exercise-induced fatigue and muscle pain. Although his serum CK was elevated, there were no documented episodes of rhabdomyolysis, and dystrophin immunostaining and Western blot were normal.31 These reports suggest that mutations involving the proximal rod domain do not significantly alter protein function and yield a mild phenotype.11,13
Deletions involving the distal rod domain of the DMD gene have also been described in association with the milder pseudometabolic phenotype. These reports included symptomatic males as well as asymptomatic and manifesting carrier females and were associated with abnormal dystrophin on Western blot, unlike our patients.16–21 Very few missense mutations in the DMD gene have been reported to date.32–37 Most of these mutations localized to the actin-binding and carboxy-terminal domains and were associated with the more severe DMD or BMD phenotypes.
Our 3 patients further expand the reported cases of dystrophinopathy causing pseudometabolic features. They all harbor an identical mis-sense mutation (c.1724T>C) and 2 of them (cases 1 and 2) who underwent muscle biopsy had normal dystrophin immunostaining and normal dystrophin quantity and molecular size on immunoblot. However, dystrophin assays in muscle (immunostaining and Western blot) are not sensitive enough to identify mild abnormalities in affected males (personal correspondence with Basil T. Darras, MD, Harvard Medical School).
To our knowledge, this is the first detailed report of the clinical features associated with this specific missense change in the DMD gene. This mutation has been reported twice in the Leiden Muscular Dystrophy database,38 once in a male patient with recurrent rhabdomyolysis without further clinical details, and in a second patient with no clinical details. The missense mutation seen in our patients causes an amino acid substitution of leucine to proline at codon 575 within the proximal rod domain. This amino acid substitution is predicted to be “probably damaging” by the polymorphism phenotyping tool (Polyphen). Polyphen is a tool that predicts possible impact of an amino acid substitution on the structure and function of a human protein.39–41 Amino acid alignment around codon 575 in the DMD gene shows that the leucine at codon 575 is highly conserved across species. However, the specific number of control chromosomes that did not have this missense mutation is not known, as the official single-nucleotide polymorphism calls for the X chromosome have not yet been released (personal communication with Paul Flicek, 1000 Genomes Project). Although missense mutations in the DMD gene are rare and little is known about the phenotypic associations of these mutations, the location of the consequent amino acid change within the rod domain in our patients is likely associated with their milder phenotypic presentation, as discussed earlier. Further longitudinal follow-up studies in our patients will help clarify the long-term prognosis of this amino acid substitution. We considered the possibility that the mutation was an incidental finding, representing a benign polymorphism. However, a causative association is supported by the following observations: similarity of the clinical symptoms and course in our 3 patients; abnormal muscle histology suggestive of a destructive process; absence of the mutation in asymptomatic male siblings; exclusion of other metabolic disorders; Polyphen prediction that the mutation could be “probably damaging”; and the absence of this variant in the healthy population. Our report has several potential weaknesses.
Because the case reports are retrospective, only limited patient information was available. Furthermore, we were unable to obtain the original immunohistochemical and immunoblot images, as the testing was performed by commercial laboratories. Imunohistochemical analysis was limited to the carboxy-terminus and rod domain. Finally, our analysis of other proteins in the dystrophin-associated glycoprotein complex was limited to dysferlin, merosin, and a-sarcoglycan immunostaining. The possibility that abnormalities in these or other associated proteins could explain our patients’ symptoms cannot be entirely excluded.
In conclusion, we have presented 3 boys with an identical DMD missense mutation and similar clinical presentations highly suggestive of a metabolic myopathy. This report represents the first detailed clinical description of this specific missense mutation. Our cases highlight the need to consider dystrophinopathy in the differential diagnosis of patients who present with complaints suggestive of a metabolic myopathy, particularly when metabolic studies have been unrevealing. Further studies and additional cases will be necessary to more fully understand the pathogenicity and natural history of disease associated with this particular mutation.
Abbreviations
- BMD
Becker muscular dystrophy
- CK
creatine kinase
- CPT
carnitine palmitoyl transferase
- DMD
Duchenne muscular dystrophy
- H&E
hematoxylin and eosin
References
- 1.Dabby R, Sadeh M, Herman O, Berger E, Watemberg N, Hayek S, et al. Asymptomatic or minimally symptomatic hyperCKemia: histopathologic correlates. Isr Med Assoc J. 2006;8:110–113. [PubMed] [Google Scholar]
- 2.Melis MA, Cau M, Muntoni F, Mateddu A, Galanello R, Boccone L, et al. Elevation of serum creatine kinase as the only manifestation of an intragenic deletion of the dystrophin gene in three unrelated families. Eur J Paediatr Neurol. 1998;2:255–261. doi: 10.1016/s1090-3798(98)80039-1. [DOI] [PubMed] [Google Scholar]
- 3.Morrone A, Zammarchi E, Scacheri PC, Donati MA, Hoop RC, Servidei S, et al. Asymptomatic dystrophinopathy. Am J Med Genet. 1997;69:261–267. doi: 10.1002/(sici)1096-8628(19970331)69:3<261::aid-ajmg9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Ramelli GP, Joncourt F, Luetschg J, Weis J, Tolnay M, Burgunder JM. Becker muscular dystrophy with marked divergence between clinical and molecular genetic findings: case series. Swiss Med Wkly. 2006;136:189–193. doi: 10.4414/smw.2006.11213. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman EP, Kunkel LM. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989;2:1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- 6.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 7.Angelini C, Fanin M, Pegoraro E, Freda MP, Cadaldini M, Martinello F. Clinical-molecular correlation in 104 mild X-linked muscular dystrophy patients: characterization of sub-clinical phenotypes. Neuromuscul Disord. 1994;4:349–358. doi: 10.1016/0960-8966(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 8.Koenig M, Beggs AH, Moyer M, Scherpf S, Heindrich K, Bettecken T, et al. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989;45:498–506. [PMC free article] [PubMed] [Google Scholar]
- 9.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman EP, Fischbeck KH, Brown RH, Johnson M, Medori R, Loike JD, et al. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne’s or Becker’s muscular dystrophy. N Engl J Med. 1988;318:1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- 11.Angelini C, Fanin M, Freda MP, Martinello F, Miorin M, Melacini P, et al. Prognostic factors in mild dystrophinopathies. J Neurol Sci. 1996;142:70–78. doi: 10.1016/0022-510x(96)00144-x. [DOI] [PubMed] [Google Scholar]
- 12.Beggs AH, Hoffman EP, Snyder JR, Arahata K, Specht L, Shapiro F, et al. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991;49:54–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Gospe SM, Jr, Lazaro RP, Lava NS, Grootscholten PM, Scott MO, Fischbeck KH. Familial X-linked myalgia and cramps: a nonprogressive myopathy associated with a deletion in the dystrophin gene. Neurology. 1989;39:1277–1280. doi: 10.1212/wnl.39.10.1277. [DOI] [PubMed] [Google Scholar]
- 14.Ceulemans BP, Storm K, Reyniers E, Jr, Callewaert L, Martin JJ. Muscle pain as the only presenting symptom in a girl with dystrophinopathy. Pediatr Neurol. 2008;38:64–66. doi: 10.1016/j.pediatrneurol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Collins AL, Leyland KG, Kennedy CR, Robinson D, Spratt HC. An inherited dystrophin deletion without muscle weakness. J Med Genet. 1994;31:505. doi: 10.1136/jmg.31.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doriguzzi C, Palmucci L, Mongini T, Chiado-Piat L, Restagno G, Ferrone M. Exercise intolerance and recurrent myoglobinuria as the only expression of Xp21 Becker type muscular dystrophy. J Neurol. 1993;240:269–271. doi: 10.1007/BF00838159. [DOI] [PubMed] [Google Scholar]
- 17.Figarella-Branger D, Baeta Machado AM, Putzu GA, Malzac P, Voelckel MA, Pellissier JF. Exertional rhabdomyolysis and exercise intolerance revealing dystrophinopathies. Acta Neuropathol. 1997;94:48–53. doi: 10.1007/s004010050671. [DOI] [PubMed] [Google Scholar]
- 18.Kleinsteuber K, Rocco P, Herrera L, Vainzof M, Birke ME, Yanez M, et al. Post exercise myalgias as presentation form of dystrophinopathy [in Spanish] Rev Med Chil. 2000;128:772–777. [PubMed] [Google Scholar]
- 19.Malapert D, Recan D, Leturcq F, Degos JD, Gherardi RK. Sporadic lower limb hypertrophy and exercise induced myalgia in a woman with dystrophin gene deletion. J Neurol Neurosurg Psychiatry. 1995;59:552–554. doi: 10.1136/jnnp.59.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Arjona MB, Rodriguez-Uranga JJ, Giles-Lima M, Fernandez-Garcia R, Chinchon-Lara I, Antinolo G, et al. Spanish family with myalgia and cramps syndrome. J Neurol Neurosurg Psychiatry. 2005;76:286–289. doi: 10.1136/jnnp.2004.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serratrice J, Chabrol B, Attarrian S, Figarella-Branger D. Pseudometabolic distrophinopathy without immunohistochemical anomaly [in French] Rev Neurol (Paris) 2000;156:175–178. [PubMed] [Google Scholar]
- 22.Bradley WG, Jones MZ, Mussini JM, Fawcett PR. Becker-type muscular dystrophy. Muscle Nerve. 1978;1:111–132. doi: 10.1002/mus.880010204. [DOI] [PubMed] [Google Scholar]
- 23.Medori R, Brooke MH, Waterston RH. Two dissimilar brothers with Becker’s dystrophy have an identical genetic defect. Neurology. 1989;39:1493–1496. doi: 10.1212/wnl.39.11.1493. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman EP, Kunkel LM, Angelini C, Clarke A, Johnson M, Harris JB. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology. 1989;39:1011–1017. doi: 10.1212/wnl.39.8.1011. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson LV, Johnson MA, Bushby KM, Gardner-Medwin D, Curtis A, Ginjaar IB, et al. Integrated study of 100 patients with Xp21 linked muscular dystrophy using clinical, genetic, immunochemical, and histopathological data. Part 2. Correlations within individual patients. J Med Genet. 1993;30:737–744. doi: 10.1136/jmg.30.9.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumari D, Gupta M, Goyle S. Detection of deletion in the dystrophin gene of a patient with quadriceps myopathy. Neurol India. 2000;48:68–71. [PubMed] [Google Scholar]
- 27.Sunohara N, Arahata K, Hoffman EP, Yamada H, Nishimiya J, Arikawa E, et al. Quadriceps myopathy: forme fruste of Becker muscular dystrophy. Ann Neurol. 1990;28:634–639. doi: 10.1002/ana.410280506. [DOI] [PubMed] [Google Scholar]
- 28.von Mitzlaff HC, Liechti-Gallati S, Rosler KM, Burgunder JM. Quadriceps myopathy as dystrophin-associated myopathy [in German] Schweiz Med Wochenschr. 1993;123:1865–1869. [PubMed] [Google Scholar]
- 29.Wada Y, Itoh Y, Furukawa T, Tsukagoshi H, Arahata K. “Quadriceps myopathy”: a clinical variant form of Becker muscular dystrophy. J Neurol. 1990;237:310–312. doi: 10.1007/BF00314749. [DOI] [PubMed] [Google Scholar]
- 30.Ishigaki C, Patria SY, Nishio H, Yabe M, Matsuo M. A Japanese boy with myalgia and cramps has a novel in-frame deletion of the dystrophin gene. Neurology. 1996;46:1347–1350. doi: 10.1212/wnl.46.5.1347. [DOI] [PubMed] [Google Scholar]
- 31.Saad FA, Vita G, Toffolatti L, Danieli GA. A possible missense mutation detected in the dystrophin gene by double-strand conformation analysis (DSCA) Neuromuscul Disord. 1994;4:335–341. doi: 10.1016/0960-8966(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg LR, Hausmanowa-Petrusewicz I, Fidzianska A, Duggan DJ, Steinberg LS, Hoffman EP. A dystrophin missense mutation showing persistence of dystrophin and dystrophin-associated proteins yet a severe phenotype. Ann Neurol. 1998;44:971–976. doi: 10.1002/ana.410440619. [DOI] [PubMed] [Google Scholar]
- 33.Hamed S, Sutherland-Smith A, Gorospe J, Kendrick-Jones J, Hoffman E. DNA sequence analysis for structure/function and mutation studies in Becker muscular dystrophy. Clin Genet. 2005;68:69–79. doi: 10.1111/j.1399-0004.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 34.Lenk U, Hanke R, Thiele H, Speer A. Point mutations at the carboxy terminus of the human dystrophin gene: implications for an association with mental retardation in DMD patients. Hum Mol Genet. 1993;2:1877–1881. doi: 10.1093/hmg/2.11.1877. [DOI] [PubMed] [Google Scholar]
- 35.Lenk U, Oexle K, Voit T, Ancker U, Hellner KA, Speer A, et al. A cysteine 3340 substitution in the dystroglycan-binding domain of dystrophin associated with Duchenne muscular dystrophy, mental retardation and absence of the ERG b-wave. Hum Mol Genet. 1996;5:973–975. doi: 10.1093/hmg/5.7.973. [DOI] [PubMed] [Google Scholar]
- 36.Prior TW, Bartolo C, Papp AC, Snyder PJ, Sedra MS, Burghes AH, et al. Identification of a missense mutation, single base deletion and a polymorphism in the dystrophin exon 16. Hum Mol Genet. 1994;3:1173–1174. doi: 10.1093/hmg/3.7.1173. [DOI] [PubMed] [Google Scholar]
- 37.Prior TW, Papp AC, Snyder PJ, Burghes AH, Bartolo C, Sedra MS, et al. A missense mutation in the dystrophin gene in a Duchenne muscular dystrophy patient. Nat Genet. 1993;4:357–360. doi: 10.1038/ng0893-357. [DOI] [PubMed] [Google Scholar]
- 38.Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34:135–144. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 39.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucl Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunyaev S, Ramensky V, Bork P. Towards a structural basis of human non-synonymous single nucleotide polymorphisms. Trends Genet. 2000;16:198–200. doi: 10.1016/s0168-9525(00)01988-0. [DOI] [PubMed] [Google Scholar]
- 41.Sunyaev S, Ramensky V, Koch I, Lathe W, III, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597. doi: 10.1093/hmg/10.6.591. [DOI] [PubMed] [Google Scholar]