Abstract
OBJECTIVE
To determine whether walking at specific ranges of absolute and relative (V*) velocity would aid efficient capture of gait trial data with low ground reaction force (GRF) variance in a heterogenous sample of dogs.
ANIMALS
17 clinical normal dogs of various breeds, ages, and sexes.
PROCEDURES
Each dog was walked across a force platform at its preferred velocity, with controlled acceleration within 0.5 m/s2. Ranges in V* were created for height at the highest point of the shoulders (withers; WHV*). Variance effects from 8 walking absolute velocity ranges and associated WHV* ranges were examined by means of repeated-measures ANCOVA.
RESULTS
The individual dog effect provided the greatest contribution to variance. Narrow velocity ranges typically resulted in capture of a smaller percentage of valid trials and were not consistently associated with lower variance. The WHV* range of 0.33 to 0.46 allowed capture of valid trials efficiently, with no significant effects on peak vertical force and vertical impulse.
CONCLUSIONS AND CLINICAL RELEVANCE
Dogs with severe lameness may be unable to trot or may experience a decline in mobility with gait trial repetition. Gait analysis involving evaluation of individual dogs at their preferred absolute velocity, such that dogs are evaluated at a similar V*, may facilitate efficient capture of valid trials without significant GRF effects. Use of individual velocity ranges derived from a WHV* range of 0.33 to 0.46 can account for heterogeneity and appears suitable for use in clinical trials involving dogs at a walking gait.
Force platform gait analysis provides valuable objective analysis of lameness in clinical trials involving dogs. Peak vertical force and VI are GRFs that correlate with limb function.1–3 The PVF represents the maximal load exerted by the paw during the stance phase, whereas VI represents the area under the force-versus-time curve. Both variables are commonly used for assessment of treatment effects in clinical trials. Factors affecting limb kinetics, kinematics, and subject comfort during locomotion have been correlated with observed lameness and altered PVF and VI.4–6
Limiting variance improves data quality and aids in interpretation of gait analysis results. Variance in GRF values can be affected by dog body weight and conformation, velocity, gait trial repetition, and individual interday variability.7–11 Recommendations for minimizing this variance have largely been based on observations from small homogeneous groups of clinically normal dogs. Normalization of GRFs to body weight and use of narrow velocity ranges (within 0.3 m/s) with controlled acceleration within 0.5 m/s2 have been recommended.9,4,12
Greater GRF variance may be identified in heterogeneous versus homogeneous dog populations because of morphologic diversity.4,13 Therefore, variance associated with body weight, conformation, and velocity should be accounted for when GRF values are measured in dogs of various shapes and sizes.12,14 However, collection of gait data may become inefficient in heterogeneous dog groups when narrow velocity ranges are used. Wider ranges in trotting velocity can improve the efficiency of gait data capture without substantial effects on PVF and VI variance in heterogeneous groups.15
Another approach to reduce variance in GRF values is to normalize the velocity of each subject to their body size by use of a morphometric trait such as height at the highest point of the shoulders (withers).14 Relative velocity (V*) or Froude number is a dimensionless value by which velocity is rescaled to body size on the basis of the theory of dynamic similarity.14 In heterogeneous populations, it is advantageous to evaluate individual dogs at their preferred absolute velocity, such that dogs are assessed at a more consistent velocity or V*; a high proportion of total trials yield valid data when velocity or V* ranges span the preferred velocity of the dog.15,16
Research into the effect of gait and subject velocity on GRFs in dogs has suggested that trotting is more sensitive than walking for evaluating GRFs for lameness detection.17 However, dogs with higher grades of lameness or more complicated multilimb lameness may not achieve a trotting velocity or may experience a rapid decline in mobility with trial repetition. With improved understanding of walking gait analysis, clinical trial design and gait analysis methods could be optimized for evaluation of dogs with more severe lameness. The purpose of the study reported here was to determine whether specific absolute and relative walking velocity ranges7,18–23 would aid efficient trial capture with low GRF variance for gait analysis in a heterogeneous group of dogs. We hypothesized that wide velocity ranges would yield efficient trial capture with low GRF variance for walking gait analysis. Use of a heterogeneous group of clinically normal dogs was intended to inform knowledge of canine gait analysis as well as aid design of future clinical trials involving heterogeneous groups of lame dogs. Ethically, it is preferable to evaluate lame dogs at their habitual velocity by means of force platform gait analysis.
Materials and Methods
Animals
Dogs owned by clients of the University of Wisconsin-Madison UW Veterinary Care hospital were recruited for inclusion in the study during the fall of 2015. Eligible dogs were identified by medical record searches and clients were contacted via email advertisement. To be eligible for inclusion, dogs were required to be > 15 months of age with no history of orthopedic disease. The same veterinarian (AP) examined each dog and measured its height at the point of the scapula by use of a metal T-square ruler while the dog was standing square. Dogs were excluded when an orthopedic abnormality was identified, lameness was observed, or the dog failed to habituate to the gait analysis.
Force platform gait analysis was performed at the University of Wisconsin-Madison UW Veterinary Care hospital with Institutional Animal Care and Use Committee approval (protocol Nos. V1070 and V1600; dates of approval, May 8, 2013, and July 9, 2013, respectively). Consent was obtained from owners of all participating dogs.
Gait analysis
All gait trials were performed by use of a biomechanical platform designed to measure 3-D forces and impulses.a A force platform mask was used to reduce the working surface area and enable data acquisition from dogs with a shorter walking stride length (Figure 1). Dogs were initially evaluated without use of the force platform mask, which was used to avoid additional footfalls if a dog’s preferred walking stride length resulted in > 2 footfalls/trial. New clinical exams and gait analysis were obtained from all dogs that had participated in previous gait analysis research.15,16
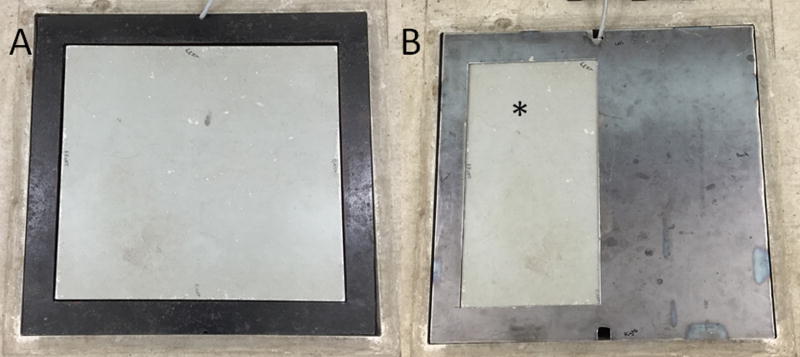
Figure 1.
Photographs of the force platforma (A; 50.8 × 46.4 cm) and custom force platform mask (B) used for performance of gait analysis of dogs. The custom mask was made to reduce the effective area of the platform to 25.4 cm × 45.8 cm. *Denotes area where a matching metal insert was placed, creating a uniform height and surface for footfalls.
Using a subset of study dogs (n = 11), we determined that the force platform mask had no substantial impact GRF data collection. Use of the force platform mask resulted differences in GRF comparable to the individual interweek variability previously reported (approx 10% of body weight for PVF and < 5% of body weight for SI).11 Velocity was measured by 3 photoelectric cells mounted 1 m apart from each other and the manufacturer of gait analysis software reports this set up as being accurate to 0.01 m/s.b The force platform system was validated daily for measurement of GRF by use of weights. Photocells were validated daily for measurement of velocity by use of a pendulum.
The same handler guided all dogs across the platform at their preferred walking velocity. Both the handler and an observer evaluated each pass to confirm foot strikes and gait. A valid trial was defined as contact of a thoracic limb paw with the platform, followed by the ipsilateral pelvic limb paw with acceleration within 0.5 m/s2 at a walk. A minimum of 5 valid trials from each ipsilateral limb pair in a single session was required for data from that session to be included in the study.
For each session, trials were performed until either 30 minutes had elapsed or a sufficient number of valid trials were obtained after dogs had habituated to the force platform. Sessions were limited to 30 minutes and then repeated, if needed.
The force platform was connected to a commercially available data acquisition system that interfaced with gait analysis computer software.b Data were collected at 1,000 Hz without filtering. Values of PVF and VI were normalized as %BW as follows:
where m is body mass (kg) and g is gravitational acceleration (9.81 m/s2).
Values of WHV* (withers-height Froude number) were calculated for each valid trial by use the following equation:
where H represents withers height (m).14
Selection of absolute and relative velocity ranges
Published velocity ranges were identified for evaluation of their valid trial capture rate and GRF variance effects. A literature search was performed by use of the PubMed search engine and the term “gait analysis + dog” to identify published reports of studies involving force platform gait analysis of walking dogs. Seven distinct walking velocity ranges were identified for trial validation,7,18–23 and variance effects associated with these ranges were determined using a previously described method.15,16 After data acquisition, trials were reviewed and data from valid trials were coded with one or more of the seven velocity ranges of interest. During statistical analysis, a novel velocity range was created on the basis of the initial results and analyzed in the subsequently developed statistical model. Five WHV* ranges were created to approximate absolute velocity ranges that yielded valid trial capture (ie, proportion of the total number of valid trials) > 40% to evaluate velocity ranges yielding superior performance in more detail.
Statistical analysis
Data from 1 session/dog were used for analysis. Values for PVF and VI for the left and right limb pairs from 5 valid gait trials, obtained at velocities that were the closest to the overall mean velocity, were compared between limb pairs by use of the Student t test for paired data. A symmetry index was calculated for the thoracic and pelvic limb by use of the following equation:
where PVF1 is the higher value and PVF2 is the lower value.17 A symmetry index value of 0 indicated perfect symmetry. If the value was > 15% or significant differences in GRF were detected between limb pairs, the dog was excluded. Dunn–Šidák correction for multiple independent tests was performed, and values of P < 0.003 were considered significant.
Data were confirmed to approximate a normal distribution by evaluation of histogram plots of dependent variables (thoracic limb PVF and VI; pelvic limb PVF and VI). Repeated-measures ANCOVA was performed, in which dog, trial number, limb (left vs right), and absolute velocity or WHV* were analyzed for effects on GRFs. Subsequently, variance effects of walking absolute velocity and associated WHV* ranges were examined in the statistical model, as described previously,15,16 to determine velocity range performance. Factor effect sizes were calculated. Valid trials that closely approximated the overall preferred WHV* (mean WHV* of all valid trials) were evaluated. A value range of 0.38 to 0.39 for WHV* was used to select these trials.
All analyses were performed by use of statistical software.c Data are reported as mean ± SD. Results were considered significant at P < 0.05.
Results
Animals
Twenty-six dogs were initially considered for inclusion in the study. Gait analysis data from 17 dogs (1 session/dog) met the inclusion criteria. Nine dogs did not meet the inclusion criteria despite appearing clinically normal on orthopaedic examination and having a low symmetry index. Dogs were excluded because of failure to habituate to walking gait analysis within the allotted time or insufficient yield of valid trials.
Mean ± SD body weight of the included dogs was 27.9 ± 6.9 kg (range, 13.1 to 36.8 kg). Mean height at the withers was 0.60 ± 0.07 m (range, 0.47 to 0.71 m). Represented breeds included mixed breed (n = 7), Labrador Retriever (4), Siberian Husky (2), and 2 each of Australian Shepherd, Smooth Coated Collie, Greyhound, and Nova Scotia Duck Tolling Retriever. Nine dogs were neutered males, and 8 dogs were spayed females. The force platform mask was used for all dogs except the Australian Shepherd, the Greyhound, and 1 Labrador Retriever.
The habituation period for the gait analysis varied among dogs. A small number of dogs appeared to habituate after < 6 trials across the platform, with most dogs requiring additional trials.
Effect of absolute velocity range and V* range on gait trial capture
A total of 378 gait trials were deemed valid, and the mean ± SD number of valid trials per dog was 13.2 ± 8.6. Mean absolute velocity for all valid trials was 0.92 ± 0.15 m/s. Mean walking absolute velocity of each dog ranged from 0.75 to 1.25 m/s. Mean WHV* for all valid trials was 0.39 ± 0.06. Mean WHV* of each dog ranged from 0.31 to 0.53. Narrow absolute velocity and WHV* ranges were observed to yield a lower trial capture rate than wider ranges (Table 1).
Table 1.
Absolute velocity (m/s) and WHV* (unitless) ranges reported for dogs walking on a force platform and mean ± SD associated trial capture rates when these rates were used for force plate analysis of a heterogeneous group of 17 orthopedically normal, walking dogs.
| Source | Absolute velocity range | Trial capture rate for absolute velocity range |
WHV* range | Trial capture rate for WHV* range (%) |
|---|---|---|---|---|
| Light et al, 201020 | 0.60–0.90 | 51.6 ± 34.6 | 0.25–0.37 | 48.2 ± 36.3 |
| Abdelhadi et al, 201321 | 0.70–0.90 | 47.6 ± 30.5 | 0.29–0.37 | 45.8 ± 33.1 |
| Krotscheck et al, 201423 | 0.75–1.25 | 86.0 ± 16.3 | 0.31–0.52 | 87.6 ± 18.0 |
| 0.80–1.10† | 66.4 ± 26.7 | 0.33–0.46† | 74.6 ± 25.9 | |
| Fisher et al, 201322 | 0.85–0.95‡ | 20.4 ± 17.7 | ||
| Besancon et al, 200419 | 0.90–1.10 | 41.3 ± 25.4 | 0.37–0.46 | 54.0 ± 32.2 |
| Evans et al, 200318 | 1.00–1.30 | 27.5 ± 32.3 | ||
| Budsberg et al, 19877 | 1.00–2.00 | 29.1 ± 36.2 |
Trial capture rate represents the percentage of all valid gait trials as reported by Hans et al.15
Absolute and respective WHV* ranges represent those proposed by the authors.
The published velocity range is 0.90m/s ± 0.05m/s
Values of WHV* were calculated as V/(g × H)1/2, where V is the velocity (m/s), g is the gravitational acceleration (9.81 m/s2), and H represents height at the withers (m). WHV* ranges were only studied for absolute velocity ranges with a trial capture rate of > 40%
The absolute velocity range 0.75 to 1.25 m/s yielded the best trial capture rate (325/378 [86%]), with a mean + SD trial capture rate per dog of 84 ± 16% (range, 46% to 100%). The absolute velocity (range) with the worst capture rate was 0.85 to 0.95 m/s (77/378 [20%]), with a mean trial capture rate per dog of 21 ± 18% (range, 0% to 53%). Of the 7 previously reported walking velocity ranges, 4 resulted in capture of > 40% of valid gait trials per dog (Table 1). The WHV* range of 0.31 to 0.52 yielded the best trial capture rate (331/378 [88%]), with a mean trial capture rate per dog of 84 ± 18% (range, 42% to 100%). The WHV* range with the worst capture rate was 0.29 to 0.37 (173/378 [46%]), with a mean trial capture rate per dog of 46 ± 33% (range, 0% to 97%). Every WHV* range that was analyzed resulted in a mean trial capture rate of > 45% per dog (Table 1).
Effect of absolute velocity and V* ranges on vertical GRFs
Individual dog, absolute velocity, and WHV* had significant effects on PVF and VI for thoracic and pelvic limbs (Table 2). The trial number effect was not significant. The limb effect was only significant for pelvic limb VI, but the effect size of this relationship was small. Analysis of effect sizes revealed that the magnitude of variance effects from greatest to least were dog, absolute velocity or WHV*, trial number, and limb (left vs right), except for pelvic limb PVF, for which the order was dog, trial number, absolute velocity or WHV*, and limb.
Table 2.
Summary of variance effects of variables included in ANCOVA models of the effects of absolute velocity and WHV* on PVF and VI in the thoracic and pelvic limbs of a heterogeneous group of 17 orthopedically normal evaluated while walking over a force plate.
| Variable | Thoracic limb PVF | Thoracic limb VI | Pelvic limb PVF | Pelvic limb VI | ||||
|---|---|---|---|---|---|---|---|---|
| ES | 95% CI | ES | 95% CI | ES | 95% CI | ES | 95% CI | |
| Absolute velocity | ||||||||
| Dog | 0.611† | 0.529–0.644 | 0.530† | 0.436–0.569 | 0.437† | 0.332–0.480 | 0.611† | 0.529–0.644 |
| Absolute velocity | 0.112† | 0.055–0.178 | 0.302† | 0.223–0.375 | 0.109† | 0.053–0.175 | 0.112† | 0.0553–0.178 |
| Trial No. | 0.103 | 0.000–0.072 | 0.171 | 0.626–0.160 | 0.114 | 0.000–0.086 | 0.103 | 0.000–0.072 |
| Limb (left vs right) | 0.003 | 0.000–0.026 | 0.005 | 0.000–0.031 | 0.003 | 0.000–0.026 | 0.003† | 0.000–0.026 |
| WHV* | ||||||||
| Dog | 0.598† | 0.000–0.072 | 0.435† | 0.330–0.478 | 0.419† | 0.312–0.462 | 0.502† | 0.404–0.542 |
| WHV* | 0.109† | 0.053–0.175 | 0.300† | 0.221–0.373 | 0.104† | 0.049–0.168 | 0.235† | 0.000–0.309 |
| Trial No. | 0.104 | 0.000–0.072 | 0.172 | 0.025–0.160 | 0.113 | 0.000–0.085 | 0.077 | 0.000–0.034 |
| Limb (left vs right) | 0.003 | 0.000–0.026 | 0.005 | 0.000–0.373 | 0.003 | 0.000–0.026 | 0.015 | 0.000–0.052 |
Narrow velocity ranges were not consistently associated with lower variance (Table 3). The absolute velocity ranges 0.80 to 1.10 m/s and 0.85 to 0.95 m/s were associated with low PVF variance. Absolute velocity ranges 0.75 to 1.25 m/s and 0.80 to 1.10 m/s were associated with low VI variance. Absolute velocity ranges 0.75 to 1.25 m/s and 0.80 to 1.10 m/s yielded nonsignificant effects for all 4 GRFs.
Table 3.
Summary of variance effects of variables included in ANCOVA models of the effects of absolute velocity and WHV* ranges on PVF and VI in the thoracic and pelvic limbs of the dogs in Table 2.
| Variable | Thoracic Limb PVF | Thoracic Limb VI | Pelvic Limb PVF | Pelvic Limb VI | ||||
|---|---|---|---|---|---|---|---|---|
| ES | 95% CI | ES | 95% CI | ES | 95% CI | ES | 95% CI | |
| Absolute velocity range | ||||||||
| Dog | 0.623† | 0.541–0.655 | 0.514† | 0.416–0.553 | 0.457† | 0.352–0.499 | 0.061† | 0.520–0.639 |
| Trial No. | 0.098 | 0.000–0.063 | 0.191 | 0.040–0.183 | 0.158 | 0.011–0.143 | 0.075 | 0.000–0.028 |
| Limb (left vs right) | 0.004 | 0.000–0.028 | 0.006 | 0.000–0.034 | 0.006 | 0.000–0.034 | 0.019† | 0.001–0.059 |
| Range | ||||||||
| 0.60–0.90 m/s | 0.025† | 0.002–0.068 | 0.096† | 0.043–0.161 | 0.007 | 0.000–0.036 | 0.058† | 0.018–0.011 |
| 0.70–0.90 m/s | 0.009 | 0.000–0.041 | 0.050† | 0.014–0.103 | 0.002 | 0.000–0.022 | 0.041† | 0.009–0.091 |
| 0.75–1.25 m/s | 0.009 | 0.000–0.040 | 0.003 | 0.000–0.026 | 0.006 | 0.000–0.033 | 0.003 | 0.000–0.027 |
| 0.80–1.10 m/s | 0.004 | 0.000–0.030 | 0.003 | 0.000–0.027 | 0.002 | 0.000–0.024 | 0.002 | 0.000–0.022 |
| 0.85–0.95 m/s‡ | 0.005 | 0.000–0.032 | 0.008 | 0.000–0.037 | 0.00005 | 0.000–0.009 | 0.007 | 0.000–0.037 |
| 0.90–1.10 m/s | 0.019† | 0.001–0.058 | 0.004 | 0.000–0.029 | 0.015† | 0.000–0.051 | 0.0002 | 0.000–0.014 |
| 1.00–1.30 m/s | 0.012† | 0.000–0.047 | 0.058† | 0.018–0.011 | 0.065† | 0.022–0.123 | 0.018† | 0.001–0.056 |
| 1.00–2.00 m/s | 0.030† | 0.004–0.075 | 0.071† | 0.026–0.130 | 0.069† | 0.025–0.128 | 0.033† | 0.006–0.080 |
| WHV* range | ||||||||
| Dog | 0.612† | 0.529–0.645 | 0.464† | 0.361–0.506 | 0.456† | 0.352–0.498 | 0.537 | 0.443–0.575 |
| Trial No. | 0.091 | 0.000–0.055 | 0.183 | 0.034–0.174 | 0.149 | 0.005–0.013 | 0.078 | 0.000–0.034 |
| Limb (left vs right) | 0.002 | 0.000–0.022 | 0.004 | 0.0–0.029 | 0.005 | 0.000–0.031 | 0.012† | 0.000–0.046 |
| Range | ||||||||
| 0.25–0.37 | 0.013† | 0.000–0.048 | 0.108† | 0.052–0.174 | 0.002 | 0.000–0.024 | 0.087† | 0.037–0.149 |
| 0.29–0.37 | 0.002 | 0.000–0.022 | 0.054† | 0.016–0.108 | 0.001 | 0.000–0.021 | 0.046† | 0.012–0.098 |
| 0.31–0.52 | 0.001 | 0.000–0.017 | 0.002 | 0.000–0.021 | 0.013† | 0.000–0.048 | 0.0003 | 0.000–0.015 |
| 0.33–0.46 | 0.001 | 0.000–0.020 | 0.0003 | 0.000–0.014 | 0.003 | 0.000–0.026 | 0.001 | 0.000–0.020 |
| 0.37–0.46 | 0.009 | 0.000–0.040 | 0.001 | 0.000–0.017 | 0.006 | 0.000–0.033 | 0.018 | 0.000–0.022 |
See Table 2 for remainder of key.
The WHV* range 0.33 to 0.46 was associated with low variance and nonsignificant effects for GRFs (Table 3). The WHV* range 0.31 to 0.52 was also associated with low variance but significantly influenced pelvic limb PVF.
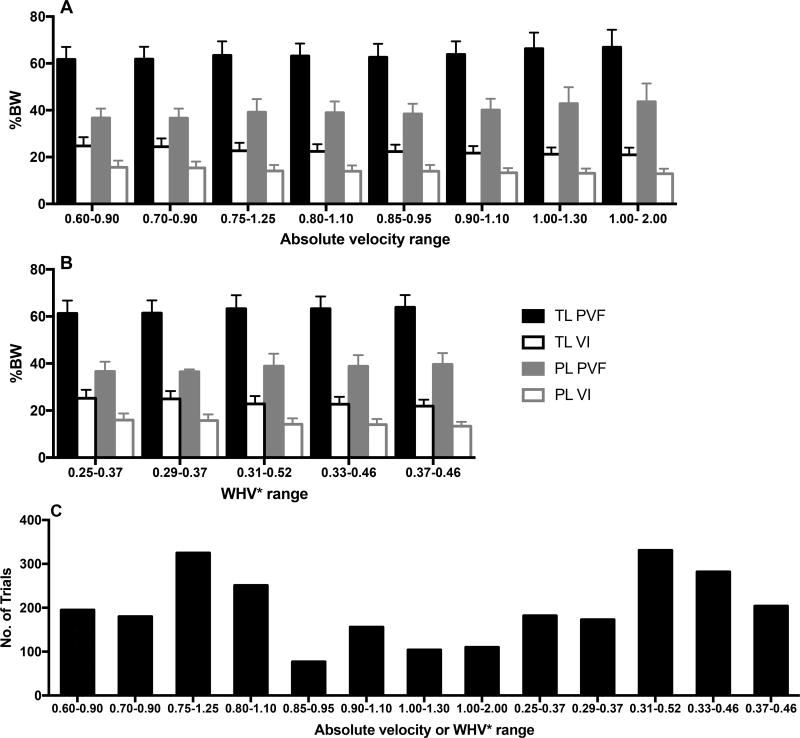
Mean PVF and VI varied mildly across absolute velocity and WHV* ranges (Figure 2). Higher PVFs and lower VIs were identified with increasing velocity. Mean thoracic limb PVF and VI at the overall preferred WHV* were 63.86 ± 5.10 (range, 53.52 to 80.35) and 23.12 ± 2.52 (range, 18.83 to 28.69), respectively (Table 4). Mean pelvic limb PVF and VI were 38.66 ± 3.99 (range, 30.36 to 49.29) and 13.96 ± 2.02 (range, 10.29 to 18.05), respectively. Data obtained at the overall preferred WHV* were derived from 41 of 378 trials (11%) for 12 of 17 dogs.
Figure 2.
Mean ground reaction forces for specific absolute velocity (A) and WHV* (B) ranges for the thoracic (TL) and pelvic (PL) limbs and associated trial capture rates (C) for a heterogeneous group of 17 orthopedically normal dogs evaluated while walking over a force plate. Values of WHV* were calculated as V/(g × H)1/2, where V is the velocity (m/s), g is the gravitational acceleration (9.81 m/s2), and H represents height at the withers (m). In panels A and B, error bars represent SD. %BW = Percentage of body weight.
Table 4.
Summary of GRFs (percentage of body weight) of 12 of the dogs in Table 2 walking at their overall preferred WHV*.
| Dog No. | No. of trials | Thoracic limb PVF |
Thoracic limb VI | Pelvic limb PVF | Pelvic limb VI |
|---|---|---|---|---|---|
| 1 | 6 | 70.78 | 20.28 | 39.59 | 10.71 |
| 2 | 1 | 55.83 | 19.96 | 42.89 | 16.00 |
| 3 | 8 | 66.65 | 22.56 | 36.03 | 12.69 |
| 4 | 1 | 68.89 | 28.69 | 32.84 | 15.11 |
| 5 | 3 | 61.33 | 26.40 | 34.16 | 14.14 |
| 6 | 1 | 60.33 | 22.05 | 36.36 | 15.53 |
| 7 | 4 | 57.36 | 22.95 | 36.79 | 16.71 |
| 8 | 3 | 63.87 | 23.90 | 42.70 | 14.51 |
| 9 | 8 | 63.27 | 25.21 | 41.87 | 15.55 |
| 10 | 3 | 57.95 | 19.80 | 38.76 | 13.25 |
| 11 | 2 | 64.35 | 23.74 | 36.23 | 14.11 |
| 12 | 1 | 61.60 | 23.61 | 45.70 | 13.10 |
| Mean ± SD | — | 63.86 ± 5.10 | 23.12 ± 2.52 | 38.66 ± 3.99 | 13.96 ± 2.02 |
Mean individual dog GRFs are reported when a dog had ≥ 2 valid trials.
%BW = Percentage of body weight. — = Not applicable.
The mean ± SD WHV* of all trials was 0.387 ± 0.060. Data from trials that closely approximated the overall preferred WHV* were analyzed using a value of 0.385 ± 0.005. Data represent 41 of 378 valid trials.
See Table 2 for remainder of key.
Discussion
Factors contributing to GRF variability include dog morphology, trial velocity, trial repetition, and day-to-day variation.7–11 Guidelines for minimizing variance in results of force platform gait analysis include normalization of GRFs to body weight and use of narrow velocity ranges with acceleration within 0.5 m/s2.4,9,12 However, normalization of GRFs to body weight is not sufficient to control for all variability associated with morphology,14 which has been identified as the largest source of variance in gait data obtained from walking dogs and is inherent to heterogeneous populations.15 Our data were normalized to body weight to help account for that heterogeneity. Morphometric GRF normalization by use of WHV* specifically can further reduce variance,16,24 and we therefore examined WHV* in our analysis.
Trial velocity is a predetermined experimental design parameter. Mean absolute velocity and WHV* in the present study were 0.92 m/s and 0.39, respectively. Several absolute velocity and WHV* ranges were associated with low variance. In general, these ranges spanned their respective mean values. The WHV* range of 0.33 to 0.46 resulted in efficient trial capture with no significant effects on GRFs. The absolute velocity range 0.80 to 1.10 m/s had no significant effects on all 4 measured GRFs, but resulted in less efficient trial capture. Similar to reported findings for trotting dogs,16 results of the study reported here suggested use of individual absolute velocity ranges would yield efficient trial capture without significant effects on GRF,16 particularly, the WHV* range of 0.33 to 0.46 in single platform studies.
Individual absolute velocity ranges (m/s) can be readily calculated from a V* range by use of the derived equation V = V* (g × H)1/2. In heterogeneous populations, absolute and V* ranges with inefficient trial capture likely prevent individual dogs from locomotion at their preferred velocity. Evaluation of dogs at their preferred velocity or V* may be advantageous in a treatment study, given that an increase in preferred velocity indicates an improvement in mobility.15
Although gait analysis of lameness while dogs are trotting is more sensitive than when they are walking,17 analysis of dogs while walking is useful in those with severe lameness, where collection of trotting GRF data is not possible. Dogs with more severe lameness or more complicated multiple limb lameness may not be able to trot well. Trial repetition during gait analysis of lame dogs may confound GRF measurement if lameness is exacerbated by the analysis.13,18 We found that the magnitude of trial repetition variance approximated the variance associated with subject velocity. This observation and past work,16 suggests that trial repetition during walking gait analysis should be minimized.
Another approach to force platform gait analysis for dogs is to measure GRFs at a single preferred V*. When GRFs obtained around the overall mean WHV* were analyzed for the dogs of the present study, we were only able to obtain data from 12 of 17 dogs derived from 41 of 378 (11%) trials. Additionally, restricting data analysis to trials obtained at the mean WHV* failed to yield uniform GRFs. Therefore, in this heterogeneous group of dogs, there appeared to be sufficient variation in preferred WHV* among individual dogs that trial data collection involving a single WHV* rather than a range is not recommended.
During force platform gait analysis, the thoracic limb of dogs makes first contact with the force platform and, thus, may be more susceptible to variation, particularly in the first 3 trials in gait analysis.15,16 Although we did not identify significant trial repetition effects on walking GRFs in the present study, appropriate habituation of dogs to the force platform is good practice.15 We defined exclusion criteria and set the asymmetry cutoff at < 15% to allow retention of dogs with a preference for weight-bearing in left or right limbs.25,26
The present study had several limitations. Use of photoelectric cells for measurement of trunk velocity is a standard method7; however, other methods exist for velocity measurement (eg, limb velocity) that could be explored in future studies. In addition, alternative methods exist for calculating V*, such as the percentage of withers height covered per second. Many factors may have influenced the failure of a given dog to meet the study inclusion criteria, such as habituation to the force platform or yielding a sufficient number of valid trials. Stride length was found to be shorter when dogs were walking versus trotting, and a force platform mask was required for data collection from most dogs. Some dogs had signs of a preference for a pacing gait, were always distracted, or were unable to walk without tension on the leash, resulting in failure to obtain a sufficient number of valid trials. Consequently, additional habituation may be required to allow collection of valid trial data from individual dogs walking at their preferred velocity or V*. Nine dogs did not complete a sufficient number of valid trials for inclusion, despite appearing clinically normal. Provision of a longer habituation period may have improved the valid trial capture rate for individual dogs.
Other considerations include the fact that GRFs are susceptible to nonspecific day-to-day variability of low magnitude.11 Repeated force platform sessions with the same dogs may have been useful for evaluating the repeatability of results. In addition, only vertical GRFs were used in data analysis because PVF and VI best correlate with limb function2,3,17; however, analysis of other GRFs could be considered for future studies. For instance, analysis of the cranial-caudal dimension and breaking forces could improve understanding of lameness that predominately effects specific phases of the gait cycle.11,27,28
Results of the study reported here suggested that analysis at a walking gait may be advantageous for evaluation of dogs with more severe grades of lameness. Variance in walking and trotting force platform gait analysis appears to be impacted by several factors. Absolute velocity range and WHV* range can impact GRF variance. When designing force platform gait analysis trials, selection of a wider velocity range or V* range may improve the quality of the GRF data collected. Use of individual velocity ranges derived from a WHV* range of 0.33 to 0.46 improved the trial capture rate and minimized the variance associated with walking gait analysis in a heterogeneous group of orthopedically normal dogs. Studies involving heterogeneous groups of lame dogs are needed to further investigate effects of trial repetition on lameness exacerbation and to evaluate whether these reported findings for orthopedically normal dogs can be applied to lame dogs as well.
Acknowledgments
Dr. Sample was supported by the National Institutes of Health (grant No. K010D0197343). The authors thank Drs. Nicola Volstad and Eric Hans for assistance with the conception of this study and Amanda Simons for coordinating appointments for enrolled dogs.
ABBREVIATIONS
- CI
Confidence interval
- GRF
Ground reaction force
- PVF
Peak vertical force
- %BW
Percentage of body weight
- V*
Relative velocity
- VI
Vertical impulse
- WHV*
Relative velocity at the height of the withers
Footnotes
OR6-6-1000 biomechanics platform with SGA6-4 signal conditioner and amplifier, Advanced Mechanical Technologies, Phoenix, Ariz.
Acquire v7.30, Sharon Software Inc, Dewitt, Mich.
Stata, version 14.0, Stata Corp LP, College Station, Tex.
References
- 1.Evans R, Horstman C, Conzemius M. Accuracy and optimization of force platform gait analysis in Labradors with cranial cruciate disease evaluated at a walking gait. Vet Surg. 2005;34:445–449. doi: 10.1111/j.1532-950X.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 2.Fanchon L, Grandjean D. Accuracy of asymmetry indices of ground reaction forces for diagnosis of hind limb lameness in dogs. Am J Vet Res. 2007;68:1089–1094. doi: 10.2460/ajvr.68.10.1089. [DOI] [PubMed] [Google Scholar]
- 3.Beraud R, Moreau M, Lussier B. Effect of exercise on kinetic gait analysis of dogs afflicted by osteoarthritis. Vet Comp Orthop Traumat. 2010;23:87–92. doi: 10.3415/VCOT-09-06-0068. [DOI] [PubMed] [Google Scholar]
- 4.Budsberg SC, Rytz U, Johnston SA. Effects of acceleration on ground reaction forces collected in healthy dogs at a trot. Vet Comp Orthop Traumat. 1999;12:15–19. [Google Scholar]
- 5.Voss K, Damur DM, Guerrero T, et al. Force plate gait analysis to assess limb function after tibial tuberosity advancement in dogs with cranial cruciate ligament disease. Vet Comp Orthop Traumat. 2008;21:243–249. [PubMed] [Google Scholar]
- 6.Malek S, Sample SJ, Schwartz Z, et al. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet Res. 2012;8:185. doi: 10.1186/1746-6148-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budsberg SC, Verstraete MC, Soutas-Little RW. Force plate analysis of the walking gait in healthy dogs. Am J Vet Res. 1987;48:915–918. [PubMed] [Google Scholar]
- 8.Jevens DJ, Hauptman JG, DeCamp CE, et al. Contributions to variance in force-plate analysis of gait in dogs. Am J Vet Res. 1993;54:612–615. [PubMed] [Google Scholar]
- 9.Riggs CM, DeCamp CE, Soutas-Little RW, et al. Effects of subject velocity on force plate-measured ground reaction forces in healthy greyhounds at the trot. Am J Vet Res. 1993;54:1523–1526. [PubMed] [Google Scholar]
- 10.McLaughlin RM, Roush JK. Effects of subject stance time and velocity on ground reaction forces in clinically normal greyhounds at the trot. Am J Vet Res. 1994;55:1666–1671. [PubMed] [Google Scholar]
- 11.Nordquist B, Fischer J, Kim SY, et al. Effects of trial repetition, limb side, intraday and inter-week variation on vertical and craniocaudal ground reaction forces in clinically normal Labrador Retrievers. Vet Comp Orthop Traumat. 2011;24:435–444. doi: 10.3415/VCOT-11-01-0015. [DOI] [PubMed] [Google Scholar]
- 12.Bertram JE, Lee DV, Case HN, et al. Comparison of the trotting gaits of Labrador Retrievers and Greyhounds. Am J Vet Res. 2000;61:832–838. doi: 10.2460/ajvr.2000.61.832. [DOI] [PubMed] [Google Scholar]
- 13.Mölsä SH, Hielm-Björkman AK, Laitinen-Vapaavouri OM. Force-platform analysis in clinically healthy Rottweilers: Comparison with Labrador Retrievers. Vet Surg. 2010;39:701–707. doi: 10.1111/j.1532-950X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 14.Voss K, Galeandro L, Wiestner T, et al. Relationships of body weight, body size, subject velocity, and vertical ground reaction forces in trotting dogs. Vet Surg. 2010;39:863–869. doi: 10.1111/j.1532-950X.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 15.Hans EC, Zwarthoed B, Seliski J, et al. Variance associated with subject velocity and trial repetition during force platform gait analysis in a heterogeneous population of clinically normal dogs. Vet J. 2014;202:498–502. doi: 10.1016/j.tvjl.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volstad N, Nemke B, Muir P. Variance associated with the use of relative velocity for force platform gait analysis in a heterogeneous population of clinically normal dogs. Vet J. 2016;207:80–84. doi: 10.1016/j.tvjl.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voss K, Imhof J, Kaestner S, et al. Force plate gait analysis at the walk and trot in dogs with low-grade hindlimb lameness. Vet Comp Orthop Traumatol. 2007;20:299–304. doi: 10.1160/vcot-07-01-0008. [DOI] [PubMed] [Google Scholar]
- 18.Evans R, Gordon W, Conzemius M. Effect of velocity on ground reaction forces in dogs with lameness attributable to tearing of the cranial cruciate ligament. Am J Vet Res. 2003;64:1479–1481. doi: 10.2460/ajvr.2003.64.1479. [DOI] [PubMed] [Google Scholar]
- 19.Besancon MF, Conzemius MG, Evans RB, et al. Distribution of vertical forces in the pads of Greyhounds and Labrador Retrievers during walking. Am J Vet Res. 2004;65:1497–1501. doi: 10.2460/ajvr.2004.65.1497. [DOI] [PubMed] [Google Scholar]
- 20.Light VA, Steiss JE, Montgomery RD, et al. Temporal-spatial gait analysis by use of a portable walkway system in healthy Labrador Retrievers at a walk. Am J Vet Res. 2010;71:997–1002. doi: 10.2460/ajvr.71.9.997. [DOI] [PubMed] [Google Scholar]
- 21.Abdelhadi J, Wefstaedt P, Galindo-Zamora V, et al. Load redistribution in walking and trotting Beagles with induced forelimb lameness. Am J Vet Res. 2013;71:34–39. doi: 10.2460/ajvr.74.1.34. [DOI] [PubMed] [Google Scholar]
- 22.Fischer S, Anders A, Nolte I, et al. Compensatory load redistribution in walking and trotting dogs with hind limb lameness. Vet J. 2013;197:746–752. doi: 10.1016/j.tvjl.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Krotscheck U, Todhunter RJ, Nelson SA, et al. Precision and accuracy of ground reaction force normalization in a heterogeneous population of dogs. Vet Surg. 2014;43:437–445. doi: 10.1111/j.1532-950X.2014.12176.x. [DOI] [PubMed] [Google Scholar]
- 24.Voss K, Wiestner T, Galeandro L, et al. Effect of dog breed and body conformation on vertical ground reaction forces, impulses, and stance times. Vet Comp Orthop Traumatol. 2011;24:106–112. doi: 10.3415/VCOT-10-06-0098. [DOI] [PubMed] [Google Scholar]
- 25.Colborne GR. Are sound dogs mechanically symmetric at trot? No actually. Vet Comp Orthop Traumatol. 2008;21:294–301. [PubMed] [Google Scholar]
- 26.Colborne GR, Good L, Cozens LE, et al. Symmetry of hind limb mechanics in orthopedically normal trotting Labrador Retrievers. Am J Vet Res. 2011;72:336–344. doi: 10.2460/ajvr.72.3.336. [DOI] [PubMed] [Google Scholar]
- 27.Budsberg SC, Jevens DJ, Brown J, et al. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am J of Vet Res. 1993;54:1569–1574. [PubMed] [Google Scholar]
- 28.Moreau M, Dupuis J, Bonneau NH, et al. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323–329. doi: 10.1136/vr.152.11.323. [DOI] [PubMed] [Google Scholar]