Abstract
Introduction
To investigate the clinical predictive values of the apparent diffusion coefficient (ADC) as a biomarker in radiation response of brain metastases.
Method
Forty-one patients with brain metastases treated with stereotactic radiosurgery (SRS) were imaged at baseline, one month post SRS, and six months post SRS using diffusion weighted MRI. The mean of ADC for metastases and tumor volume was calculated. A diffusion index (DI) was generated using the sum of 1/ADC among all the voxels in a tumor. Tumor response status was determined by lesion volume measured at six month post-SRS, or the last available follow-up MRI. Logistic regression analysis was used to account for factors associated with tumor response at baseline and one month post SRS.
Results
A higher ADC mean distinguished responders from non-responders only at six month post SRS (p < 0.05). However, a lower DI distinguished a responder from non-responders on the baseline, one month post SRS and six months post SRS, indicating better diagnostic performance of the DI with regard to a non-invasive biomarker in monitoring SRS treatment response. A multivariate logistic regression analysis identified the DI as a predictor of tumor response at baseline and one month post SRS (p = 0.002 and p = 0.001, respectively). However, logistic regression analysis identified the ADC mean as a predictor of tumor response only at six months post SRS (p = 0.019).
Conclusion
Our results support the hypothesis that ADC and tumor volume generated DT at baseline, one month and six months post SRS may be a promising biomarker predicting brain metastases’ response. Specifically, a lower DI at baseline and one month distinguished responders from non-responders.
Keywords: Brain metastases, Stereotactic radiosurgery, Treatment response, Diffusion weighted imaging
1. Introduction
Brain metastases, the main cause of death for cancer patients, are common among malignant tumors in adults with an incident rate of up to 30% [1], [2]. The main treatment options for brain metastases include whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), and/or surgical resection [3], [4], [5]. High radiation energy leads to tumor cell necrosis, while lower radiation energy leads to chromosome cleavage, organelle damage, as well as restraining cell division. This also has the secondary effect of tumor vascular endothelial cell hyperplasia, vascular wall thickening, and hyaline degeneration, followed by thrombosis and vascular occlusion [6], [7]. SRS has several advantages over other treatment options for brain metastases and it is becoming the preferred treatment choice for brain metastases. One of the major advantages of SRS is a minimally invasive outpatient procedure with no significant recovery time. In addition, compared with WBRT, SRS provides more potent biologic doses of radiation, which may be beneficial, especially in tumor types considered to be radioresistant. Furthermore, potential neurologic toxicities associated with WBRT may be avoided with SRS. Currently, the candidacy for SRS is largely determined by the number of metastases and the best evidence for SRS is in patients with less than three to four lesions [1], [8].
The evaluation of tumor response to radiotherapy depends mainly on morphological changes (i.e. size reduction or growth inhibition of the tumor) provided by a conventional MRI during long-term follow-ups, which could provide early evidence of treatment response [7], [9], [10], [11]. Response criteria for tumors in general have been traditionally based on three-dimensional (3-D) volume-based assessments [12]. The most commonly accepted criteria for the evaluation of treatment response are the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines [12]. However, the limitations of RECIST, and any linear dimension-based criteria for that matter, include generalizing the complexity of tumor structure to tumor volume, and the difficulty in estimating the maximum tumor volume for irregular or confluent lesions [13], as discrepancies in scan planes and patient positioning can result in erroneous measurements.
Non-invasive methods for monitoring tumor response to treatment are mainly imaging based. For example, diffusion-weighted MRI (DW-MRI) measures the impediment to diffusion of water molecules in tissue. The apparent diffusion coefficient (ADC), a quantitative parameter measured on a DW-MRI, is sensitive to changes in the number of water molecular between the intra-/extracellular space which is related to undergoing biologic changes in response to treatment [14]. Therefore, the purpose of this study is to determine if quantitative parameters from DWI are capable of predicting SRS treatment response in patients with brain metastases.
2. Methods
2.1. Patients
Demographic data is shown in Table 1. From 2014–2015, 41 consecutive patients (20 men, 21 women, mean age = 61.2 ± 6.5 years old) who underwent SRS were recruited for this study. The primary tumors were from lung (21), breast (10), renal (4), head and neck (4) and soft tissue (1) (Table 1). The clinical symptoms included headaches, dizziness, paralysis of limbs, nausea, vomiting, lethargy, etc. All patients were treated with SRS with a mean dose of 21 ± 5 Gy; the margin dose of the lesion ranged from 14 to 18 Gy, and the average target volume was 2.38 cm3. As a part of the SRS procedure, all patients received local anesthesia to facilitate the painless application of the stereotactic head frame. This study was approved by the Institutional Review Board, and informed consent was obtained from all patients. This study was also compliant with all patient confidentiality regulations.
Table 1.
Showing patient demographics data.
| Responders (n = 25) | Non-responders (n = 16) | p Value | |
|---|---|---|---|
| Age, y mean(SD) | 60.0 (8.6) | 62.3 (9.4) | >0.0.5 |
| Sex, male, n (%) | 17 (68) | 10 (63) | >0.05 |
| Total radiation dose (SD) | 19.8 (5.0) | 22 (4.9) | >0.05 |
| Primary tumor site n (%) | |||
| Lung | 15(60) | 6 (38) | >0.05 |
| Breast | 5 (20) | 5 (31) | >0.05 |
| Genitourinary | 2 (8) | 2 (13) | >0.05 |
| Head and neck | 2 (8) | 2 (13) | >0.05 |
| Sarcoma | 1 (4) | 1 (6) | >0.05 |
| Mean total volume (cc) of treated metastases (SD) | 3.4 (0.7) | 3.8 (0.8) | >0.05 |
2.2. MR data acquisition
All patients underwent MRI scans at three time points: baseline (one day before radiotherapy), early-mid-treatment (one month after start of radiotherapy), and post-treatment (six months after radiotherapy). MRI scans were performed on all participants using a 3T MRI scanner (Siemens TrioTim) and a 12-channel head coil. All patients were supine, with their heads fixed by a sponge. Axial spin-echo T1-weighted images were acquired with TR/TE = 360/8 ms, slice thickness = 1.5 mm, field of view (FOV) = 26 cm × 26 cm, matrix size = 256 × 256, number of excitations (NEX) = 4. Fast spin-echo T2–weighted images were acquired with TR/TE = 2800/90 ms, slice thickness = 3.0 mm, interslice gap = 0, FOV = 26 cm × 26 cm, matrix size = 288 × 256, and NEX = 4. DWI was performed before administration of a contrast agent in the transverse plane by using a single-shot SE-planar imaging sequence with diffusion gradients in three orthogonal directions, and two diffusion weightings (b = 0 s/mm and 1000s/mm). DWIs were acquired with TR/TE = 6000/70 ms, slice thickness = 3.0 mm, FOV = 26 cm × 26 cm, matrix size = 128 × 128, NEX = 4. Finally, a volumetric three-dimensional gadolinium-enhanced T1 fast-spoiled gradient echo (FSPGR) was acquired with TR/TE = 8.5/4.2 ms, flip angle = 20°, FOV = 22 cm × 22 cm, matrix size = 270 × 270, slice thickness = 1.5 mm and NEX = 1. An initial loading dose of 3 mL of gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy) was administered which, after five minutes, was followed by another bolus injection with the remaining dose (for a total of 0.3 mL/kg or 1.5 times a single dose) during image acquisition.
2.3. MRI data processing
DWI images were processed using DTI Studio v2.4 [15] (Johns Hopkins University, Baltimore, MD) to generate eigenvalues (λ1, λ2 and λ3). ADC values were created for quantitative analysis by applying the following equation:
| ADC = (λ1 + λ2 + λ1)/3 | (1) |
2.4. Imaging analysis
Regions of interest (ROIs) were drawn manually for the tumor-enhanced regions of the tissues in the axial Gd-enhanced T1-weighted images. ROIs in the tumors included areas with maximal degrees of contrast enhancement on Gd-enhanced T1-weighted images while avoiding necrosis, cystic areas, hemorrhage and calcification. ROIs were set in all slices of tumor that included the tumor parenchyma to the full extent.
Mean ADC value (ADCi) and the cross-sectional area (areai) of the tumor ROI on each slice (i representing the slice number) was calculated using Image J software (NIH, USA). Subsequently, the ADCmean of the entire tumor was calculated as the weighted average for all ADCi values in each tumor using Eq. (1):
| (1) |
We calculated weighted averages as this is mathematically identical to calculating averages of ADC values directly from all voxels within the entire tumor volume. The volume of the tumor on DWI images was calculated using Eq. (2):
| (2) |
We also introduced the concept of a diffusivity index (DI), which is the sum of 1/ADC among all the voxels in a tumor calculated via the home-made MATLAB script using Eq. (3):
| DI = tumor volume/ADCmean | (3) |
where the volume of the tumor is calculated using Eq. (2); where the DI is the mean of 1/ADC for all voxels on each ROI area. This equation would be mathematically identical to calculating the sums of 1/ADC values directly from all voxels within the entire tumor volume.
2.5. Tumour response evaluation
Tumor response was assessed based on the volumetric T1 post-gadolinium MRI using three-dimensional (3-D) volume-based criteria [16]: (l) complete response (CR) − lesions disappeared completely or little traces remained; (2) partial response (PR) − lesions were reduced by more than 50%, did not intensify, or did not form cysts; (3) stability disease (SD) − lesions were reduced by less than 50%, or increased by less than 25%; (4) progressive disease (PD) − lesions increased by more than 25%. Using these criteria, (1), (2), (3) were considered as an effective treatment (response group), and (4) was considered an ineffective treatment (non-response group). Accordingly, patients were assessed by volume criteria at the six-month post SRS MRI, last follow-up MRI, and the MRI prior to salvage surgery or further radiation. Clinical outcomes were blinded and stored separately from segmented volumes.
2.6. Statistical analysis
Statistical analyses were conducted with SPSS Statistics (version 21.0.0.1, SPSS, Inc., 2010, Chicago, IL). A p value of <0.05 was considered to indicate statistical significance.
Continuous variables were presented as mean ± standard deviation (SD). Categorical data were presented as frequencies and percentages. The Mann–Whitney U test was used to evaluate statistical differences of continuous variables, and Fisher’s exact test was applied to evaluate statistical differences in categorical variables. To evaluate longitudinal changes of diffusion parameters, a one-way ANOVA test was used to analyze longitudinal changes of ADCmean, tumor volume, and the DI, in terms of tumor responders and non-responders. ADCmean, tumor volume and the DI were compared for tumor responders versus non-responders at baseline, one-month and six-month post-treatment using the Student t-test.
A univariate logistic regression analysis was used to account for tumor responders and non-responders with adjustment for baseline characteristics including: age, gender, baseline volume, radiation dose, steroid administration. Type I errors from multiple tests in the univariate analysis was controlled using a Bonferroni adjusted p value <0.005.
3. Results
3.1. Lesion characterization and treatment response evaluation
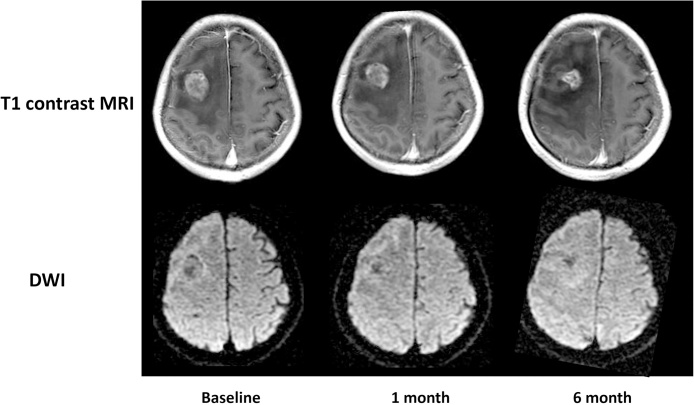
According to REIST criteria [12], 25 patients were defined as the tumor response group (Fig. 1), whereas 16 patients were defined as non-responders (Fig. 2). There were no significant differences in terms of sex or patient age between responders and non-responders (all p > 0.05). The mean tumor volume was 3.5 ± 0.8 mm3 at baseline, 2.6 ± 1.3 mm3 at one month post SRS and 2.7 ± 2.0 mm3 at six months post SRS. Longitudinally, significantly decreased tumor volume were found in one and six months post SRS compared to baseline (p = 0.009 and p = 0.017, respectively).
Fig. 1.
Treatment response group.
Gd-T1WI and DWI in a 52-year-old man with brain metastasis from lung adenocarcinoma. The contrast-enhanced T1WI showed a well-enhanced lesion in the right parietal lobe before radiotherapy. The maximum tumor volume (3.1 cm3 at baseline) markedly decreased at one month after radiotherapy (2.5 cm3) and six months (1.4 cm3) after radiotherapy. The ADC values in tumor parenchyma steadily increased from 0.75 × 10−3mm2/s at baseline, 0.74 × 10−3 mm2/s at one month after radiotherapy, and 0.81 × 10−3 mm2/s at six months after radiotherapy.
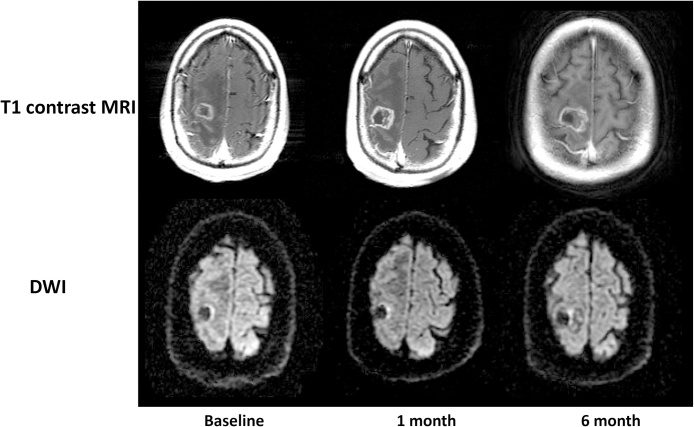
Fig. 2.
Treatment non-response group.
Gd-T1WI and DWI in a 77-year-old man with brain metastasis from renal cell carcinoma. The lesion volume was 2.6 cm3 at baseline, 2.9 cm3 and 3.5 cm3 at six months after radiotherapy. The ADC values in tumor parenchyma were 0.71 × 10−3 mm2/s before radiotherapy, 0.74 × 10−3 mm2/s at one month and 0.69 × 10−3 mm2/s at six months after radiotherapy.
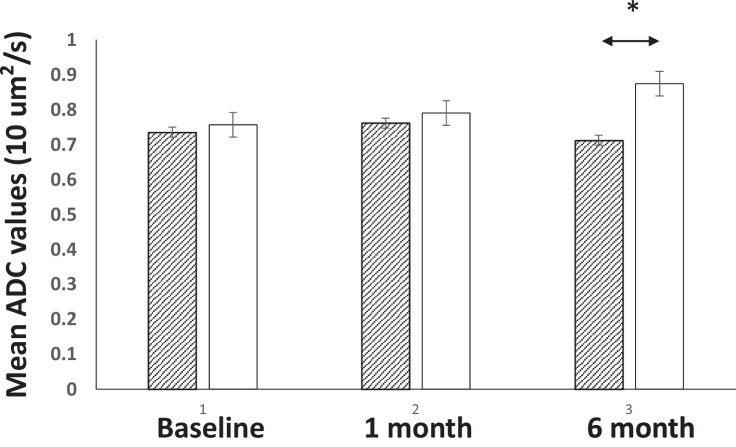
3.2. Longitudinal changes of ADCmean
ADCmeans for all tumors were 0.75 ± 0.08 × 10−3 mm2/s at baseline, and 0.78 ± 0.08 × 10−3 mm2/s for one month post SRS, and 0.81 ± 0.12 × 10−3 mm2/s for six months post SRS, respectively. Longitudinally, significantly increased ADCmean values were found between six months post SRS vs. the baseline.
Longitudinal changes of ADCmean in brain metastases are shown in Fig. 3. Higher ADCmeans were found at all time points in responders vs. non-responders. Six months after SRS, the differences reach statistical significance, (0.87 ± 0.07 × 10−3 vs., 0.71 ± 0.10 mm2/s, p < 0.05). In the non-responders’ group, no significant changes of ADCmean were found among all time points. However, significantly higher ADCmeans were found for six months post SRS compared to the baseline (p < 0.05) in the responders group.
Fig. 3.
Longitudinal changes of ADC values and mean tumor volumes among response vs., non-response group.
Only significantly differences are found for six months after radiotherapy, where the responder group has a significantly higher mean ADC compared to the non-responder group (0.87 ± 0.07 vs., 0.71 ± 0.09 × 10−3 mm2/s).
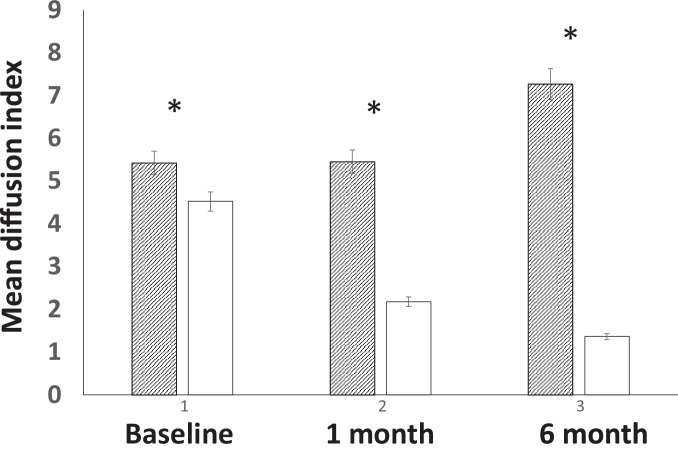
3.3. Diffusion index differentiates response and non-response group at baseline
The DI was calculated as the sum of 1/ADC among all the voxels in a tumor. Therefore, the index is mathematically identical to calculating all voxel diffusion parameters within the entire tumor volume. Overall, mean DI was 4.8 ± 1.4 at baseline, 3.5 ± 2.0 at one month post SRS, and 3.6 ± 3.2 at six months post SRS. Longitudinally, a significantly decreased DI was found for one and six months post SRS treatment compared to the baseline (all p < 0.05).
Cross-sectional and longitudinal DI values between responders vs. non-responders in brain metastases are showed in Fig. 4. In comparison to responders vs. non-responders, significantly lower DI values were found among the baseline, one month post SRS and six months post SRS, with a maximum difference for six months post SRS (7.3 ± 2.2 vs., 1.4 ± 0.5, p < 0.001). Interestingly, significantly lower DI was demonstrated at the baseline, indicating that the DI may reflect the potential biological characteristics of brain metastases and their response to SRS treatment.
Fig. 4.
Longitudinal changes of the diffusion index between response vs. non-response group.
A gradually increased diffusion index was demonstrated among the non-responder group vs. a gradually decreased diffusion index in the responder group. In addition, a significantly decreased diffusion index was found at baseline, one month and six months.
The DI characterizes longitudinal changes of diffusion properties in the responders’ group. In the responders’ group, the ANOVA test indicates gradually decreased DI values in one and six months post SRS compared to the baseline (2.2 ± 0.6 vs., 4.5 ± 1.1, p < 0.001; 1.4 ± 0.5 vs., 4.5 ± 1.1, p < 0.001). However, the ANOVA test indicates gradually increased DI values in the non-responders’ group. A significantly higher DI was found for six months post SRS compared to the baseline and one month post SRS (7.3 ± 2.2 vs., 5.4 ± 1.7, 5.5 ± 1.8, p = 0.03; 7.3 ± 2.2 vs., 5.5 ± 1.8, p = 0.03, respectively).
3.4. Diffusion index predicts SRS treatment response in brain metastases
Table 2 indicates the logistic regression analysis of ADCmean, tumor volume and DI with regard to their predictor values for SRS treatment response. A multivariate general estimating equation analysis identified the DI at all times as predictors of tumor response (p = 0.028, 0.002 and 0.001, respectively), and only ADCmean at six months post SRS demonstrates a significant predictor for tumor response (p = 0.019), whereas lower tumor volumes after SRS treatment are predictors for tumor response (p = 0.001 and p = 0.003, respectively). Therefore, lower DI values at baseline, one month and six month post SRS distinguished responders from non-responders and may be promising biomarkers for radiation response.
Table 2.
Showing Multivariate Regression Analysis of the Relationships Between Independent Variables; meanADC, tumor volume and diffusion index, and dependent variables of treatment response classification (response vs., non-response).
| Variables | Response vs. Non-response |
||
|---|---|---|---|
| Effect Estimate (B) | 95% CI | P value | |
| ADCmean (baseline) | 3.3 | −8.3–12.7 | 0.421 |
| ADCmean (1 month) | 4.1 | −6.4–13.0 | 0.289 |
| ADCmean (6 months) | 25.3 | 12.7–15.6 | 0.019 |
| Volume (baseline) | −0.8 | −1.8–0.1 | 0.064 |
| Volume (1 month) | −4.7 | −16.8 to −3.4 | 0.001 |
| Volume (6 months) | −48.9 | −5.4–2.6 | 0.037 |
| DI (baseline) | −0.50 | −1.14 to −0.05 | 0.028 |
| DI (1 month) | −3.6 | −9.5 to −2.4 | 0.002 |
| DI (6 months) | −26.1 | −27.8 to −10.5 | 0.001 |
Abbreviations: DIdiffusion index; CIconfidence interval.
4. Discussion
After SRS treatment, we have demonstrated that the higher ADCmean indicate better SRS treatment response. In addition, ADCmean and tumor volume fails to distinguish between SRS responders when compared to non-responders as early as baseline and one month post SRS in patients with brain metastases. Finally, the DI generated from ADC and tumor volume measurement shows distinguishable values at baseline and one month post SRS, and is a prediction factor for prognosis evaluations. Further evaluation of the DT as a biomarker for clinical application in brain metastases is required.
With fast imaging techniques such as echo planar imaging (EPI) and parallel imaging techniques, DWI can be performed with diagnostic quality at high b-values [9], which leads to a heightened interest in investigating its role in oncologic imaging. It appears that DWI has the potential to characterize tumors without the need of a contrast injection, thus it may have a potential role in MR imaging for malignancies [9]. ADC reflects the mean diffusivities in different directions. Dynamic change in ADC reflects the water distribution between the extra- and intra-cellular space. Histopathology analysis indicated that the ADC increases results from treatment-induced cell loss, cell necrosis, and shrinkage of the cytoplasm [17]. These effects reduce cell density, rupture membranes and remove proteins and other macromolecule restrictions, allowing intracellular water to leak out, thus creating space for extra-cellular water motion to increase so that the diffuse motion is sped up. Therefore, increasing ADC values and generating low signal intensity was in the DWI, which makes early assessment of SRS for metastases possible [6], [9]. Increased ADC shows prognostic values for cancer treatment. Huang et al. [18], found that ADC values were obviously higher one week post SRS than the baseline in patients with brain metastases. They also found that the values continued to increase, indicating positive response to treatment, which is consistent with our results. However, decreased or non-changes of ADC values may indicate a non-response to treatment, or a poor prognosis. It has been demonstrated that decreased ADC values in treated brain tumors may indicate tumor recurrence or non-response to treatment. The phenomenon may be as a result of increased cellularity of tumor cells, which restricted water diffusion in the brain. Consistent with previous studies, our results indicate that ADC values were stable, or decreased, post SRS. However, in the response group, ADC values gradually increased post SRS treatment. In addition, patients in the non-response group have a significantly higher ADCmean compared to the responders. However, the ADCmean fails to differentiate between the response vs. non-response group one month post SRS treatment, which may lower its diagnostic performance for early prediction SRS treatment response in brain metastases. The possible reason for this is that the ADCmean is calculated by taking averages of mean ADC values for each image, regardless of the cross-sectional size of the tumor on each image [19]. We feel that a weighted average, taking into consideration the tumor cross-sectional size on each image, is a more accurate calculation to reflect the true mean ADC value of all voxels in the entire tumor.
We introduce diffusion index values, defined as the quotient of tumor volume divided by ADC values, to study predicted values either in predicted SRS treatment response or characterize brain metastases at the baseline. Gu et al. [20], use a similar method to calculate a tumor diffusion index. They found significant correlation between TLG, which is a product of SUVmean, and tumor volume measured by PET. TLG reflects the total amount of glycolysis in a given tumor, and has been used clinically as a surrogate biomarker for monitoring treatment response [21], [22], [23]. For example, Guillem et al. found that a reduction of 30.5% of TLG in rectal cancer could predict no-evidence-of-disease status and freedom from recurrence with a sensitivity of 90% and specificity of 80% [22]. The correlation between TDI and TLG is no surprise as the ADC and SUV values, as well as the tumor volumes measured by MR and PET, are correlated. Since a tumor’s response to treatment may be reflected in changes of both size and function, we feel that DT values may have a potential role in monitoring treatment response similar to TLG. However, longer-term studies with a larger patient population are needed to clarify the diagnostic performance of DT.
5. Conclusion
In conclusion, our investigation represents a comprehensive analysis of the DWI with ADC measurements following radiation for brain metastases. Our results support the hypothesis that ADC and tumor volume generated a diffusion index at baseline, one month and six months post SRS, which may be a promising biomarker predicting brain metastasis’ response. Specifically, a lower DI at baseline and one month distinguished responders from non-responders. The changes in the DI may correlate with pathological changes post SRS, including radiation-induced cytotoxic edema, increased tissue necrosis, and a decrease in tumor blood flow. Further evaluation of the DI as a biomarker for clinical application in brain metastases is required to identify its biological correlations.
One of the limitations of this study is that the group of patients is relatively inhomogeneous due to different types of primary tumor. Therefore, a more uniform patient population would be helpful to fully understand the clinical meaning of the DI. Another limitation is that the exact correlation between the DI and pathological conditions is not clear due to the lack of pathological data. However current data has shown that the DI may be regarded as a new biomarker to reflect treatment response post SRS.
Disclosure of interest
The authors declare that they have no competing interest.
Grant supports
-
1.
Medical Engineering Cross Research Foundation of Shanghai Jiao Tong University(YG2014MS50)
-
2.
Foundation of Shanghai Municipal Commission of Health and Family Planning (201540231)
-
3.
The National Basic Research Program (973) of China (No. 2011CB013300)
Contributor Information
Jianming Ni, Email: jianmingniwuxi@gmail.com.
Jianrong Xu, Email: xu_jianr@163.com.
References
- 1.Chang S.D., Adler J.R., Jr. Current treatment of patients with multiple brain metastases. Neurosurg. Focus. 2000;9(2):e5. [PubMed] [Google Scholar]
- 2.Ranjan T., Abrey L.E. Current management of metastatic brain disease. Neurotherapeutics. 2009;6(3):598–603. doi: 10.1016/j.nurt.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura H. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am. J. Neuroradiol. 2000;21(8):1540–1546. [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson A.M. MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology. 1999;211(3):807–814. doi: 10.1148/radiology.211.3.r99jn48807. [DOI] [PubMed] [Google Scholar]
- 5.Tung G.A. MR imaging of pituitary adenomas after gamma knife stereotactic radiosurgery. AJR Am. J. Roentgenol. 2001;177(4):919–924. doi: 10.2214/ajr.177.4.1770919. [DOI] [PubMed] [Google Scholar]
- 6.Chenevert T.L., McKeever P.E., Ross B.D. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin. Cancer Res. 1997;3(9):1457–1466. [PubMed] [Google Scholar]
- 7.Moffat B.A. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. MAGMA. 2004;17(3–6):249–259. doi: 10.1007/s10334-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 8.Chang E.L. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 9.Chenevert T.L. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J. Natl. Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 10.Franzin A. Stereotactic drainage and Gamma Knife radiosurgery of cystic brain metastasis. J. Neurosurg. 2008;109(2):259–267. doi: 10.3171/JNS/2008/109/8/0259. [DOI] [PubMed] [Google Scholar]
- 11.Hawighorst H. Serial MR imaging of intracranial metastases after radiosurgery. Magn. Reson. Imaging. 1997;15(10):1121–1132. doi: 10.1016/s0730-725x(97)00178-1. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer: national Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Hopper K.D. Analysis of interobserver and intraobserver variability in CT tumor measurements. AJR Am. J. Roentgenol. 1996;167(4):851–854. doi: 10.2214/ajr.167.4.8819370. [DOI] [PubMed] [Google Scholar]
- 14.Padhani A.R. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006;81(2):106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Follwell M.J. Volume specific response criteria for brain metastases following salvage stereotactic radiosurgery and associated predictors of response. Acta Oncol. 2012;51(5):629–635. doi: 10.3109/0284186X.2012.681066. [DOI] [PubMed] [Google Scholar]
- 17.Thoeny H.C. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234(3):756–764. doi: 10.1148/radiol.2343031721. [DOI] [PubMed] [Google Scholar]
- 18.Huang C.F. Diffusion magnetic resonance imaging as an evaluation of the response of brain metastases treated by stereotactic radiosurgery. Surg. Neurol. 2008;69(1):62–68. doi: 10.1016/j.surneu.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Ho K.C. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(2):200–208. doi: 10.1007/s00259-008-0936-5. [DOI] [PubMed] [Google Scholar]
- 20.Gu J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol. Imaging Biol. 2011;13(5):1020–1028. doi: 10.1007/s11307-010-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costelloe C.M. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J. Nucl. Med. 2009;50(3):340–347. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 22.Guillem J.G. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J. Am. Coll. Surg. 2004;199(1):1–7. doi: 10.1016/j.jamcollsurg.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi U. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247(1):189–196. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]