Abstract
Stimulants are the main pharmacological treatment for patients with attention-deficit/hyperactivity disorder (ADHD). Their current prescription rates are rising, both in children, adolescents and adults. Related to the impulse control phenotype, both preclinical and clinical studies have demonstrated lower γ-amino butyric acid (GABA) levels in prefrontal brain regions in ADHD. Whereas stimulant treatment increases GABA levels, preclinical studies have suggested that stimulant treatment effects may be age-dependent.
As the long-term consequences of stimulant use in ADHD children and adolescents have so far been poorly studied, we used magnetic resonance spectroscopy to assess GABA+ and glutamate + glutamine (Glx) levels in the medial prefrontal cortex (mPFC) of adult ADHD patients, both before and after an oral methylphenidate (MPH) challenge. Three groups were studied: 1) ADHD patients who were first treated with stimulants before 16 years of age, i.e. during periods of ongoing brain development (early-stimulant-treated, EST); 2) patients first treated with stimulants in adulthood (i.e. > 23 years) (late-stimulant-treated, LST), and 3) stimulant-treatment-naive (STN) ADHD patients.
Reduced basal GABA+ levels were found in EST compared to LST patients (p = 0.04), while after an MPH challenge, only the EST patients showed significant increases in GABA+ (p = 0.01). For Glx, no differences were found at baseline, nor after an MPH challenge.
First stimulant exposure at a young age is thus associated with lower baseline levels of GABA+ and increased responsivity in adulthood. This effect could not be found in patients that started treatment at an adult age. Hence, while adult stimulant treatment seems to exert no major effects on GABA+ levels in the mPFC, MPH may induce long-lasting alterations in the adult mPFC GABAergic system when treatment was started at a young age.
Keywords: ADHD, GABA, Glutamate, Spectroscopy, Methylphenidate, Development
Highlights
-
•
Early stimulant exposure results in lower baseline PFC GABA levels in adulthood.
-
•
Exposure at young age alters the GABAergic response to stimulants in adulthood.
-
•
First exposure to stimulants in adulthood exerts no major effects.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) has an estimated worldwide prevalence of 7.2% (Thomas et al., 2015) and is defined by symptoms of hyperactivity, inattentiveness, difficulty in controlling one's actions and impulsivity. These symptoms cause substantial impairment in daily functioning and while they often emerge at pre-school age, they can persist into adulthood (Tarver et al., 2014). The most common pharmacotherapy for ADHD is stimulant medication with e.g. methylphenidate (MPH), which targets dopamine (DA) imbalances (Volkow et al., 2002) and is highly effective in alleviating ADHD symptoms (MTA-Cooperative-Group, 1999).
Nowadays, parallel to the increased prevalence of ADHD over the past decade, stimulant medication prescription rates have risen strongly (Thomas et al., 2015). Although the efficacy and safety of stimulants prescribed to children diagnosed with ADHD has been extensively documented (Faraone and Buitelaar, 2010), it is currently unclear whether these drugs induce long-term effects, especially when they are first given during sensitive periods of ongoing human brain development, as is the case for children and adolescents.
In addition to the DA neurotransmitter system, the γ-amino butyric acid (GABA) system has also been implicated in ADHD pathophysiology. Various measures of inhibitory control, one of the main problems of children with ADHD (Vaidya et al., 1998), have e.g. been correlated with brain GABA levels in healthy adults (Boy et al., 2010, Boy et al., 2011, Marenco et al., 2010). In fact, recent preclinical studies support a link between alterations in GABA and behavioral changes relevant for ADHD; e.g. micro-infusions of a GABA receptor antagonist in the rat medial prefrontal cortex (mPFC) cause attentional deficits (Pezze et al., 2014), while reductions in extracellular GABA levels have been found in a rodent ADHD model (Sterley et al., 2013).
These findings are supported by human studies, in which behavioral impulsivity is correlated with decreases in prefrontal GABA levels in healthy volunteers (Boy et al., 2011). In addition, school-age children diagnosed with ADHD show reduced short inter-cortical inhibition in a paired-pulse transcranial magnetic stimulation protocol, a process known to be mediated by GABAergic cortical neurons (Gilbert et al., 2011, Ziemann et al., 1996). Moreover, reduced cortical GABA levels are found in children with ADHD (Edden et al., 2012), and also in adult ADHD patients, subcortical GABA levels are altered (Bollmann et al., 2015, Ende et al., 2015, Schür et al., 2016).
Whereas these alterations suggest a role for the GABAergic system in ADHD symptomatology, effects of concurrent stimulant treatment could confound these findings. ADHD medication increases GABA in animal models (Freese et al., 2012, Goitia et al., 2013, Sotomayor-Zárate et al., 2010) and Bollmann et al. (Bollmann et al., 2015) have demonstrated that age is a factor that needs to be considered as the GABAergic system continues to develop throughout childhood and adolescence (Andersen, 2003, Luján et al., 2005). Hence, an aberration from the normal development of the GABAergic system could result in a later imbalance between excitatory and inhibitory neurotransmission, that could play a role in the pathophysiology and persistence of ADHD (Ferreira et al., 2009, Luján et al., 2005).
Age-dependent and long-lasting effects of stimulant treatment have already been reported in rodents; compared to rats that started MPH treatment in adulthood, juvenile rats that were MPH-treated showed a diminished cocaine sensitivity in adulthood (Andersen et al., 2002), notably parallel to reductions in DA transporter density (Moll et al., 2001) and in extracellular DA levels (Cornish and Kalivas, 2001, Howard et al., 1997).
In summary, whereas both preclinical and clinical data suggest a role for the GABAergic system in ADHD (symptoms), the influence and interaction of age and stimulant treatment on GABA levels in the human PFC was poorly studied. We therefore used edited magnetic resonance spectroscopy (MRS) to investigate GABA+ levels in adult ADHD patients before and after an MPH challenge. In order to study the age-dependent effects of stimulant treatment on the GABAergic system we investigated ADHD patients who; 1) either started with stimulant treatment at a young age (i.e. < 16 years), 2) who were first treated only later in life (> 23 years), or 3) who had never received stimulant treatment.
2. Methods
2.1. Participants
A group of 44 male ADHD patients between 23 and 40 years of age were included (mean age 29.11 ± 4.90 years). Participants were recruited via outpatient clinics, newspaper advertisement and databases containing prescription data (Pharmo Institute Utrecht). All patients had an established clinical diagnosis of ADHD (all subtypes) made by a specialized physician using the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Fourth Edition (American Psychiatric Association, 1994). Exclusion criteria were; 1) an IQ < 80, 2) a history of brain trauma or neurological disease, 3) magnetic resonance imaging (MRI) contra-indications, 4) alcohol and/or drug dependence according to DSM-IV criteria as assessed using the MINI (Sheehan et al., 1998), 5) contra-indications for MPH treatment (e.g. use of mono-amine oxidase (MAO) inhibitors or antipsychotics), 6) current or previous treatment with related DAergic medication before the age of 23, such as neuroleptics, D2/D3 agonists or antipsychotics, and current or previous DA-system-related drug dependence (e.g. amphetamine), and 7) prenatal use of MPH by the mother of the patient.
Patients were stratified in three groups: 1) an early stimulant treated group (EST): i.e. patients who were treated for the first time with stimulants starting before the age of 16; 2) a late stimulant treated group (LST): i.e. patients were treated first with stimulants after 23 years of age and 3) a stimulant treatment-naive group (STN): ADHD patients with no history of stimulant medication. Prescription history was based on self-report and verified with available prescription data from pharmacies and treating physicians. Current ADHD symptom severity was measured with the ADHD rating scale (Kooij et al., 2005). Education was determined based on a rating scale (Verhage, 1964). All participants signed written informed consent. The study was carried out in accordance with the Declaration of Helsinki (2012) and was approved by the Medical Ethical Committee of the Amsterdam Medical Center.
2.2. Procedures
All participants underwent an MRS scan session, in which GABA levels were assessed in a single voxel in the mPFC. This region was chosen because the mPFC plays an important role in behavioral inhibition, which is a prominent symptom in ADHD (Castellanos and Proal, 2012). For a subgroup of the participants (N = 38), the MRS scan session was followed by an oral MPH challenge of 0.5 mg/kg MPH (with a maximum dose of 40 mg), 5 min after the MRS scan. MPH (0.5 mg/kg) has been administered as an oral bolus in previous MRI studies up to 50 mg, which was well tolerated (Rao et al., 2000, Silveri et al., 2004). This subgroup underwent a second MRS scan 90 min after MPH administration, which is when the maximum uptake of MPH in the striatum is reached (Kollins, 2003).
During the first scan session, a T1-weighted MRI scan was obtained to assess tissue composition differences in the voxels between the different groups. Participants classified as being on stimulant medication, were medication-free for at least one week before the scan, in order to prevent acute effects of stimulant treatment on our GABA measurements. Participants were further instructed to abstain from nicotine and caffeine on the study day, alcohol at least 24 h before the study, and other drugs of abuse for at least one week before the study.
2.3. MRI acquisition
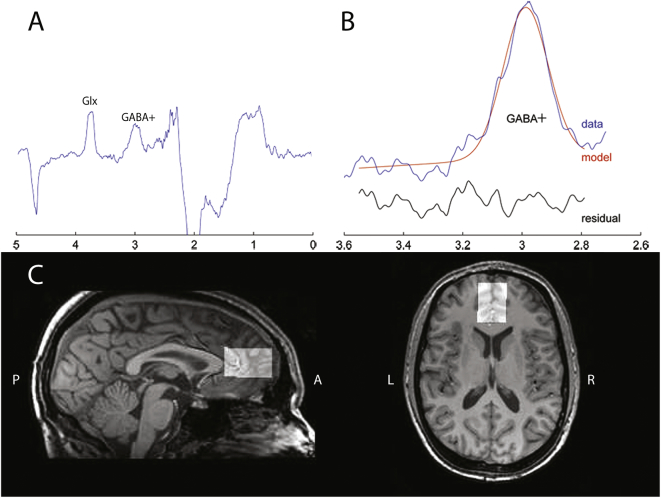
Data were acquired using a 3.0 T Philips Achieva MR Scanner (Philips Medical Systems, Best, The Netherlands), using a SENSE 8-channel receive-only head coil and body coil transmission. The anatomical 3D-fast field echo T1-weighted scan was obtained with the following scan parameters: TR/TE = 9.8/4.6 ms, FOV = 256 × 256 × 120 mm, voxel size = 0.875 × 0.875 × 1.2 mm in each session. J-difference edited MRS spectra were acquired using a MEGA-PRESS sequence (Mescher et al., 1998) from a 2.5 × 3.5 × 2.5 cm3 voxel in the mPFC with the following parameters: TR/TE = 2000/68 ms, number of signal averages = 2, dynamic scans = 160, 14 ms editing pulses placed at 1.9 ppm (ON) and 7.46 ppm (OFF) with 1024 data points and 2 kHz spectral width, for an approximately 10 min acquisition. The voxel was placed manually and anterior of the genu of the corpus callosum. It was oriented along the anterior-posterior commissure and centered on the interhemispheric fissure (see Fig. 1C).
Fig. 1.
Spectrum, segmentation data and position of the voxel.
A) Representative example of a typical MEGA-PRESS difference spectrum. The GABA+ peak is around 3.0 ppm, the glutamate (Glx) peak is around 3.7 ppm.
B) Illustration of the curve fitting of the GABA+ peak using Gannet. The red line represents the result of the curve-fitting, the blue line shows the post-phase and frequency aligned GABA+ data, the black line is the residual difference between the data and the curve-fit.
C) Illustration of the position of the voxel in the mPFC in a sagittal and transverse T1-weighted image of a representative subject.
2.4. Image analysis
Edited MRS spectra were analyzed using the Gannet GABA analysis toolbox (Edden et al., 2014) (see Fig. 1A and B). Coil-combination, phasing, apodization and frequency correction were performed automatically in this toolbox. Water-scaled GABA concentrations were calculated according to standard procedures, as described in detail elsewhere (Mullins et al., 2014). In short, the time-domain data is processed into a frequency-domain GABA-edited spectrum. Using a nonlinear, least-squares fitting, the GABA concentration at 3 ppm is estimated (Edden et al., 2014). The assessment of GABA using MEGA-PRESS however results in co-editing of macro-molecules such as proteins, which contribute to the edited GABA peak at 3.0 ppm. GABA levels are quantified against the unsuppressed water signal from the same region with estimated relaxation values for water and GABA (Edden et al., 2014). As the editing pulse at 1.9 ppm is known to co-edit macromolecule signals at 1.7 ppm (Mullins et al., 2014), the water-scaled GABA findings described in this paper represent GABA and related macromolecules and are therefore referred to as GABA+ levels. GABA+ fit errors were calculated with the Gannet GABA analysis toolbox to assess the data quality of the spectra.
The SPM8 toolbox was used with MATLAB (The Mathworks, Natick, MA) to co-register the T1-weighted scan to the MRS scan in the Gannet toolbox. Co-registration of the T1-weighted image to the MRS spectrum allowed for a calculation of both the grey and white matter and CSF fraction within the voxel. For the subjects that underwent MRS scans before and after MPH, a low-resolution T1 obtained at the second scan session that was registered, together with the MRS voxel, to the high resolution T1 from the first session, in order to estimate the overlap between the voxels during both scans. In addition to water-scaled GABA+, the co-edited water-scaled glutamate + glutamine (Glx) signal was assessed to investigate differences in baseline level and after administration of the MPH challenge.
Exclusion criteria for bad data quality were based on visual inspection of the GABA+ edited difference spectrum, frequency drifts of the residual water spectrum, the creatine signal before and after frequency and phase correction, and the fit of the GABA+, the water and creatine signal, in addition to quantitative measurements of the provided fit error and expected full-width/half-maximum of the signal peaks, and on visual inspection of the voxel position.
2.5. Statistical analyses
The IBM SPSS Statistics package Version 20 (SPSS, Chicago, IL) was used to conduct statistical tests for all data. Data were checked for normality and equality-of-variance. Due to missing values for some of the subjects after the MPH challenge, linear mixed model analyses were used to assess the main effect of group and time-point, and an interaction between group and time-point on the GABA+ and Glx levels. An unstructured covariance matrix was assumed and a fixed intercept was used and the model was estimated using maximum likelihood. p-Values < 0.05 were considered statistically significant and follow-up pairwise comparisons were corrected for multiple testing using Tukey corrections. Pearson correlations were used to assess correlations between GABA+ and Glx levels as well as age, symptom severity and time since last stimulant exposure to check for possible confounding effects.
3. Results
3.1. Sample characteristics
As shown in Table 1, mean age and educational level did not differ significantly between the three groups (age: F(2,41) = 1.47, p = 0.24; education: F(2,40) = 2.54, p = 0.09). Symptom severity did differ significantly between the three groups (F(2,40) = 3.44, p = 0.04) with higher scores indicating a more severe symptomatology, although post-hoc Tukey test showed no differences between the individual groups. Inherent to the study design, mean age of first stimulant exposure was significantly lower in the EST subjects compared to the LST subjects (t = − 10.52, p < 0.01). Treatment duration differed significantly between the EST and LST group (t = 3.89, p < 0.01), as well as time since last stimulant exposure (t = 6.83, p < 0.01; Table 1). 44 subjects completed the first scan session, of which 38 subjects received the MPH challenge and underwent the second scan session. For the GABA+ analyses, 3 participants were excluded from both scan sessions due to bad data quality or voxel misplacement. In addition, 2 participants were excluded from the second MRS scan session due to bad data quality. This resulted in a final group for the GABA+ analyses of 41 subjects at baseline and 33 subjects who completed the second MRS scan session.
Table 1.
Sample characteristics at baseline.
| STN |
Exposed |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 19 |
N = 25 |
||||||||
| EST (N = 14) |
LST (N = 11) |
||||||||
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| Age (y) | 29.71 | 5.02 | 23–39 | 27.32 | 3.28 | 23–35 | 30.36 | 6.08 | 24–40 |
| Educational levela | 5.79 | 0.86 | 4–7 | 5.15 | 0.56 | 4–6 | 5.73 | 1.01 | 4–7 |
| ADHD symptom severityb | 29.63 | 9.62 | 9–44 | 23.64 | 8.42 | 9–35 | 21.40 | 7.73 | 10–39 |
| Age of diagnosis (y) | 29.63 | 5.02 | 22–39 | 8.85 | 3.60 | 3–14 | 29.06 | 5.25 | 24–39⁎ |
| Age of first stimulant exposure (y) | – | – | – | 9.92 | 2.99 | 4–14 | 28.64 | 5.22 | 23–39⁎⁎ |
| Treatment duration (m) | – | – | – | 90.92 | 59.74 | 18–228 | 14.62 | 34.95 | 4–120⁎⁎ |
| Time since last stimulant exposure (m) | – | – | – | 96.04 | 52.13 | 0–168 | 0.77 | 1.73 | 0–6⁎⁎ |
SD = standard deviation.
y = years.
m = months.
p < 0.05 for EST versus STN and LST subjects.
p < 0.05 for EST versus LST subjects.
For the additional Glx analyses, 6 subjects were excluded due to bad data quality or voxel misplacement from both scan sessions. In addition, 3 subjects were excluded from the Glx measurements from the second MRS scan. This resulted in a final group for the Glx analyses of 38 subjects at baseline and 29 subjects after the MPH challenge.
3.2. Morphological differences in mPFC voxel
No significant differences were found in grey matter, white matter or cerebrospinal fluid (CSF) fraction in the MRS voxel between the groups when studied at baseline (Table 2). Comparison of the voxel position during the first and second MRS scan resulted in a mean overlap of 86.77% for all participants who underwent the second MRS scan session.
Table 2.
Differences in mPFC voxel morphology at baseline.
| STN |
Exposed |
|||||
|---|---|---|---|---|---|---|
| N = 17 |
N = 24 |
|||||
| EST (N = 13) |
LST (N = 11) |
|||||
| Mean | SD | Mean | SD | Mean | SD | |
| Grey matter fraction | 0.65 | 0.02 | 0.64 | 0.03 | 0.63 | 0.03 |
| White matter fraction | 0.18 | 0.04 | 0.20 | 0.03 | 0.18 | 0.03 |
| CSF fraction | 0.17 | 0.04 | 0.17 | 0.03 | 0.18 | 0.03 |
CSF = cerebrospinal fluid.
mPFC = medial prefrontal cortex.
SD = standard deviation.
3.3. GABA+ levels
Linear mixed model analyses showed a trend significant interaction between group and time-point (F(2,38) = 2.55, p = 0.09) and a significant main effect of group on GABA+ levels (F(2,36) = 5.43, p = 0.01). Addition of possible covariates such as age, ADHD symptom severity or age of diagnosis did not affect these results, nor did the covariates showed a significant main effect. Post-hoc analyses revealed differences at baseline in the estimated GABA+ levels between the STN, EST and LST subjects (F(2,0.70) = 3.75, p = 0.03; Fig. 2, Fig. A.1). Statistically significant lower estimated GABA+ levels were found in EST when compared to LST subjects (p = 0.04). In addition, trend significant differences in GABA+ levels were found after the MPH challenge (F(2,0.32) = 3.16, p = 0.057). Furthermore, estimated GABA+ levels increased significantly after the MPH challenge only in the EST group (t = − 3.20, p = 0.01), whereas the STN and LST subjects did not show such a change in estimated GABA+ levels after the MPH challenge (Fig. 2, Fig. A.1). There were no significant differences in GABA+ fit errors between the three groups (F(2,38) = 2.58, p = 0.09).
Fig. 2.
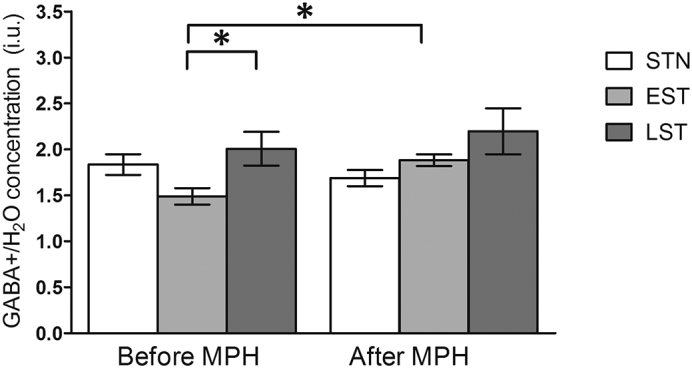
Representative water-scaled estimated GABA+ levels in non-, early and late exposed subjects, before and after MPH challenge. Data are represented as mean, error bars represent the standard error of the mean. *p < 0.05. Before the MPH challenge, the EST and LST subjects differed significantly (p = 0.04), whereas only the EST subjects showed increased estimated GABA+ levels after the MPH challenge (p = 0.01).
Fig. A.1.
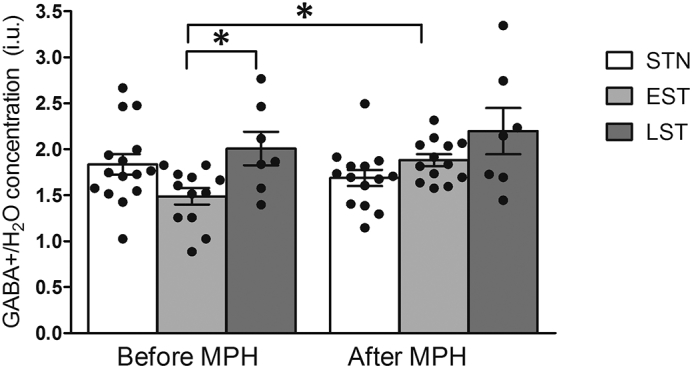
Representative water-scaled estimated GABA+ levels in non-, early and late exposed subjects, before and after MPH challenge. Data are represented as individual data points, bars represent mean, error bars represent the standard error of the mean. *p < 0.05. Before the MPH challenge, the EST and LST subjects differed significantly (p = 0.04), whereas only the EST subjects showed increased estimated GABA+ levels after the MPH challenge (p = 0.01).
At baseline, no correlation was present between age and estimated GABA+ levels (r = 0.26, p = 0.10). Also, ADHD symptom severity did not correlate with estimated GABA+ levels (r = 0.14, p = 0.40) in all subjects, nor in the unexposed subjects (r = 0.37, p = 0.16) or previously exposed subjects (r = − 0.21, p = 0.35).Additionally, treatment duration was not correlated to GABA+ levels in any of the previously exposed subjects (r = − 0.02, p = 0.93). However, time since last stimulant exposure correlated significantly with estimated GABA+ levels in the previously exposed subjects (r = − 0.50, p = 0.01).
3.4. Glx levels
No significant interaction between group and time-point was found on Glx levels (F(2,30.00) = 0.10, p = 0.91) in the linear mixed models, nor was there a main effect of group (F(2,34.04) = 1.47, p = 0.24) or time-point (F(1,30.00) = 0.69, p = 0.41 (Fig. A.2).
Fig. A.2.
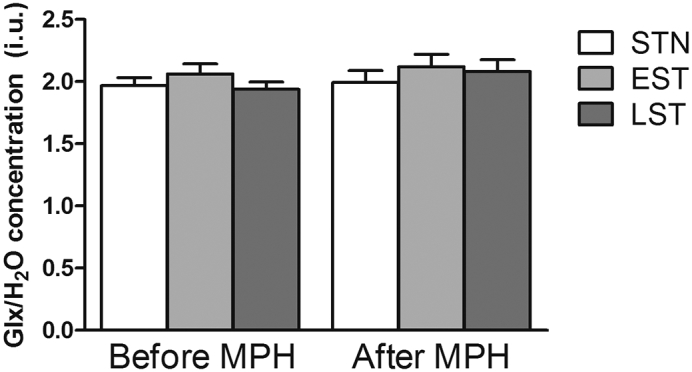
Representative water-scaled estimated Glx levels in non-, early and late exposed subjects, before and after MPH challenge. Data are represented as mean, error bars represent the standard error of the mean.
4. Discussion
In this study, estimated GABA+ levels, and changes in GABA+ levels in response to MPH, were compared between 3 groups of ADHD patients. One group was exposed to stimulants for the first time during a period of ongoing brain development (EST), another during a period when brain maturation is nearly completed (LST), and one was treatment naive. Estimated GABA+ levels differed significantly at baseline between the three groups with the EST group showing significantly lower mPFC estimated GABA+ levels relative to LST subjects. Moreover, a significant increase in GABA+ levels was found after an acute MPH challenge only in the EST, but not LST, subjects. Together, this indicates that alterations have occurred in the mPFC GABAergic system, selectively in those ADHD patients who were first exposed to stimulant treatment early in their lives, i.e. during childhood/adolescence.
The current increase in estimated GABA+ levels found after an MPH challenge in EST subjects is in accordance with studies in healthy rats (Freese et al., 2012, Goitia et al., 2013). In these studies, a challenge with MPH increased the mRNA levels of glutamic acid decarboxylase (GAD) that encodes the protein converting glutamate into GABA (Freese et al., 2012). Also, a single administration of MPH increased GABAergic neurotransmission in healthy mice (Goitia et al., 2013).
In stimulant treatment-naive ADHD subjects, such increases in estimated GABA+ levels were not observed after an acute MPH challenge. A possible explanation could be that the animal studies mentioned above were conducted in wild-type animals and are therefore difficult to extrapolate to a disease state like human ADHD, aside from the translation of ADHD-like animal models to human disease states in general, which is complicated in itself (Wickens et al., 2011). Alternatively, species-specific differences in the GABAergic system have been reported, e.g. in the sub-regional distribution of GABA receptors and in their subunit compositions (Young and Chu, 1990), that could possibly underlie the discrepancy between our current findings and the preclinical studies.
In our study, the mPFC was chosen as region of interest since dysfunction of the prefrontal-striatal circuitry is thought to underlie at least some of the executive deficits in ADHD (Castellanos et al., 2006). Hence, down-regulation of GABA in the mPFC could contribute to alterations in ADHD related functions like inhibition, behavioral impulsivity, decision making and working memory (Boy et al., 2011, Lew and Tseng, 2014). A related study by Bollmann et al. (2015) did not find differences in GABA levels in a frontal voxel, but this voxel was positioned more laterally than our current voxel. Nevertheless, these authors found differences in GABA levels between adult ADHD patients and controls in the basal ganglia, which is also part of the prefrontal-striatal circuitry. Future studies, e.g. using chemical shift imaging (CSI), could help shed light on the regional differences in GABA and on whether modulation occurs by disease and/or pharmacotherapy.
The results from our study suggest that stimulants have different effects when acting on the developing or the mature brain. Drug treatment during periods of ongoing brain development has been hypothesized to induce long-lasting changes in later neurotransmitter sensitivity, also known as chemical programming (Andersen and Navalta, 2004). In this respect, the chronic increase in GABA+ levels present during MPH treatment in childhood could have caused a lasting down-regulation of endogenous GABA+ levels as an adaptive response.
Alternatively, MPH treatment was shown before to increase DA levels (Volkow et al., 1998) that could subsequently affect DAergic neurotransmission. In turn, as proposed recently, this could have increased GABA release from dopaminergic axons (e.g. (Freese et al., 2012, Tritsch et al., 2012)). Chronic MPH treatment may therefore have led to lasting increases in DA and GABA levels, and could eventually have resulted in a down-regulation of their respective receptors. While speculative, this could have decreased the sensitivity and thereby lower the overall activity of both the DA and GABA systems. Changes in receptor down-regulation could be long-lasting, especially when MPH treatment occurs in an immature brain, but this possibility remains to be tested in detail.
We further show that only the EST subjects displayed an increased GABA+ response after an MPH challenge. This suggests that compensatory effects may have occurred in response to e.g. reductions in basal GABA+ levels, also temporal aspects could explain the differences between EST and LST subjects. Although the wash-out period lasted one week, the differences in GABA+ levels may also have resulted from a differential sensitivity to recent stimulant treatments. However, no differences were present in baseline GABA+ levels between the LST and STN subjects, which argues against this explanation. Thus, as only EST subjects showed an increase in estimated GABA+ levels after the MPH challenge, this indicates the differences we observed between EST and LST subjects, is most likely explained by the age at first exposure.
Previous literature has shown that, next to GABA, also glutamate may be altered in ADHD, although some studies could not be confirmed later (Bollmann et al., 2015, Carrey et al., 2007, Ferreira et al., 2009, Moore et al., 2006). In our current study, we did not find differences in baseline Glx levels, nor did we find any changes in Glx after an MPH challenge. This suggests that in contrast to GABA+, the prefrontal glutamatergic system does not seem to be affected by early stimulant exposure, nor by a single MPH challenge, which further adds specificity to the current GABA+ effects.
Our current study has several strengths and limitations. It is the first to investigate the influence of age of first stimulant treatment on later GABA+ and Glx levels and responses in adult ADHD patients. As stimulants are currently prescribed to many children diagnosed with ADHD, and in view of its putative imprinting effects (Andersen, 2005), our results provide relevant data to the timely discussion on this topic in both science and society. Although the early MPH treatment improves ADHD symptoms, lasting changes in the GABAergic system could have been induced that could e.g. underlie later changes in impulsivity, that in turn, could make patients more vulnerable for risk-taking behavior and/or later drug (ab)use. As such, these data may help to better evaluate decisions regarding treatment of children and adults with ADHD.
The current addition of an MPH challenge in our study provided useful information about the responsiveness of the GABAergic system. The sample studied here is unique, as it also contained ADHD patients who were stimulant treatment-naive, which allowed us to compare the specific actions of stimulant treatment in relation to first age of treatment. However, no healthy control group was included, and we can thus not draw any conclusions about possible ‘normalizing’ effects of acute MPH on GABA+ levels now. Additionally, this study did not include an additional control group with patients diagnosed with ADHD at young age but who did not receive any treatment, which would have extended the interpretation of our results with age of diagnosis next to age of first treatment.
MRS is currently the only technique that allows to reliably measure brain metabolites in the human brain in vivo. Due to technical limitations, a large voxel is needed to obtain a sufficiently high signal-to-noise ratio to fit and estimate GABA levels accurately. Whereas current GABA measurements do not have a high spatial resolution, recent advancements in 7T protocols, as well as in CSI for an increased coverage at 3T, should allow to draw more firm conclusions in the near future (Stagg et al., 2011).
A potential limitation of our study is that the subjects diagnosed early or late in life with ADHD could possibly represent different subgroups. A very recent longitudinal study in New Zealand indicated that adults with ADHD do not always meet childhood criteria for ADHD (Moffitt et al., 2015). Additionally, the variance of the GABA+ levels in the LST group was large, indicating that the LST group might be quite heterogeneous. Hence, a subset of our ADHD patients could in principle have a different neurobiological profile, which may relate to different GABA+ or Glx levels. While we did not find changes in basal levels of the latter, further research is warranted to investigate this alternative hypothesis.
Second, although our study is the first to investigate effects of age of first stimulant treatment on GABA+ levels in adult ADHD patients, our sample, inherent to the retrospective nature of our design, was quite heterogeneous in terms of symptom severity, treatment duration and time since last exposure. Nevertheless, while we focused on effects of ADHD medication, neither current ADHD symptom severity, nor treatment duration, nor age at diagnosis, nor time since last exposure significantly affected our results. Moreover, a prospective study design that would overcome such issues would have been very difficult, if not impossible, to execute. Additionally, exploratory analyses revealed no relations between baseline mPFC GABA+ levels and ADHD symptom severity. Our results do therefore not report on the relation between GABA+ levels and ADHD symptomatology.
5. Conclusion
In conclusion, our results demonstrate that MPH effects on GABA+ levels in ADHD patients are influenced by whether a subject had first started stimulant treatment in childhood or in adulthood. Our data thus suggest that long-lasting alterations may have occurred in GABAergic neurotransmission in the mPFC, selectively in subjects who had been first exposed to stimulant treatment early during childhood, but not in those who started medication only from later in their lives onward. Future studies are therefore warranted to assess the underlying mechanisms as well as the consequences of these lower GABA+ levels on cognitive and behavioral problems in ADHD.
The following are the supplementary data related to this article.
Acknowledgements
The authors would like to thank the participants of the study. The study was funded by personal research grants awarded to PL and LR from the Academic Medical Center and the Swammerdam Institute for Life Sciences, both from the University of Amsterdam and 11.32050.26 ERA-NET PRIOMED CHILD FP 6 (EU) and by an Amsterdam Brain & Cognition Project grant to PL and LR. PL is further supported by ISAO/Alzheimer Nederland and NWO. This study applies tools developed under NIH R01 EB016089 and P41 EB015909. The funders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript. The authors declare no conflict of interest.
Contributor Information
Michelle M. Solleveld, Email: m.m.solleveld@amc.uva.nl.
Anouk Schrantee, Email: a.g.schrantee@amc.uva.nl.
Liesbeth Reneman, Email: l.reneman@amc.uva.nl.
Paul J. Lucassen, Email: p.j.lucassen@uva.nl.
References
- American Psychiatric Association . 4th edition. 1994. Diagnostic and Statistical Manual of Mental Health Disorders. (Washington DC) [Google Scholar]
- Andersen S.L. Vol. 27. 2003. Trajectories of brain development: point of vulnerability or window of opportunity? pp. 3–18. (Neuroscience and Biobehavioral Reviews). [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Stimulants and the developing brain. Trends Pharmacol. Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Navalta C.P. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int. J. Dev. Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Arvanitogiannis A., Pliakas A.M., LeBlanc C., Carlezon W. a. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Bollmann S., Ghisleni C., Poil S.-S., Martin E., Ball J., Eich-Höchli D., Edden R. a E., Klaver P., Michels L., Brandeis D., O'Gorman R.L. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl. Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F., Evans C.J., Edden R.A.E., Singh K.D., Husain M., Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy F., Evans C.J., Edden R.A.E., Lawrence A.D., Singh K.D., Husain M., Sumner P. Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol. Psychiatry. 2011;70:866–872. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey N.J., MacMaster F.P., Gaudet L., Schmidt M.H. Striatal creatine and glutamate/glutamine in attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2007;17:11–17. doi: 10.1089/cap.2006.0008. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn. Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cornish J.L., Kalivas P.W. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J. Addict. Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Edden R.A.E., Crocetti D., Zhu H., Gilbert D.L., Mostofsky S.H. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2012;69:750–753. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A.E., Puts N.A.J., Harris A.D., Barker P.B., Evans C.J. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G., Cackowski S., VanEijk J., Sack M., Demirakca T., Kleindienst N., Bohus M., Sobanski E., Krause-Utz A., Schmahl C. Impulsivity and aggression in female BPD and ADHD patients: association with ACC glutamate and GABA concentrations. Neuropsychopharmacology. 2015;41:1–30. doi: 10.1038/npp.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V., Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur. Child Adolesc. Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Ferreira P.E.M.S., Palmini A., Bau C.H.D., Grevet E.H., Hoefel J.R., Rohde L.A., Anés M., Ferreira E.E., Belmonte-De-Abreu P. Differentiating attention-deficit/hyperactivity disorder inattentive and combined types: a 1H-magnetic resonance spectroscopy study of fronto-striato-thalamic regions. J. Neural Transm. 2009;116:623–629. doi: 10.1007/s00702-009-0191-3. [DOI] [PubMed] [Google Scholar]
- Freese L., Muller E.J., Souza M.F., Couto-Pereira N.S., Tosca C.F., Ferigolo M., Barros H.M.T. GABA system changes in methylphenidate sensitized female rats. Behav. Brain Res. 2012;231:181–186. doi: 10.1016/j.bbr.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Gilbert D.L., Isaacs K.M., Augusta M., Macneil L.K., Mostofsky S.H. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitia B., Raineri M., González L.E., Rozas J.L., Garcia-Rill E., Bisagno V., Urbano F.J. Differential effects of methylphenidate and cocaine on GABA transmission in sensory thalamic nuclei. J. Neurochem. 2013;124:602–612. doi: 10.1111/jnc.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S.G., Fisher R., Landry C.F. Alterations in the spontaneous release of dopamine and the density of the DA D2 receptor mRNA after chronic postnatal exposure to cocaine. Brain Res. Bull. 1997;43:101–106. doi: 10.1016/s0361-9230(96)00427-3. [DOI] [PubMed] [Google Scholar]
- Kollins S.H. Comparing the abuse potential of methylphenidate versus other stimulants: a review of available evidence and relevance to the ADHD patient. J. Clin. Psychiatry. 2003;64:14–18. [PubMed] [Google Scholar]
- Kooij J.J.S., Buitelaar J.K., van den Oord E.J., Furer J.W., Rijnders C.A.T., Hodiamont P.P.G. Internal and external validity of attention-deficit hyperactivity disorder in a population-based sample of adults. Psychol. Med. 2005;35:817–827. doi: 10.1017/s003329170400337x. [DOI] [PubMed] [Google Scholar]
- Lew S.E., Tseng K.Y. Dopamine modulation of GABAergic function enables network stability and input selectivity for sustaining working memory in a computational model of the prefrontal cortex. Neuropsychopharmacology. 2014;39:3067–3076. doi: 10.1038/npp.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R., Shigemoto R., López-Bendito G. Glutamate and GABA receptor signalling in the developing brain. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Marenco S., Savostyanova A.A., Van Der Veen J.W., Geramita M., Stern A., Barnett A.S., Kolachana B., Radulescu E., Zhang F., Callicott J.H., Straub R.E., Shen J., Weinberger D.R. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35:1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M., Merkle H., Kirsch J., Garwood M., Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Houts R., Asherson P., Belsky D.W., Corcoran D.L., Hammerle M., Harrington H., Hogan S., Meier M.H., Polanczyk G.V., Poulton R., Ramrakha S., Sugden K., Williams B., Rohde L.A., Caspi A. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am. J. Psychiatry. 2015;172:967–977. doi: 10.1176/appi.ajp.2015.14101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll G.H., Hause S., Rüther E., Rothenberger A., Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J. Child Adolesc. Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Moore C.M., Biederman J., Wozniak J., Mick E., Aleardi M., Wardrop M., Dougherty M., Harpold T., Hammerness P., Randall E., Renshaw P.F. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: a proton magnetic resonance spectroscopy study. Am. J. Psychiatry. 2006;163:316–318. doi: 10.1176/appi.ajp.163.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA-Cooperative-Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch. Gen. Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Mullins P.G., McGonigle D.J., O'Gorman R.L., Puts N. a J., Vidyasagar R., Evans C.J., Edden R. a E., Brookes M.J., Garcia A., Foerster B.R., Petrou M., Price D., Solanky B.S., Violante I.R., Williams S., Wilson M. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze M., McGarrity S., Mason R., Fone K.C., Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J. Neurosci. 2014;34:7931–7946. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.M., Salmeron B.J., Durgerian S., Janowiak J.A., Fischer M., Risinger R.C., Conant L.L., Stein E.A. Effects of methylphenidate on functional MRI blood-oxygen-level-dependent contrast. Am. J. Psychiatry. 2000;157:1697–1699. doi: 10.1176/appi.ajp.157.10.1697. [DOI] [PubMed] [Google Scholar]
- Schür R.R., Draisma L.W.R., Wijnen J.P., Boks M.P., Koevoets M.G.J.C., Joëls M., Klomp D.W., Kahn R.S., Vinkers C.H. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of 1 H-MRS studies. Hum. Brain Mapp. 2016;00 doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Silveri M.M., Anderson C.M., McNeil J.F., Diaz C.I., Lukas S.E., Mendelson J.H., Renshaw P.F., Kaufman M.J. Oral methylphenidate challenge selectively decreases putaminal T2 in healthy subjects. Drug Alcohol Depend. 2004;76:173–180. doi: 10.1016/j.drugalcdep.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Sotomayor-Zárate R., Araya K.A., Pereira P., Blanco E., Quiroz G., Pozo S., Carreño P., Andrés M.E., Forray M.I., Gysling K. Activation of GABA-B receptors induced by systemic amphetamine abolishes dopamine release in the rat lateral septum. J. Neurochem. 2010;114:1678–1686. doi: 10.1111/j.1471-4159.2010.06877.x. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol. 2011;4:573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterley T.L., Howells F.M., Russell V a. Evidence for reduced tonic levels of GABA in the hippocampus of an animal model of ADHD, the spontaneously hypertensive rat. Brain Res. 2013;1541:52–60. doi: 10.1016/j.brainres.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Tarver J., Daley D., Sayal K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev. 2014;40:762–774. doi: 10.1111/cch.12139. [DOI] [PubMed] [Google Scholar]
- Thomas R., Sanders S., Doust J., Beller E., Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135 doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- Tritsch N.X., Ding J.B., Sabatini B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya C.J., Austin G., Kirkorian G., Ridlehuber H.W., Desmond J.E., Glover G.H., Gabrieli J.D. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage F. van Gorcum; Assen: 1964. Intelligence and Age: Study With Dutch People Aged 12–77. (in Dutch) [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Gatley S.J., Logan J., Ding Y.S., Hitzemann R., Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am. J. Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G., Ding Y., Gatley S.J. Mechanism of action of methylphenidate: insights from PET imaging studies. J. Atten. Disord. 2002;6(Suppl. 1):S31–S43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- Wickens J.R., Hyland B.I., Tripp G. Animal models to guide clinical drug development in ADHD: lost in translation? Br. J. Pharmacol. 2011;164:1107–1128. doi: 10.1111/j.1476-5381.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.B., Chu D. Distribution of GABA, and GABA, receptors in mammalian brain: potential targets for drug development. Drug Dev. Res. 1990;167(21):161–167. [Google Scholar]
- Ziemann U., Lönnecker S., Steinhoff B.J., Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]