Abstract
This study used polysaccharide degrading enzymes and protein precipitation to extract polyphenols from oats and to determine their bioactivity. Duplicate oat brans were treated with viscozyme (Vis), cellulase (Cel) or no enzyme (control, CTL) then, proteins were removed in one set (Vis1, Cel1, CTL1) and not in the other (Vis2, Cel2, CTL2). HPLC analyses showed that for cellulase treated brans, precipitation of proteins increased phenolic acids and avenanthramides by 14%. Meanwhile, a decreased of 67% and 20% respectively was found for viscozyme and control brans. The effect of protein precipitation on soluble polyphenols is therefore dependent of the carbohydrase, as proteins with different compositions will interact differently with other molecules. Radical scavenging data showed that Cel1 and Vis1 had higher quenching effects on ROO• radicals with activities of 22.1 ± 0.8 and 23.5 ± 1.2 μM Trolox Equivalents/g defatted brans. Meanwhile, CTL2 had the highest HO• radicals inhibition (49.4 ± 2.8%) compared to 10.8–32.3% for others. Samples that highly inhibited lipoxygenase (LOX), an enzyme involved in lipid oxidation were Cel1 (23.4 ± 2.3%) and CTL1 (18 ± 0.4%).
Keyword: Food science
1. Introduction
Cereals are known to possess various health benefits that are associated with reduce risks of developing conditions such as coronary heart diseases, stroke, hypertension, diabetes, and certain gastrointestinal disorders [1, 2]. Health-promoting effects of cereals are usually associated with their content of fibres and phenolic compounds [3] but peptides released from proteins are potentially beneficial as well [4]. Polyphenols are important food components and are characterized by their anti-oxidative, anti-atherosclerotic and anti-inflammatory activities in various in vitro and in vivo models [5, 6]. In addition to free radicals, enzymes such lipoxygenases (LOX), which oxidize unsaturated fatty acids (e.g. arachidonic acid and linoleic acid) also contributed to the increase risks of chronic diseases, specifically inflammation. LOX action generates hydroperoxides that may undergo further reactions to produce volatile carbonyls [7, 8]. Molecules that scavenge free radicals or inhibit the activity of LOX may then serve as modulators of the inflammatory process.
Oats (Avena sativa L.) are usually consumed as whole grains and provide the human body with polyphenols, fibres, vitamins, and minerals (Clemens et al., 2014). Their phenolic compounds are mostly located in the bran layer although some are present in groats and hulls [9]. Some phenols present in cereals are ferulic acid, caffeic acid, p-hydroxybenzoic acid, p-hydroxyphenylacetic acid, vanillic acid, protocatechuic acid, syringic acid, p-coumaric acid, sinapic acid, tricin, apigenin, luteolin, kaempferol, and quercetin [5]. In addition, avenanthramides, which consist of anthranilic acid and hydrocinnamic acid derivatives are present only in oats [3]. The consumption of cereal products rich in antioxidants can decrease the risk of developing diabetes and coronary heart diseases via several mechanisms including the reduction of free radicals and inflammation [3, 10]. The vast majority of polyphenols in cereals (up to 80%) are linked to cell wall polysaccharides, proteins or lipids [11] thereby limiting the amount that can be solubilized in aqueous alcohol. Fermentation, polysaccharide degrading enzymes and microwave have been used to increase the extraction yields of soluble polyphenols [12]. Amongst the enzymes, viscozyme is a multi-enzyme complex containing arabanase, beta-glucanase, cellulase, xylanase, and hemicellulose. It has been used in previous studies to breakdown non-starch polysaccharides thereby, reducing the viscosity of cereal slurries and improving the efficiency of separation of phytochemicals or proteins [13, 14]. Cellulase on the other hand breaks cellulose and has been used to enhance the extraction of polyphenols from cereal brans [15]. In the literature, polysaccharide degrading enzymes are used to either enhance the extraction of polyphenols or that of proteins but not for both in the same experiment. A procedure to do so will therefore be important to fully investigate the biochemistry and functionality of these groups molecules. Objectives of this study were to test the use of polysaccharide degrading enzymes with or without protein precipitation on the extraction of polyphenols from oat samples, quantify individual polyphenols, determine antioxidant and lipoxygenase inhibiting activities of the polyphenol-rich extracts.
2. Materials and methods
2.1. Materials and chemicals
Fine oat bran was donated Richardson Milling (Portage la Prairie, MB, Canada). Authentic phenolic acids (trans-cinnamic acid, vanillic acid, p-coumaric acid, ferulic acid), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), rutin trihydrate, mono- and di-basic potassium phosphate, 1,10-phenanthroline, cellulase (100 EGU/g), viscozyme (700 FBG/g), iron (II) sulfate heptahydrate, 30% hydrogen peroxide were purchased from Sigma Aldrich (Oakville, ON, Canada). 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) was obtained from Wako Chemicals (Cape Charles, VA, USA). HPLC-grade methanol, hexane, fluorescein and ethyl acetate were from Fisher Scientific Ltd. (Nepean, ON, Canada). A lipoxygenase inhibitor assay kit was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA).
2.2. Extraction of polyphenols
Forty grams of defatted oat flours were suspended in deionized water at a ratio of 1:10 (w/v). The pH of homogenized slurries was adjusted to 4.5 or 5.5 (Table 1) with 0.1 M HCl. These are values at which viscozyme and cellulase are most active. Polysaccharide degrading enzymes viscozyme L or cellulase was added at a concentration of 3 FBG/g and 20 EGU/g, respectively based on a previous study on their use to extract proteins [13]. A control oat flour containing no enzyme was also made. All slurries were incubated at 45 °C, 190 g for 1 h in a Max Q 8000 model system (Fisher Scientific, Nepean, ON, Canada). The pH was adjusted to 9.5 with 1.0 M NaOH and further incubated at the same temperature for 1.5 h. Supernatants were collected after centrifugation (2500 g, 20 min, 4 °C). At this stage, supernatants from one control and from one of each enzyme treated sample were kept for the extraction of polyphenols. In order to assess the effect of proteins, the pH of samples from the other set was adjusted to 4.0 and centrifuged at 10,000 g (4 °C, 40 min) to precipitate proteins. To obtain polyphenols, the pH of each supernatant was adjusted to 2.0 with 1.0 M HCl followed by partitioning with ethyl acetate (EtOAc, 3 × 400 mL) in separatory funnels. The pooled EtOAc fractions from each sample was filtered through magnesium sulfate, and dried on a rotatory evaporator (Buchi 210 model, VWR Canada, Montreal). Dried samples were recovered with 4 × 1 mL of EtOAc and dried again on a stream of nitrogen gas. Finally, they were reconstituted with 2 mL of 80% methanol, divided into 100 μL aliquots, and stored at −80 °C until further analyses.
Table 1.
Summary of the extraction of phenolic compounds with viscozyme, cellulase, and without enzyme.
| pH | Carbohydrase | Proteins precipitation | Partitioning solvent | Sample code |
|---|---|---|---|---|
| 4.5 | Viscozyme | Yes | Ethyl acetate | Vis-1 |
| 4.5 | Viscozyme | No | Ethyl acetate | Vis-2 |
| 5.5 | Cellulase | Yes | Ethyl acetate | Cel-1 |
| 5.5 | Cellulase | No | Ethyl acetate | Cel-2 |
| 4.5 | None (Control) | Yes | Ethyl acetate | CTL-1 |
| 4.5 | None (Control) | No | Ethyl acetate | CTL-2 |
2.3. Analysis of polyphenols
The total phenolic content (TPC) was determined using the Folin–Ciocalteu reagent according to a previous literature [16]. HPLC was used for the chromatographic analysis of individual polyphenols as described below. Nine hundred microliters of 0.5% acetic acid in methanol were added to each sample (100 μL) of polyphenolic extract and filtered using 0.45 μm polypropylene membrane into HPLC vials. The system was Breeze™ 2 HPLC and included 2707 autosampler, 1525 Binary HPLC pump, 2998 photodiode detector, and Empower 3 chromatography data analysis software from Waters Canada (Montreal, QC). The separation (20 μL) was done using Waters Spherisorb® 5 μm ODS2 4.6 × 150 mm analytical column at a flow rate of 0.80 mL/min. A binary mobile phase of 0.5% acetic acid in water (A) and 0.5% acetic acid in methanol (B) was used to elute the phenolic compounds from the column. The linear gradient conditions were for 0–5 min A:B (100:0), 5–30 min A:B (80:20), 30–35 min A:B (50:50), 35–55 min A:B (20:80), and 55–60 min A:B (0:100). The column was equilibrated for 10 min between injections. Peaks were detected at 280 and 315 nm and were identified by matching retention times of peaks in samples to those of standards and by comparison with literature data [16].
2.4. Oxygen radical absorbance capacity (ORAC) and hydroxyl radical scavenging assays
The antioxidant activity of extracts was determined using two methods. The peroxyl radical scavenging activity was based reported procedures [17]. All samples and standard were prepared in 75 mM potassium phosphate buffer pH 7.4. Phenolic acid extracts were diluted 180 times with buffer (equivalent to 5.56 mg defatted flour/mL) and five concentrations of Trolox (5–100 μM) were used for calibration. In a 96-well black microplate, 120 μL of fluorescein (0.08 μM), 20 μM of sample or standard were added in triplicate and the plate was sealed and incubated for 20 min at 37 °C in the FLx800 fluorimeter. After incubation, 60 μL of AAPH (150 mM) were added to every well and data were recorded at 1 min interval for a total of 50 min. Net area under the curves were used to calculate ORAC values as μM Trolox equivalents (TE)/g defatted flour.
The hydroxyl radical assay was performed based on the Fenton reaction between hydrogen peroxide and ferrous ions [18]. Samples were diluted with potassium phosphate buffer to concentrations of 83.3 mg defatted flour/mL. Hydrogen peroxide, 0.03% was prepared in water. In a 96 well-clear microplate, 50 μL of potassium phosphate buffer were added to the blank and control wells while 50 μL of sample was added to other wells in triplicates. After that, 50 μL of 3 mM 1,10-phenanthroline, 50 μL of 3 mM FeSO4.7H2O in water and 50 μL of 0.03% hydrogen peroxide (H2O2) were added to all wells except for the blank where H2O2 was replaced with water (50 μL). The plate was later sealed with a plastic film and incubated at 37 °C and 150 g for an hour and the absorbance was measured at 536 nm in a BioTek Epoch microplate reader (Fisher Scientific, Nepean, ON, Canada). The scavenging activity of samples were calculated using the following equation:
2.5. Lipoxygenase assay
Lipoxygenase catalyses the oxidation of polyunsaturated fatty acids. The inhibitory activity of extracts was measured using a 15-LOX inhibitor screening kit (Cayman Chemical, Ann Arbor, Michigan, USA) according to the manufacturer’s instructions in a 96 well plate using linoleic acid as a substrate. Preliminary screening showed that 150 times dilution of extracts with methanol was good for the assay. Methanol and nordihydroguaiaretic acid were used as blank and positive control, respectively. The assay plate was read for 10 min (30 sec intervals) at 495 nm in a BioTek Epoch microplate reader (Fisher Scientific, Nepean, ON). The percentage of inhibition of each sample was calculated using the following equation:
2.6. Statistical analysis
The software Sata® SE 14.0 (College Station, Texas 77845 USA) was used for statistical analyses. Comparison was accomplished using one-way analysis of variance (ANOVA) followed by Scheffe’s post-hoc test. Experiments were performed in triplicates and results are expressed as mean ± standard deviation. Statistical significance was set to p < 0.05 and the function Regress was used to investigate relationships between data.
3. Results and discussion
3.1. Analysis of polyphenol extracts
The total phenolic content (TPC) of extracts was determined using the Folin-Ciocalteu reagent while their composition in phenolic acids and avenanthramides (AV) was done using HPLC. Data from the Folin-Ciocalteu assay showed that TPC of cellulase (Cel-1, 98.6 ± 1.9 μM GAE/g) and viscozyme (Vis-1, 113 ± 0.9 μM GAE/g) treated brans were higher (p < 0.05) than the TPC value of control bran (CTL-1, 87.5 ± 1.3 μM GAE/g). However, when proteins were removed by centrifugation those values decreased to 90.6 ± 1.5 and 94.7 ± 1.6 μM GAE/g, respectively in enzyme samples (Cel-2, Vis-2) while increasing to 101.1 ± 1.9 μM GAE/g in the control sample (CLT-2). Several studies have reported that the use of carbohydrases can free proteins or polyphenols that were linked to the cell-wall polysaccharides [13, 19, 20] thereby increasing the extraction yield of these groups of molecules. The increase of TPC is, therefore, in agreement with literature, however, no other study has looked at the combined effect of both carbohydrase and proteins removal on the extraction of polyphenols. Although, the precipitation of proteins reduced TPC in enzyme treated brans, their value was still higher than that of the control bran implying that the procedure was appropriated for the extraction of both polyphenols and proteins from the same starting material.
Individual phenols were identified and quantified by HPLC. Retention times were 18.4 (vanillic acid), 23.7 (p-coumaric), 25.5 (ferulic acid), 33.5 min (cinnamic acid), 36.2 (AV-2c), 38.1 (AV-2p), and 39.5 min (AV-2f). The amount of each molecule is shown in Table 2. Cinnamic acid (0.25–0.82 μg/g) was the least abundant in each sample while most abundant were AV-2f, AV-2p, or ferulic acid. The next least compound was AV-2c and surprisingly it was not detected in the two viscozyme treated brans. It is believed that AV-2c was not solubilized in 100% aqueous environment of this study because, it was detected in a previous work that used the same enzyme and aqueous methanol [16]. The effect of protein removal varied dependent on the initial treatment. HPLC data showed that in brans treated with cellulase, the precipitation of proteins (Cel-1) increased the amount of every identified polyphenols. Meanwhile, when viscozyme was used the precipitation step (Vis-1) decreased by up to 5.2-fold the concentration of phenolic acids and AVs. In contract to cellulase which has only a carbohydrase activity, viscozyme is a multi-complex enzyme with mainly carbohydrase activity but also some protease activity [21]. Despite the decrease of compounds observed during HPLC analysis of Vis-1 sample, its TPC value was the highest and this can be explained by the presence of polyphenols released but not included in the HPLC analysis or by the presence of peptides containing phenol moieties. There was no correlation between TPC values from the Folin-Ciocalteu test and the total of polyphenols measured by HPLC mainly because of the outlier HPLC data from the analysis of Vis-1. In the absence of this outlier value, TPC data significantly correlated (R2 = 0.82) with total HPLC AVs but not with AVs plus free acids. Concentrations of identified phenolic acids are similar to those of a previous study [22] meanwhile, those of AVs are low [16]. In the literature, mixtures of water and alcohols are often used while in this work, 100% water was used for solubilisation before partition with ethyl acetate in order to precipitate proteins in their native forms. This resulted in lower extraction of avenanthramides. Contents of AV-2f and AV-2p are within a range of those reported in oat species and cultivars [23].
Table 2.
Amounts of individual phenolic acids and avenanthramides quantified by HPLC in the defatted oat bran extracts in the presence of carbohydrases (cellulase, viscozyme) and with or without the removal of proteins. Concentrations are μg/g of defatted sample. Values with different letters within the same row indicate significant differences (p < 0.05).
| Phenol | Cellulase1‡ | Cellulase2* | Viscozyme1‡ | Viscozyme2* | Control1‡ | Control2* |
|---|---|---|---|---|---|---|
| VA | 14.33 ± 0.23d | 12.72 ± 0.17b | 6.14 ± 0.02a | 18.59 ± 0.37a | 13.45 ± 0.26c | 13.37 ± 0.20c |
| CA | 0.66 ± 0.01b | 0.64 ± 0.04b | 0.25 ± 0.01a | 0.82 ± 0.09c | 0.79 ± 0.04c | 0.76 ± 0.03c |
| p-CA | 6.88 ± 0.19c | 6.41 ± 0.14b | 4.58 ± 0.02a | 12.90 ± 0.15f | 8.05 ± 0.15e | 7.63 ± 0.20d |
| FA | 22.99 ± 0.27c | 20.70 ± 0.06b | 16.93 ± 0.10a | 37.17 ± 0.50f | 26.24 ± 0.42e | 25.66 ± 0.33d |
| AV2p‖ | 18.17 ± 0.36c | 14.93 ± 0.07b | 3.93 ± 0.01a | 20.33 ± 0.47d | 14.74 ± 0.07b | 25.92 ± 0.11e |
| AV2f‖ | 22.14 ± 0.90e | 19.06 ± 0.17c | 4.39 ± 0.04a | 20.99 ± 0.54d | 15.73 ± 0.15b | 26.26 ± 0.15f |
| AV2c‖ | 1.01 ± 0.14a | 1.63 ± 0.07b | n.d. | n.d. | 2.10 ± 0.15c | 2.67 ± 0.12d |
| Total | 86.16 ± 2.10 | 76.09 ± 0.72 | 36.22 ± 0.21 | 110.80 ± 2.12 | 81.10 ± 1.25 | 102.26 ± 1.14 |
n.d.: not detected. ‡Proteins precipitated before partitioning with ethyl acetate, *No precipitation of proteins, ‖Expressed as μg ferulic acid equivalents/g.
3.2. Antioxidant activities
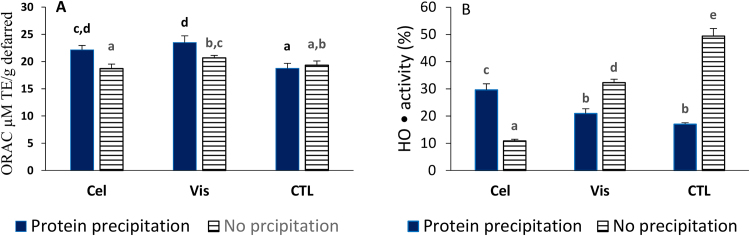
Peroxyl radical (ROO•) and hydroxyl (HO•) radicals are two reactive species commonly produced in foods and biological systems and can cause damages to molecules such as proteins, polypeptides, and DNA [24, 25]. Their scavenging activities were determined using the oxygen radical absorbance capacity (ORAC) assay and the Fenton reaction between hydrogen peroxide and ferrous ions, respectively. In the ORAC assay (Fig. 1A), Vis-1 had the highest activity 23.5 ± 1.2 TE μM/g. Vis-1 also had the highest total phenolic content (TPC) based on Folin-Ciocalteu assay, but the lowest concentration of selected phenolic acids and avenanthramides that were quantified by HPLC. The second most active was Cel-1 (22.1 ± 0.8 μM TE/g). It was found that for both enzyme treated brans, removing proteins increased the ROO• scavenging activity but had not effect on control samples. This showed that the process used in this work could yield proteins as well as phenolic extracts with good peroxyl radical scavenging activities. In contrast to ORAC, the effect of proteins precipitation had different effects on HO• (Fig. 1B). It increased the HO• activity of cellulase but decreased the activity of viscozyme and control brans. Higher HO• inhibitory activities were associated with CTL-2 (49.4 ± 2.9%) and Vis-2 (32.3 ± 1.2%) samples which did not undergo protein precipitation steps followed by Cel-1 in which proteins were precipitated (29.6 ± 2.2%).
Fig. 1.
Oxygen radical absorbance capacity (ORAC, A) and hydroxyl radical (B) scavenging activities of ethyl acetate extracts from defatted oat samples. Values represent means ± STDEV (n = 3). Cel: treated with cellulase; Vis: treated with viscozyme; CTL: control not treated with enzyme. Means sharing same letters are not significantly different from each other at the 0.05 probability level (p > 0.05).
Peroxyl data significantly correlated with TPC values, but hydroxyl data did not because of difference in mechanisms. The ORAC measures both the degree of inhibition and the inhibition time of ROO• while HO• assay measures both direct scavenging and metal chelating ability of a chemical [26, 27]. Differences are also due the oxidative power of the radical and chemical structures of molecules being investigated. The presence of ortho-hydroxyl groups can enhance the activity of polyphenols because of their ability to chelate metal like ferrous ions used in the HO• assay or to donate two protons and transform themselves into non-radical quinones. Two molecules identified in the presence study, vanillic acid and AV-2c had ortho-hydroxyl groups, however their concentrations were low and no relationship with activities was found. Only AV-2p and AV-2f had moderate but non-significant correlations with HO• activities, R2 of 0.50 and 0.34, respectively. ORAC values from this study are lower compared to those reported for medium oat bran flours that were treated with the same enzymes but compounds were solubilized with aqueous methanol and no precipitation of proteins [20].
3.3. Lipoxygenase activity of polyphenol extracts
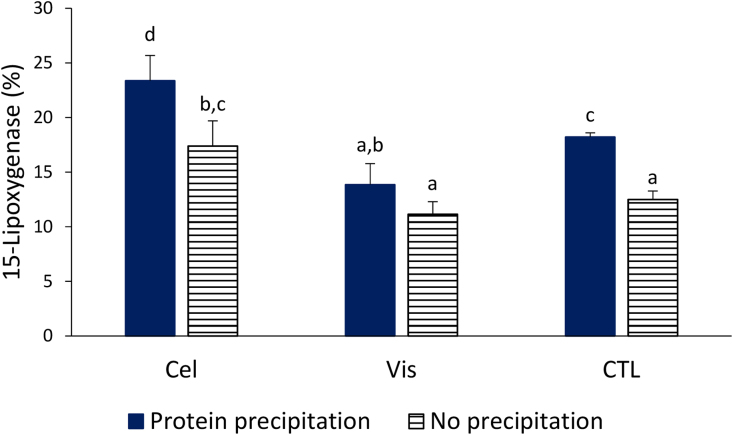
In addition to their ability to quench radicals, polyphenols can affect the activity of enzymes such as lipoxygenase (LOX) and cyclooxygenase that modulate the inflammatory process. LOX oxidized unsaturated fatty acids (e.g. arachidonic and linolenic) to hydroperoxy-eicosatetraenoic acids and leukotrienes both of which are active mediators of many inflammatory events [28, 29]. An increase formation of leukotrienes is associated with asthma, atherosclerosis and other chronic inflammatory diseases [30]. The effect of polyphenol extract to inhibit 15-lipoxygenase (15-LOX) was then assessed and data are presented in Fig. 2. In the assay, an inhibitory activity is manifested by a reduced concentration of hydroperoxyl-eicosatetraenoic acids. It was found that in each of the three pairs of samples, the precipitation of proteins prior to extraction of polyphenols resulted in an increased of inhibitory activity (Fig. 2). The highest activity was due to treatment with cellulase (Cel-1, 23.4 ± 2.3% inhibition) followed by CTL-1 (18.2 ± 0.4% inhibition). LOX assay did not correlate with phenolic contents (TPC, HPLC), peroxyl or hydroxyl radical scavenging data. Mechanisms such as metal chelating and reducing rather than radical scavenging may be involved in the LOX inhibitory properties of polyphenols [31, 32]. Oat phenolic acids have been shown to possess chelating activity [5]. An antioxidant compound can also donate protons to the radical intermediates thereby reducing the formation of lipid hydroperoxides which are needed as substrates for succeeding reactions in the LOX assay [33]. LOX inhibiting properties of polyphenols from other foods have been reported. Grape seed extracts had an IC50 of 13 μg/mL [34] while an extract of oat inhibited ex-vivo the formation of the inflammatory mediator 12-hydroxyeicosatetraenoic acid through LOX pathway [35].
Fig. 2.
Inhibition of lipoxygenase enzyme by polyphenol oat bran extracts. Values represent means ± STDEV (n = 3). Cel: treated with cellulase; Vis: treated with viscozyme; CTL: control not treated with enzyme. Means sharing same letters are not significantly different from each other at the 0.05 probability level (p > 0.05).
Declarations
Author contribution statement
Nisita Ratnasari, Mallory Walters: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Apollinaire Tsopmo: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a grant from Natural Science and Engineering Research Council of Canada (grant No. 371908).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Andreou A., Feussner I. Lipoxygenases − Structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Smith C.E., Tucker K.L. Health benefits of cereal fibre: a review of clinical trials. Nutr. Res. Rev. 2011;24:118–131. doi: 10.1017/S0954422411000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimberg L.H., Gissén C., Nilsson J. Phenolic compounds in oat grains (Avena sativa L.) grown in conventional and organic systems. Ambio. 2005;34:331–337. doi: 10.1639/0044-7447(2005)034[0331:pcioga]2.0.co;2. http://www.ncbi.nlm.nih.gov/ubmed/16092265 (accessed February 7, 2017) [DOI] [PubMed] [Google Scholar]
- 4.McDermott A. Springer Science and Business Media; New York: 2009. Bioactive peptides. [Google Scholar]
- 5.Chen C.Y., Milbury P.E., Kwak H.-K., Collins F.W., Samuel P., Blumberg J.B. Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2004;134:1459–1466. doi: 10.1093/jn/134.6.1459. http://www.ncbi.nlm.nih.gov/pubmed/15173412 (accessed February 8, 2017) [DOI] [PubMed] [Google Scholar]
- 6.Kováčová M., Malinová E. Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes. Czech J. Food Sci. 2007;25:325–332. [Google Scholar]
- 7.Zoia L., Perazzini R., Crestini C., Argyropoulos D.S. Understanding the radical mechanism of lipoxygenases using 31 P NMR spin trapping. Bioorg. Med. Chem. 2011;19:3022–3028. doi: 10.1016/j.bmc.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 8.Duque A.L., Pinto M.D.C., Macias P. Lipoxygenase inhibition by red wine phenolics compounds. J. Food Biochem. 2011;35:542–555. [Google Scholar]
- 9.Gangopadhyay N., Hossain M.B., Rai D.K., Brunton N.P. A review of extraction and analysis of bioactives in oat and barley and scope for use of novel food processing technologies. Molecules. 2015;20:10884–10909. doi: 10.3390/molecules200610884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L., Perret J., Davy B., Wilson J., Melby C.L. Antioxidant Properties of Cereal Products. J. Food Sci. 2002;67:2600–2603. [Google Scholar]
- 11.Adom K.K., Liu R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002;50 doi: 10.1021/jf0205099. [DOI] [PubMed] [Google Scholar]
- 12.Stalikas C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 13.Jodayree S., Smith J.C., Tsopmo A. Use of carbohydrase to enhance protein extraction efficiency and antioxidative properties of oat bran protein hydrolysates. Food Res. Int. 2012;46:69–75. [Google Scholar]
- 14.Alrahmany R., Tsopmo A. Role of carbohydrases on the release of reducing sugar, total phenolics and on antioxidant properties of oat bran. Food Chem. 2012;132:413–418. doi: 10.1016/j.foodchem.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Chen D., Shi J., Hu X. Enhancement of polyphenol content and antioxidant capacity of oat (Avena nuda L.) bran by cellulase treatment. Appl. Biol. Chem. 2016;59 [Google Scholar]
- 16.Hitayezu R., Baakdah M.M., Kinnin J., Henderson K., Tsopmo A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal Sci. 2015;63:35–40. [Google Scholar]
- 17.Yilmaz Y., Toledo R.T. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J. Food Compos. Anal. 2006;19:41–48. [Google Scholar]
- 18.Vanvi A., Tsopmo A. Pepsin digested oat bran proteins: Separation, antioxidant activity, and identification of new peptides. J. Chem. 2016;2016 [Google Scholar]
- 19.Kapasakalidis P.G., Rastall R.A., Gordon M.H. Effect of a cellulase treatment on extraction of antioxidant phenols from black currant (Ribes nigrum L.) pomace. J. Agric. Food Chem. 2009;57:4342–4351. doi: 10.1021/jf8029176. [DOI] [PubMed] [Google Scholar]
- 20.Alrahmany R., Tsopmo A. Role of carbohydrases on the release of reducing sugar, total phenolics and on antioxidant properties of oat bran. Food Chem. 2012;132:413–418. doi: 10.1016/j.foodchem.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.-M., Lim S.-T. Enhanced antioxidant activity of rice bran extract by carbohydrase treatment. J. Cereal Sci. 2016;68:116–121. [Google Scholar]
- 22.Alrahmany R., Avis T.J., Tsopmo A. Treatment of oat bran with carbohydrases increases soluble phenolic acid content and influences antioxidant and antimicrobial activities. Food Res. Int. 2013;52:568–574. http://dx.doi.org/10.1016/j.foodres.2013.03.037 [Google Scholar]
- 23.Zhu Y., Li T., Fu X., Abbasi A.M., Zheng B., Liu R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.) J. Funct. Foods. 2015;19:439–450. [Google Scholar]
- 24.Khan R.A., Khan M.R., Sahreen S., Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J. 2012;6:12. doi: 10.1186/1752-153X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaguti M., Dinelli G., Leoncini E., Bregola V., Bosi S., Cicero A.F.G., Hrelia S. Bioactive peptides in cereals and legumes: agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014;15:21120–21135. doi: 10.3390/ijms151121120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie K.M., Chae J.M., Ainsworth E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007;2:867–870. doi: 10.1038/nprot.2007.100. [DOI] [PubMed] [Google Scholar]
- 27.Dávalos A., Gómez-Cordovés C., Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 28.Gawlik-Dziki U. Dietary spices as a natural effectors of lipoxygenase, xanthine oxidase, peroxidase and antioxidant agents. LWT - Food Sci. Technol. 2012;47:138–146. [Google Scholar]
- 29.Alitonou G., Avlessi F., Sohounhloue D., Agnaniet H., Bessiere J., Menut C. Investigations on the essential oil of Cymbopogon giganteus from Benin for its potential use as an anti-inflammatory agent. Int. J. Aromather. 2006;16:37–41. [Google Scholar]
- 30.Wisastra R., Dekker F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers (Basel) 2014;6 doi: 10.3390/cancers6031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahesha H.G., Singh S.A., Rao A.G.A. Inhibition of lipoxygenase by soy isoflavones: Evidence of isoflavones as redox inhibitors. Arch. Biochem. Biophys. 2007;461 doi: 10.1016/j.abb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Sadik C.D., Sies H., Schewe T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003;65 doi: 10.1016/s0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 33.Rackova L., Oblozinsky M., Kostalova D., Kettmann V., Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J. Inflamm. 2007;4 doi: 10.1186/1476-9255-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leifert W.R., Abeywardena M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008;28:842–850. doi: 10.1016/j.nutres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S., Gul S., Gul H., Bangash M.H. Anti-inflammatory and anti-platelet activities of Avena sativa are mediated through the inhibition of cyclooxygenase and lipoxygenase enzymes. Int. J. Endorsing Heal. Sci. 2013;1:62–65. http://applications.emro.who.int/imemrf/Int_J_Endorsing_Health_Sci_Res/Int_J_Endorsing_Health_Sci_Res_2013_1_2_62_65.pdf (accessed February 8, 2017) [Google Scholar]