Abstract
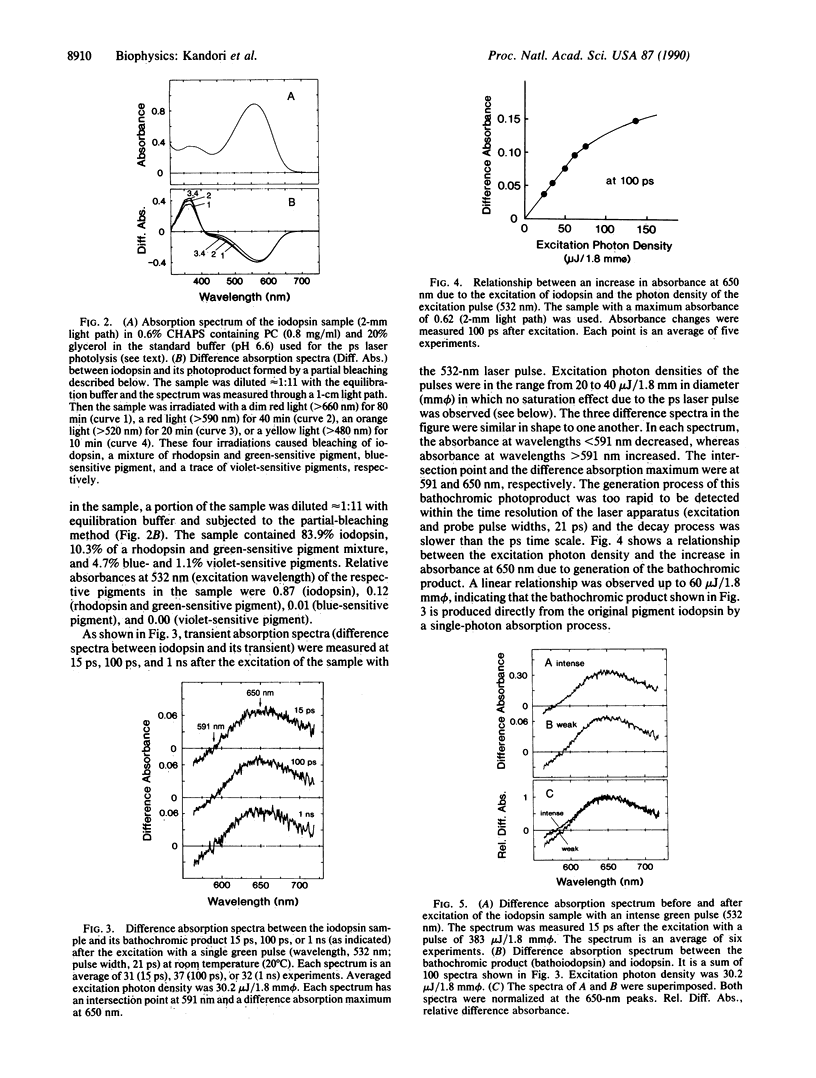
Measurement of the primary photochemical reaction of iodopsin, a chicken red-sensitive cone visual pigment, was carried out at room temperature by using picosecond (ps) laser photolysis. Excitation of iodopsin with a ps green pulse (pulse width, 21 ps) caused the instantaneous formation of a bathochromic product, which was stable on a ps time scale. This product may correspond to "bathoiodopsin," which was detected by low-temperature spectrophotometry. Although bathoiodopsin produced at the temperature of liquid nitrogen or helium reverted to the original pigment (iodopsin) on warming (above -170 degrees C), the bathoiodopsin produced at physiological temperature decayed to all-trans-retinal and R-photopsin (the protein moiety of iodopsin) presumably through several intermediates. The absorption maximum of bathoiodopsin at room temperature was at 625 nm, a wave-length slightly shorter than that measured at low temperature (lambda max, 640 nm). The extinction coefficient of bathoiodopsin at room temperature was lower than that at low temperature and close to that of the original iodopsin at room temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN P. K., WALD G. VISUAL PIGMENTS IN SINGLE RODS AND CONES OF THE HUMAN RETINA. DIRECT MEASUREMENTS REVEAL MECHANISMS OF HUMAN NIGHT AND COLOR VISION. Science. 1964 Apr 3;144(3614):45–52. doi: 10.1126/science.144.3614.45. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. Electrical signaling in vertebrate photoreceptors. Methods Enzymol. 1982;81:403–423. doi: 10.1016/s0076-6879(82)81058-6. [DOI] [PubMed] [Google Scholar]
- Chen J. G., Nakamura T., Ebrey T. G., Ok H., Konno K., Derguini F., Nakanishi K., Honig B. Wavelength regulation in iodopsin, a cone pigment. Biophys J. 1989 Apr;55(4):725–729. doi: 10.1016/S0006-3495(89)82871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fager L. Y., Fager R. S. Halide control of color of the chicken cone pigment iodopsin. Exp Eye Res. 1979 Oct;29(4):401–408. doi: 10.1016/0014-4835(79)90056-3. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Okano T., Shichida Y., Yoshizawa T., Trehan A., Mead D., Denny M., Asato A. E., Liu R. S. Comparative study on the chromophore binding sites of rod and red-sensitive cone visual pigments by use of synthetic retinal isomers and analogues. Biochemistry. 1990 Mar 27;29(12):3133–3140. doi: 10.1021/bi00464a033. [DOI] [PubMed] [Google Scholar]
- HUBBARD R., KROPF A. Chicken lumiand meta-iodopsin. Nature. 1959 Feb 14;183(4659):448–450. [PubMed] [Google Scholar]
- Horiuchi S., Yoshizawa T., Tsukamoto Y. Photic reactions of chicken iodopsin and rhodopsin at liquid helium temperature. Vision Res. 1975 Jul;15(7):819–823. doi: 10.1016/0042-6989(75)90260-6. [DOI] [PubMed] [Google Scholar]
- Imamoto Y., Kandori H., Okano T., Fukada Y., Shichida Y., Yoshizawa T. Effect of chloride ion on the thermal decay process of the batho intermediate of iodopsin at low temperature. Biochemistry. 1989 Nov 28;28(24):9412–9416. doi: 10.1021/bi00450a025. [DOI] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Nagai H., Shichida Y., Yoshizawa T. Dependency of apparent relative quantum yield of isorhodopsin to rhodopsin on the photon density of picosecond laser pulse. Photochem Photobiol. 1988 Jul;48(1):93–97. doi: 10.1111/j.1751-1097.1988.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Shichida Y., Yoshizawa T. Dependency of photon density on primary process of cattle rhodopsin. Photochem Photobiol. 1989 Feb;49(2):181–184. doi: 10.1111/j.1751-1097.1989.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Kandori H., Matuoka S., Shichida Y., Yoshizawa T., Ito M., Tsukida K., Balogh-Nair V., Nakanishi K. Mechanism of isomerization of rhodopsin studied by use of 11-cis-locked rhodopsin analogues excited with a picosecond laser pulse. Biochemistry. 1989 Jul 25;28(15):6460–6467. doi: 10.1021/bi00441a045. [DOI] [PubMed] [Google Scholar]
- Kandori H., Shichida Y., Yoshizawa T. Absolute absorption spectra of batho- and photorhodopsins at room temperature. Picosecond laser photolysis of rhodopsin in polyacrylamide. Biophys J. 1989 Sep;56(3):453–457. doi: 10.1016/S0006-3495(89)82692-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles A. The effects of chloride ion upon chicken visual pigments. Biochem Biophys Res Commun. 1976 Nov 8;73(1):56–62. doi: 10.1016/0006-291x(76)90496-4. [DOI] [PubMed] [Google Scholar]
- Okano T., Fukada Y., Artamonov I. D., Yoshizawa T. Purification of cone visual pigments from chicken retina. Biochemistry. 1989 Oct 31;28(22):8848–8856. doi: 10.1021/bi00448a025. [DOI] [PubMed] [Google Scholar]
- Shichida Y., Kato T., Sasayama S., Fukada Y., Yoshizawa T. Effects of chloride on chicken iodopsin and the chromophore transfer reactions from iodopsin to scotopsin and B-photopsin. Biochemistry. 1990 Jun 19;29(24):5843–5848. doi: 10.1021/bi00476a028. [DOI] [PubMed] [Google Scholar]
- Spalink J. D., Reynolds A. H., Rentzepis P. M., Sperling W., Applebury M. L. Bathorhodopsin intermediates from 11-cis-rhodopsin and 9-cis-rhodopsin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1887–1891. doi: 10.1073/pnas.80.7.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Wald G. Photochemistry of lodopsin. Nature. 1967 May 6;214(5088):566–571. doi: 10.1038/214566a0. [DOI] [PubMed] [Google Scholar]



