Abstract
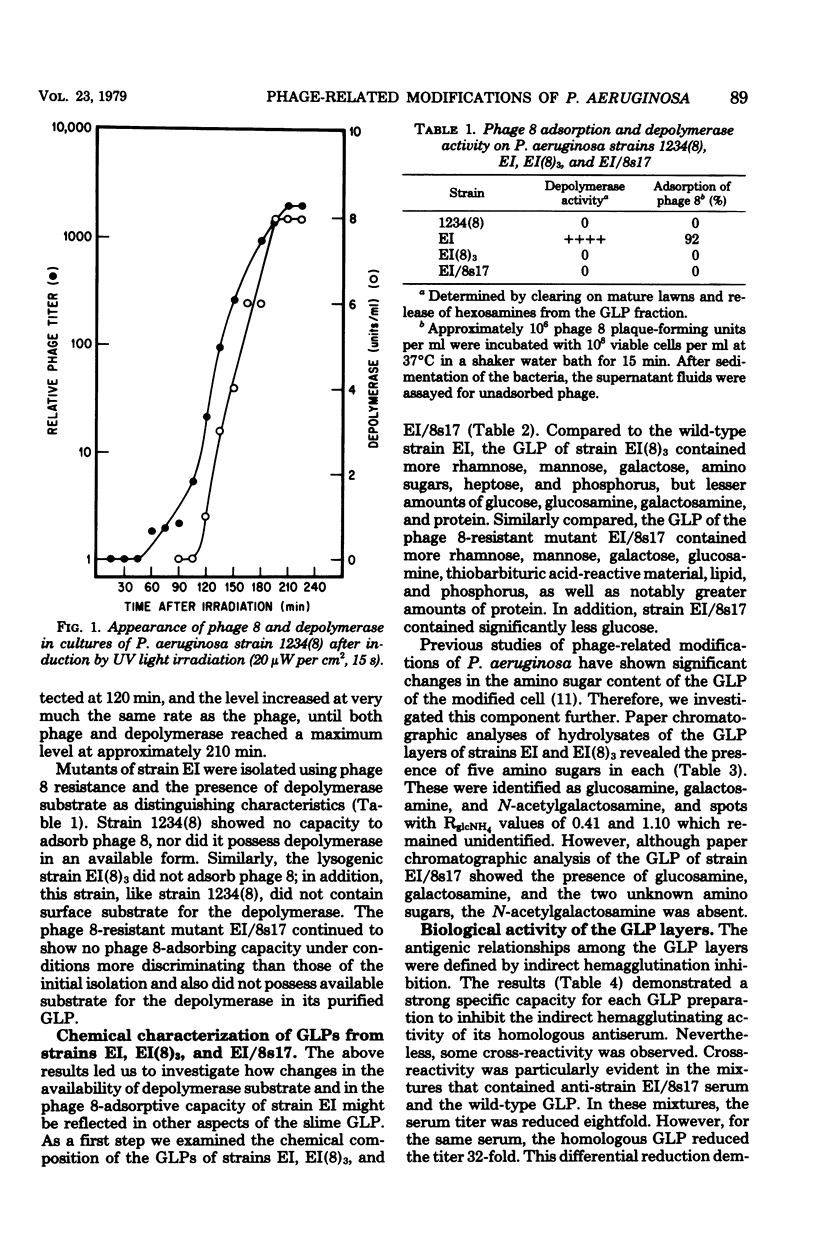
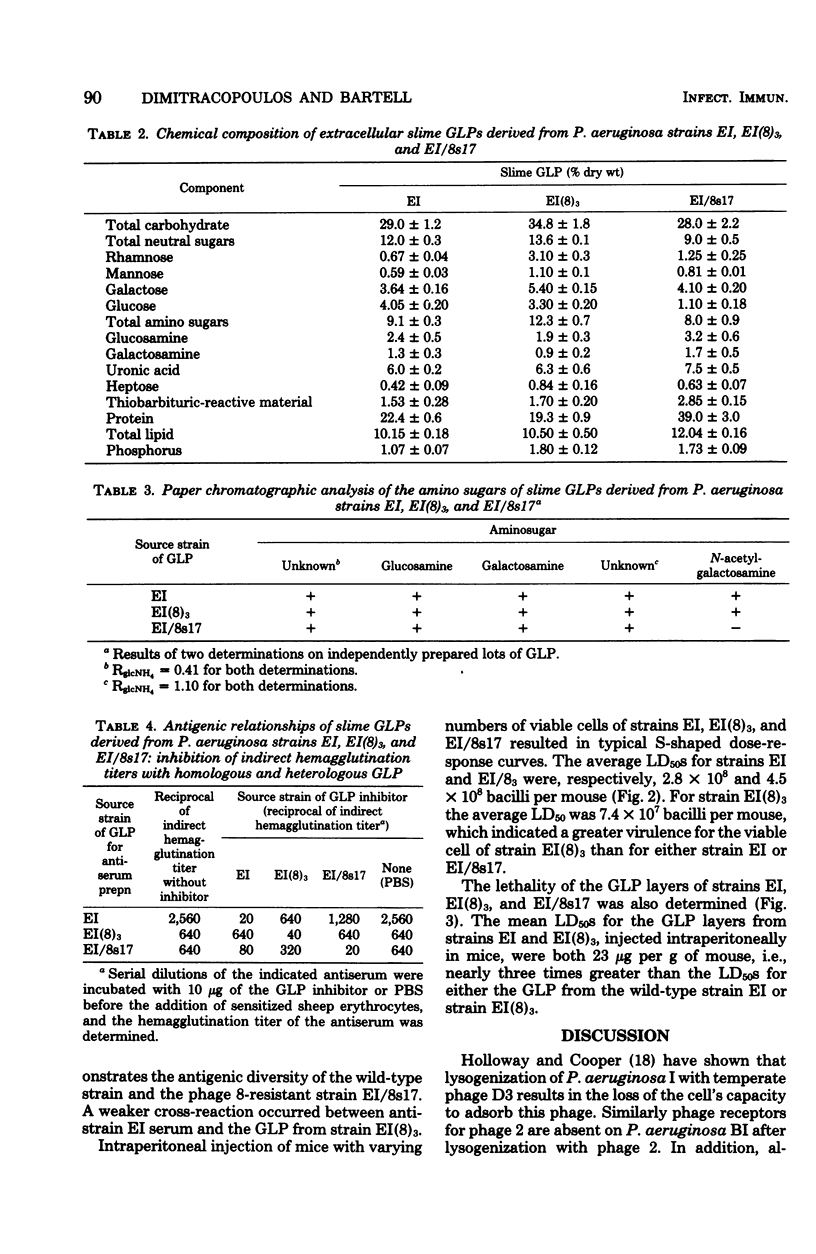
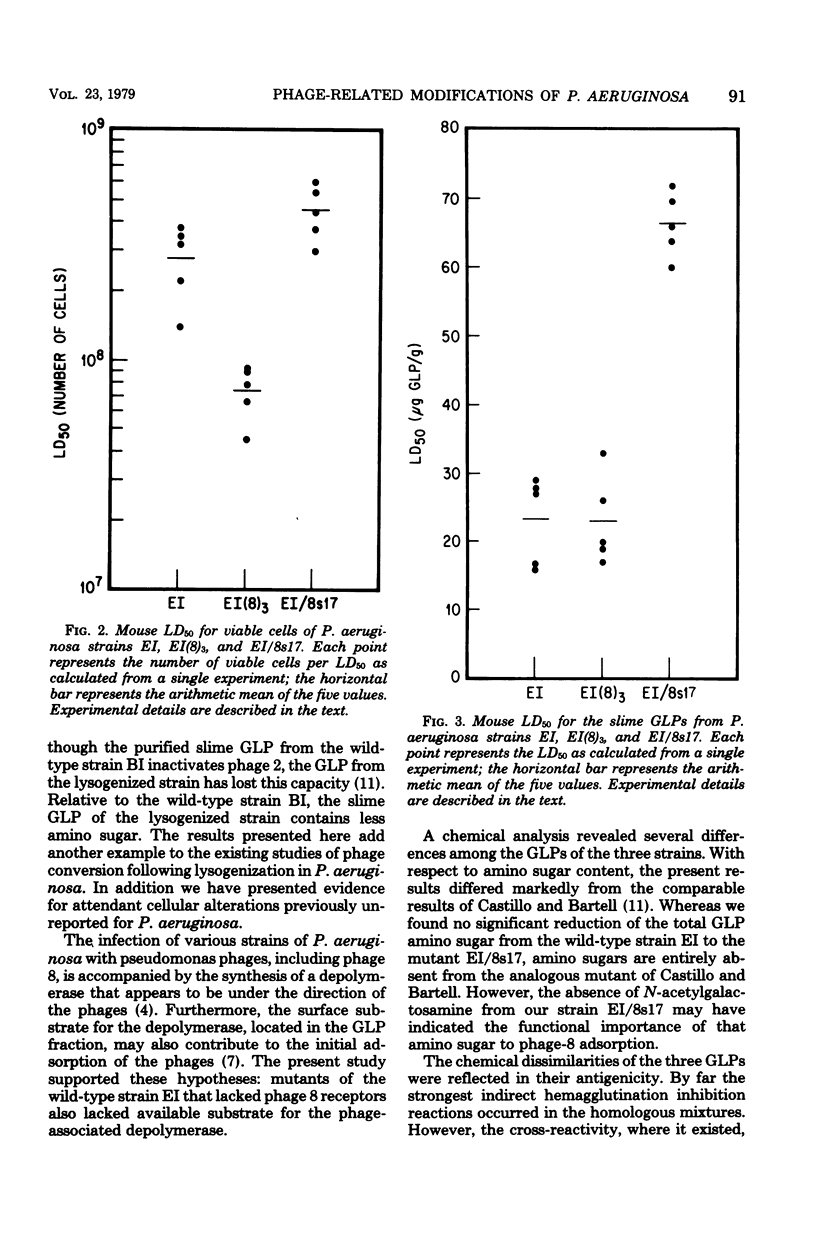
Lysogenic [EI(8)3] and phage 8-resistant mutant (EI/8S17) strains of Pseudomonas aeruginosa EI were isolated. Besides lacking the capacity to adsorb phage 8, strains EI(8)3 and EI/8s17 did not contain surface substrate for the depolymerase that is produced de novo when phage 8 infects wild-type strain. EI. The glycolipoprotein (GLP) in the wild type contains phage 8 receptors and surface substrate for the depolymerase, as well as possesses characteristics of a virulence factor; therefore, the chemical and biological characteristics of the derived strains were investigated. The neutral-sugar, amino sugar, and protein content of the GLPs from the derived strains differed quantitatively from that of the wild type. In spite of some cross-reactivity, the GLPs from all strains were antigenically distinct in the indirect hemagglutination inhibition test. In mice, the toxicity of the GLP from strain EI(8)3 equaled that of the wild type, but the GLP of strain EI/8s17 was threefold less toxic. Significantly fewer viable EI(8)3 cells were required for the mouse 50% lethal dose than for the cells of either the wild type or the phage-resistant mutant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Chudio B. Purification and Chemical Composition of the Protective Slime Antigen of Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E. Distinct slime polysaccharide depolymerases of bacteriophage-infected Pseudomonas aeruginosa: evidence of close association with the structured bacteriophage particle. J Virol. 1969 Nov;4(5):580–584. doi: 10.1128/jvi.4.5.580-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Reese J. F., Imaeda T. Interaction of Pseudomonas bacteriophage 2 with the slime polysaccharide and lipopolysaccharide of Pseudomonas aeruginosa strain B1. J Virol. 1971 Sep;8(3):311–317. doi: 10.1128/jvi.8.3.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Midtvedt T. Epidemiological markers for pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):1–9. doi: 10.1111/j.1699-0463.1975.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Castillo F. J., Bartell P. F. Studies on the bacteriophage 2 receptors of Pseudomonas aeruginosa. J Virol. 1974 Oct;14(4):904–909. doi: 10.1128/jvi.14.4.904-909.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C. The distribution of 2-keto-3-deoxy-octonic acid in bacterial walls. J Gen Microbiol. 1970 Mar;60(3):373–380. doi: 10.1099/00221287-60-3-373. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Levin R. H., Bodey G. P., Morse E. E., Freireich E. J. The nature and control of infections in patients with acute leukemia. Cancer Res. 1965 Oct;25(9):1511–1515. [PubMed] [Google Scholar]
- HOLLOWAY B. W., COOPER G. N. Lysogenic conversion in Pseudomonas aeruginosa. J Bacteriol. 1962 Dec;84:1321–1324. doi: 10.1128/jb.84.6.1321-1324.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IACOCCA V. F., SIBINGA M., BARBERO G. J. RESPIRATORY TRACT BACTERIOLOGY IN CYSTIC FIBROSIS. Am J Dis Child. 1963 Sep;106:315–324. doi: 10.1001/archpedi.1963.02080050317012. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee Y. C., McKelvy J. F., Lang D. Rapid automatic analysis of sugar components of glycoproteins. II. Neutral sugars. Anal Biochem. 1969 Mar;27(3):567–574. doi: 10.1016/0003-2697(69)90071-2. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Changes in somatic antigens of Pseudomonas aeruginosa induced by bacteriophages. J Infect Dis. 1969 Mar;119(3):237–246. doi: 10.1093/infdis/119.3.237. [DOI] [PubMed] [Google Scholar]
- Lynn M., Sensakovic J. W., Bartell P. F. In vivo distribution of Pseudomonas aeruginosa slime glycolipoprotein: association with leukocytes. Infect Immun. 1977 Jan;15(1):109–114. doi: 10.1128/iai.15.1.109-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lányi B., Lantos J. Antigenic changes in Pseudomonas aeruginosa in vivo and after lysogenization in vitro. Acta Microbiol Acad Sci Hung. 1976;23(4):337–351. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- RABIN E. R., GRABER C. D., VOGEL E. H., Jr, FINKELSTEIN R. A., TUMBUSCH W. A. Fatal pseudomonas infection in burned patients. A clinical, bacteriologic and anatomic study. N Engl J Med. 1961 Dec 21;265:1225–1231. doi: 10.1056/NEJM196112212652501. [DOI] [PubMed] [Google Scholar]
- ROBBINS P. W., UCHIDA T. Studies on the chemical basis of the phage conversion of O-antigens in the E-group Salmonellae. Biochemistry. 1962 Mar;1:323–335. doi: 10.1021/bi00908a020. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Glycolipoprotein from Pseudomonas aeruginosa as a protective antigen against P. aeruginosa infection in mice. Infect Immun. 1977 Nov;18(2):304–309. doi: 10.1128/iai.18.2.304-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z. The effect of virulence of converting the O antigen of Salmonella cholerae-suis from 627 to 617 by phage. J Gen Microbiol. 1974 Mar;81(1):217–224. doi: 10.1099/00221287-81-1-217. [DOI] [PubMed] [Google Scholar]



