Abstract
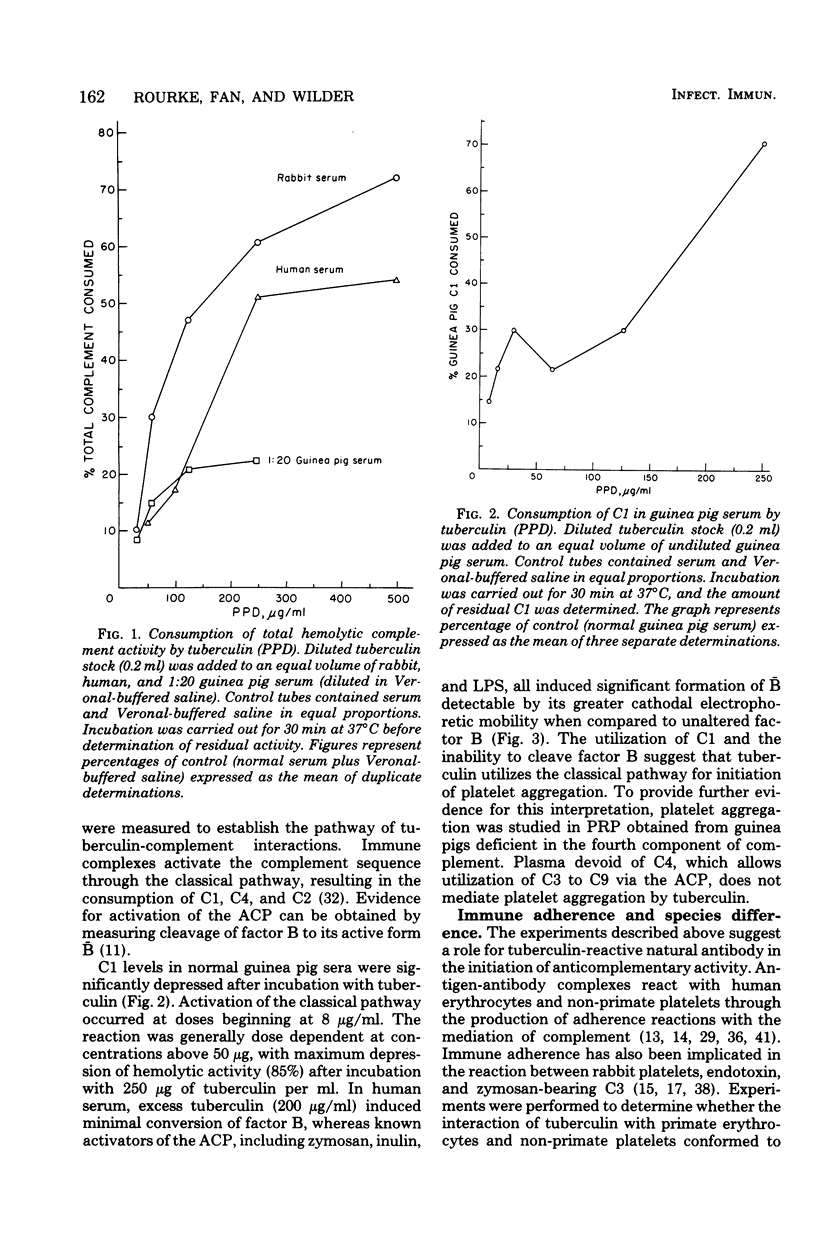
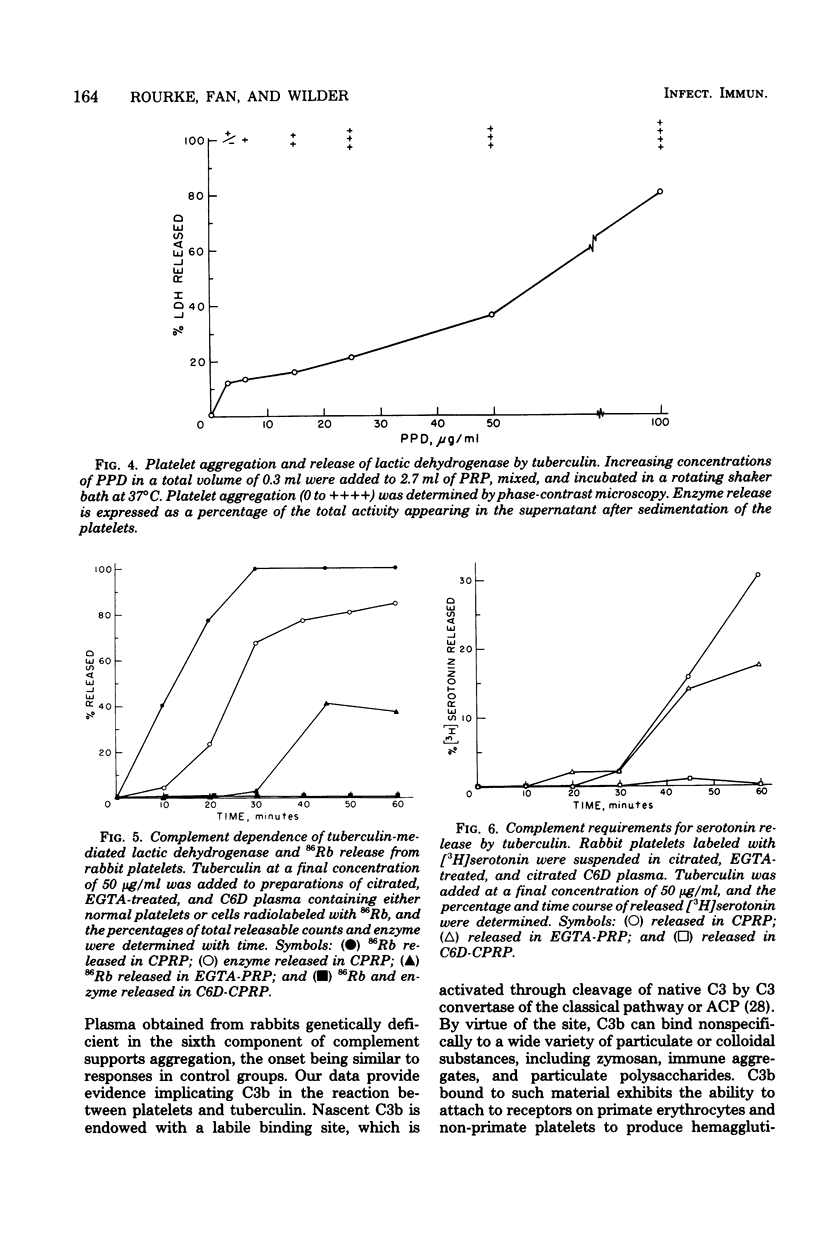
Experiments were performed to examine the interaction of tuberculin with platelets and complement. Hemolytic complement titrations show that tuberculin consumes complement in human, rabbit, and guinea pig serum. Evidence in support of classical pathway activation was provided by observation of C1 consumption and failure to detect significant conversion of alternative pathway factor B to B by immunoelectrophoresis. Platelets in plasma from guinea pigs deficient in the fourth component of complement were not affected by tuberculin. However, studies on platelet aggregation in plasma chelated with ethyleneglycolbis(beta-aminoethyl ether)-N,N-tetraacetic acid indicated that tuberculin may initiate sluggish activation of the alternative pathway. That the reaction between tuberculin and platelets is a lytic one was evidenced by observing the release of the cytoplasmic enzyme lactic dehydrogenase and efflux of rubidium-86. Studies with C6-deficient rabbits indicated that platelet release of exogenously supplied tritiated serotonin is caused by platelet lysis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babson A. L., Phillips G. E. A rapid colorimetric assay for serum lactic dehydrogenase. Clin Chim Acta. 1965 Aug;12(2):210–215. doi: 10.1016/0009-8981(65)90032-x. [DOI] [PubMed] [Google Scholar]
- Bardana E. J., Jr, McClatchy J. K., Farr R. S., Minden P. Universal occurrence of antibodies to tubercle bacilli in sera from non-tuberculous and tuberculous individuals. Clin Exp Immunol. 1973 Jan;13(1):65–77. [PMC free article] [PubMed] [Google Scholar]
- DES PREZ R. M., HOROWITZ H. I., HOOK E. W. Effects of bacterial endotoxin on rabbit platelets. I. Platelet aggregation and release of platelet factors in vitro. J Exp Med. 1961 Dec 1;114:857–874. doi: 10.1084/jem.114.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. B. Electron microscopic changes in blood platelets induced by bacterial lipopolysaccharide. Exp Mol Pathol. 1966 Dec;5(6):559–574. doi: 10.1016/0014-4800(66)90046-3. [DOI] [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O. Interaction of lipopolysaccharides and lipid A with complement. Eur J Biochem. 1971 Mar 1;19(1):143–152. doi: 10.1111/j.1432-1033.1971.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. Lysis of erythrocytes by complement in the absence of antibody. J Exp Med. 1970 Nov;132(5):898–915. doi: 10.1084/jem.132.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Hawiger J., Marney S. R., Jr, Colley D. G., Des Prez R. M. Complement-dependent platelet injury by staphylococcal protein A. J Exp Med. 1972 Jul 1;136(1):68–80. doi: 10.1084/jem.136.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Cochrane C. G. Immunological induction of increased vascular permeability. II. Two mechanisms of histamine release from rabbit platelets involving complement. J Exp Med. 1969 Jan 1;129(1):167–184. doi: 10.1084/jem.129.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. Complement-dependent platelet and polymorphonuclear leukocyte reactions. Transplant Proc. 1974 Mar;6(1):27–31. [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of release of constituents from rabbit platelets by antigen-antibody complexes and complement. I. Lytic and nonlytic reactions. J Immunol. 1970 Aug;105(2):476–489. [PubMed] [Google Scholar]
- Henson P. M. The adherence of leucocytes and platelets induced by fixed IgG antibody or complement. Immunology. 1969 Jan;16(1):107–121. [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Stormorken H. The blood platelet release reaction. Scand J Haematol Suppl. 1969;8:3–26. [PubMed] [Google Scholar]
- Kravis T. C., Henson P. M. IgE-induced release of a platelet-activating factor from rabbit lung. J Immunol. 1975 Dec;115(6):1677–1681. [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- Marx J. J., Flaherty D. K. Activation of the complement sequence by extracts of bacteria and fungi associated with hypersensitivity pneumonitis. J Allergy Clin Immunol. 1976 Apr;57(4):328–334. doi: 10.1016/0091-6749(76)90089-0. [DOI] [PubMed] [Google Scholar]
- Mergenhagen S. E., Snyderman R., Gewurz H., Shin H. S. Significance of complement to the mechanism of action of endotoxin. Curr Top Microbiol Immunol. 1969;50:37–77. doi: 10.1007/978-3-642-46169-9_2. [DOI] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Cooper R., Bardana E. J., Jr, Farr R. S. Shared antigens between Mycobacterium bovis (BCG) and other bacterial species. Science. 1972 Apr 7;176(4030):57–58. doi: 10.1126/science.176.4030.57. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F., Oades Z. G., Henson P. M. Mechanisms of lipopolysaccharide-initiated rabbit platelet responses: alternative complement pathway dependence of the lytic response. Infect Immun. 1978 Jun;20(3):744–751. doi: 10.1128/iai.20.3.744-751.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllerèberhard H. J., Dalmasso A. P., Calcott M. A. The reaction mechanism of beta-1C-globulin (C'3) in immune hemolysis. J Exp Med. 1966 Jan 1;123(1):33–54. doi: 10.1084/jem.123.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nicholson A., Brade V., Lee G. D., Shin H. S., Mayer M. M. Kinetic studies of the formation of the properdin system enzymes on zymosan: evidence that nascent C3b controls the rate of assembly. J Immunol. 1974 Mar;112(3):1115–1123. [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man. I. N Engl J Med. 1972 Sep 7;287(10):489–495. doi: 10.1056/NEJM197209072871005. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Secchi A. G., Osler A. G. The allergic response of rabbit platelets. I. Membrane permeability changes. J Immunol. 1968 Dec;101(6):1130–1139. [PubMed] [Google Scholar]
- Spielvogel A. R. An ultrastructural study of the mechanisms of platelet-endotoxin interaction. J Exp Med. 1967 Aug 1;126(2):235–250. doi: 10.1084/jem.126.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem P. Platelet count by phase contrast microscopy--new diluting fluid for better visualization. Scand J Clin Lab Invest. 1974 Apr;33(2):121–123. doi: 10.1080/00365517409082478. [DOI] [PubMed] [Google Scholar]
- TURK J. L. Immune-adherence with soluble antigens. Immunology. 1958 Oct;1(4):305–314. [PMC free article] [PubMed] [Google Scholar]
- Tauber J. W., Polley M. J., Zabriskie J. B. Nonspecific complement activation by streptococcal structures. II. Properdin-independent initiation of the alternate pathway. J Exp Med. 1976 Jun 1;143(6):1352–1366. doi: 10.1084/jem.143.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Origin and function of platelet dense bodies. Ser Haematol. 1970;3(4):17–46. [PubMed] [Google Scholar]
- Wilder M. S., Fan S. S. Rabbit platelet aggregation by tuberculin. Proc Soc Exp Biol Med. 1976 Oct;153(1):57–62. doi: 10.3181/00379727-153-39480. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Bocchini J. A., Jr, Schiffman G. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J Immunol. 1976 Feb;116(2):367–370. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative pathway by pneumococcal cell walls. J Immunol. 1977 Feb;118(2):451–454. [PubMed] [Google Scholar]