Abstract
Berberine is a widely used plant extract for gastrointestinal infections, and is reported to have potential benefits in treatment for diabetes and hypercholesterolemia. It has been suggested that interactions between berberine-containing products and cytochromes P450 (CYPs) exist, but little is known about which CYPs mediate the metabolism of berberine in vivo.
In this study, berberine metabolites in urine and feces of mice were analyzed, and the role that CYPs play in producing these metabolites were characterized in liver microsomes from mice (MLM) and humans (HLM), as well as recombinant human CYPs. Eleven berberine metabolites were identified in mice, including 5 unconjugated metabolites, mainly in feces, and 6 glucuronide and sulfate conjugates, predominantly in urine. Three novel berberine metabolites were observed. Three unconjugated metabolites of berberine were produced by MLM, HLM, and recombinant human CYPs. CYP2D6 was the primary recombinant human CYP producing these metabolites, followed by CYP1A2, 3A4, 2E1 and CYP2C19. The metabolism of berberine in MLM and HLM was decreased the most by a CYP2D inhibitor, and moderately by inhibitors of CYP1A and 3A.
CYP2D plays a major role in berberine biotransformation, therefore, CYP2D6 pharmacogenetics and potential drug-drug interactions should be considered when berberine is used.
Keywords: Berberine, liver, metabolism, mice, humans
Introduction
Berberine is a major isoquinoline alkaloid in herbs such as goldenseal, berberis, and Coptis chinensis, and has been used for about 1,000 years to treat gastrointestinal diarrhea (Lahiri and Dutta, 1967; Amin et al., 1969). Presently, berberine is widely used in oriental countries for diabetes, hypercholesterolemia, and other medical conditions (Vuddanda et al., 2010). Goldenseal, a main source of berberine (Abourashed and Khan, 2001), is ranked as the sixth most commonly used herbal supplement for children in America (Barnes et al., 2008).
Many studies have demonstrated effects of berberine in mice. In db/db mice, berberine was reported to increase insulin/Insulin-like growth factor 1 signaling, block muscle protein losses (Wang et al., 2010), reduce body weight, and improve glucose tolerance without altering food intake (Lee et al., 2006). It also activates AMP-activated protein kinase and improves insulin sensitivity in insulin resistant C57BL mice (Turner et al., 2008). However, biotransformation of berberine in mice has not been studied.
The disposition of berberine had been studied in humans and rats. After oral administration of 400 mg berberine to healthy male subjects, the Cmax in plasma was 0.4 ng/ml, tmax was 9.8 h, and t1/2 was 28 h (Hua et al., 2007), which suggests that berberine has a low systemic bioavailability and long half-life in humans. When berberine was given orally to rats, about half of the compound was absorbed by the gastrointestinal tract and was distributed to the liver (Liu et al., 2010). Metabolites of berberine in urine of humans and rats reveal a similar metabolite profile in the two species (Qiu et al., 2008), but metabolites in feces have not been examined.
The AUC0-limt and Cmax of berberine metabolites were 10- to 50-fold higher than those of the parent compound in plasma and liver of rats (Zuo et al., 2006). Therefore, extensive metabolism in liver appears to be an important reason for the low plasma concentration of berberine. It is suggested that the cytochrome P450 (CYP) superfamily of enzymes play important roles in berberine metabolism. The CYP superfamily is the major drug-metabolizing system in liver and intestine, and is involved in the elimination of more than 70–80% of clinically used drugs (Evans and Relling, 1999). Drug-drug interactions, which are caused mainly by co-administrated drugs that induce or inhibit CYPs, have been estimated to account for about 250,000 hospital admissions and about 1.3 billion dollars of health care costs per year (Sandson, 2005).
It had been reported that SKF-525A (proadifen), a CYP inhibitor, decreases the metabolism of berberine in rats (Tsai and Tsai, 2004). CYP3A1/2 and CYP2B have been suggested to metabolize berberine in liver of rats, but limited information is known about the specific CYP producing each berberine metabolite in human liver. In the current study, metabolites of berberine in urine and feces of mice have been proposed and compared with data in other species, and the roles that CYPs play in the metabolism of berberine have been characterized in MLM, HLM, and recombinant human CYPs.
Materials and Methods
Chemicals and Reagents
Recombinant human CYPs and HLM were obtained from XenoTech (Lenexa, KS, USA). berberine chloride and all other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and Treatments
Male C57BL/6 mice (22±2g, 8-weeks old) were obtained from Charles River Laboratories, Inc. (Wilmington, MA). All mice were maintained under a standard 12-h dark and 12-h light cycle with water and chow provided ad libitum. Rodent studies were in accordance with protocols approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
For the metabolomic studies, berberine (10 mg/kg) or saline was injected i.p. in mice, and the mice (four per group) were housed in separate metabolic cages for 24 h. Urine and fecal samples were collected and stored at −80°C for further analysis. Pooled MLM were prepared by differential-ultracentrifugation from livers of non-treated C57BL/6 mice (http://www.currentprotocols.com/protocol/ph0708).
Metabolism of Berberine by Microsomes of Mice and Humans as well as Recombinant Human CYPs
Incubation time as well as berberine and protein concentrations were varied to find a linear kinetic range. All the In vitro incubations were performed in duplicate. Each incubation reaction was performed in 1× phosphate-buffered saline (PBS, pH 7.4), containing 1 or 10 μM berberine and 0.1 mg HLM, MLM, or 2 pmol of each recombinant human CYP including control, CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and CYP3A4. The final volume was 200 μl. After 5 min of pre-incubation at 37°C, the reaction was initiated by adding 10 μl of 20 mM NADPH (final concentration 1.0 mM) or PBS, and shaken gently for another 45 min. All reactions were terminated by adding 200 μl of ice-cold acetonitrile.
Chemical Inhibition Analysis in HLM and MLM
Paroxetine (0, 1, 10 μM) was used as an inhibitor for CYP2D6 (Cyp2d), ketoconazole (0, 1, 10 μM) for CYP3A4 (Cyp3a), and furafylline (0, 1, 10 μM) for CYP1A2 (Cyp1A). These inhibitors were pre-incubated in the reaction system with the liver microsomes for 30 min at 37°C, and then 10 μl of 200 μM berberine (final concentration is 10 μM) was added to each reaction and gently shaken for another 45 min. All reactions were terminated by adding 200 μl of ice-cold acetonitrile.
Sample Preparation and UPLC-TOFMS Analysis
Sample preparation and UPLC-TOFMS analyses were performed as described previously with minor modifications (Li et al., 2010). Urine samples were prepared by mixing 50 μl of urine with 150 μl of 50% acetonitrile and centrifuged at 20,000 g for 10 min. Feces were homogenized after adding 1×PBS (10 μl per mg feces). Subsequently, 200 μl of acetonitrile was added to 200 μl of the resulting mixture, followed by vortexing and centrifugation at 20,000 g for 10 min. The in vitro incubation was terminated by adding 200 μl of acetonitrile and centrifuged at 20,000 g for 10 min. Each supernatant was transferred to an auto-sampler vial, and 5 μl was injected into a system combining UPLC and TOFMS (HMDS SYNAPT, Waters, Milford, MA). A 100 mm x 2.1 mm (Acquity 1.7 μm) UPLC BEH C-18 column (Waters, Milford, MA) was used to separate berberine and its metabolites. The flow rate of the mobile phase was 0.3 ml/min with a gradient ranging from 2 to 98% aqueous acetonitrile, containing 0.1% formic acid in a 10-min run. TOFMS was operated in a positive mode with electrospray ionization. The source temperature and desolvation temperature were set at 120°C and 350°C, respectively. Nitrogen was applied as the cone gas (10 l/h) and desolvation gas (700 l/h) and argon as the collision gas. TOFMS was calibrated with sodium formate and monitored by the intermittent injection of lock mass leucine enkeophalin in real time. The capillary voltage and cone voltage were set at 3.5 kV and 35 V in positive ion mode. Screening and identification of major metabolites were performed using MakerLynx software (Waters, Milford, MA), based on accurate mass measurement (mass errors less than 10 ppm). The structures of berberine and its metabolites were elucidated by tandem mass fragmentation with collision energy ramp ranging from 10 to 35 eV.
Data Analysis
Mass chromatograms and mass spectra were acquired by MassLynx software in centroid format from m/z 50 to m/z 1000. Centroid and integrated mass chromatographic data were processed by MarkerLynx software to generate a multivariate data matrix. Orthogonal projection to latent structures-discriminant analysis (OPLS-DA) was conducted on Pareto-scaled data. The corresponding data matrices were then exported into SIMCA-P+12 (Umetrics, Kinnelon, NJ) for multivariate data analysis.
Results
Identification of Major Metabolites of Berberine in Mouse Urine and Feces
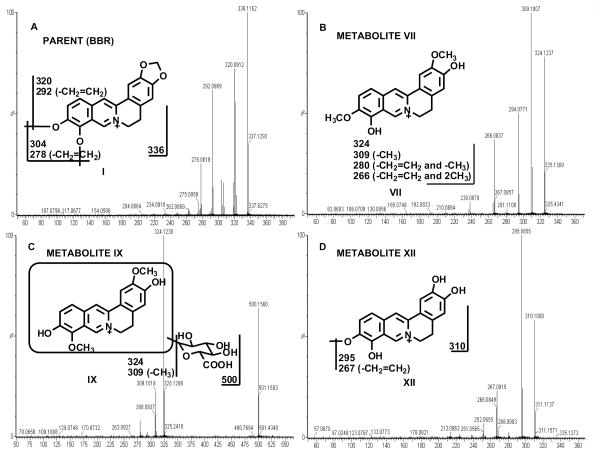
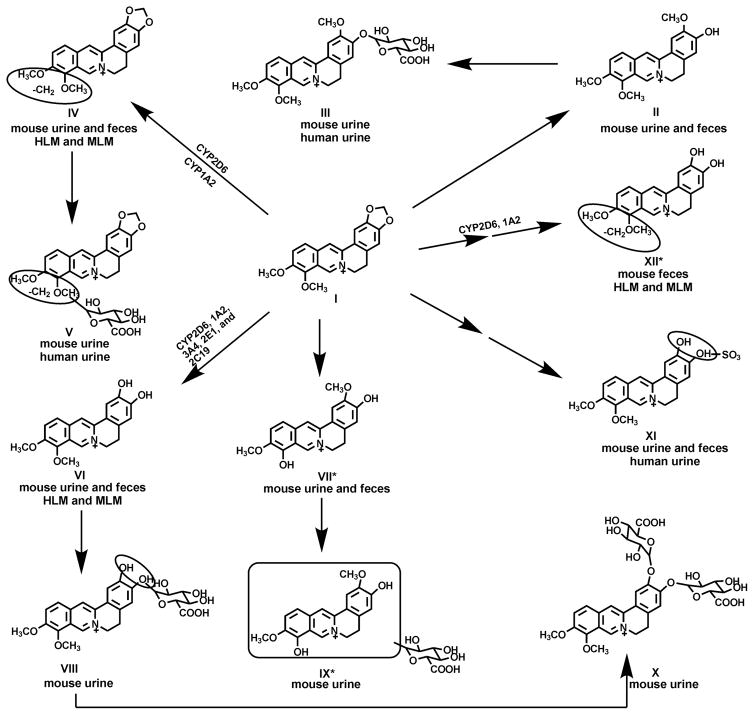
Berberine is compound I in Fig. 1A. Eleven berberine metabolites were identified in mouse urine and feces; namely metabolite II, a 1,3-dioxole ring-opened product; metabolite IV, a demethylated product; metabolite VI, a demethenylated product; metabolite VII, a demethylated/1,3-dioxole ring-opened product; metabolite XII, a demethylated/demethenylated product; metabolites III, V, VIII, and IX are 4 mono-glucuronidated products; metabolite X, a di-glucuronidated product; and metabolite XI, a sulfated product. Eight of 11 identified berberine metabolites were characterized previously from humans and rats (Zuo et al., 2006; Qiu et al., 2008), and the MS/MS spectra of these metabolites and their structural elucidations are described in detail in the supporting information (supporting Figs. 1A–1H) and chromatograms (supporting Figs. 2A–2B). The other three are novel metabolites, namely metabolite VII, metabolite IX, and metabolite XII.
Fig. 1. MS/MS Fragmentation Patterns of Berberine and Three Novel Metabolites.
(A) MS/MS fragmentation pattern for berberine. (B) MS/MS analysis of demethylated/1,3-dioxole ring-opening metabolite VII, whichhas a [M]+ ion at m/z 324, 12 Daltons less than that of berberine. (C) Mono-glucuronidated metabolite IX has a [M]+ ion at m/z 500, 176 mass units higher than that of metabolite VII. (D) Demethylated/demethenylated metabolite XII was only observed in feces, and corresponded to a [M]+ion at m/z 310. Berberine is abbreviated as BBR in all the figures in the present study.
Demethylated/1,3-dioxole ring-opened metabolite VII was observed in urine and feces. It eluted at 4.11 min and had a [M]+ ion at m/z 324, which is 12 Daltons less than that of berberine. MS/MS analysis of metabolite VII produced daughter ions at m/z 309 (loss of CH3), 294 (loss of CH2=CH2), and 266 (loss of CH2=CH2 and 2CH3). The daughter ions of metabolite VII are interpreted in the inlaid structural diagram (Fig. 1B).
Mono-glucuronidated metabolite IX was only detected in urine with a retention time of 3.56 min, having a[M]+ ion at m/z 500, 176 mass units higher than that of metabolite VII, suggesting that metabolite IX was a glucuronide product of metabolite VII. The daughter ions at m/z 324 (loss of C6H8O6) and 309 (loss of C6H8O6 and CH3) are interpreted in the inlaid structural diagram (Fig. 1C).
Demethylated/demethenylated metabolite XII was only observed in feces. Metabolite XII (at a retention time of 3.48 min) corresponds to a [M]+ ion at m/z 310, which is 26 Daltons less than that of berberine. The daughter ions at m/z 295 (loss of CH3) and 267 (loss of CH2=CH2 and CH3) are interpreted in the inlaid structural diagram (Fig. 1D).
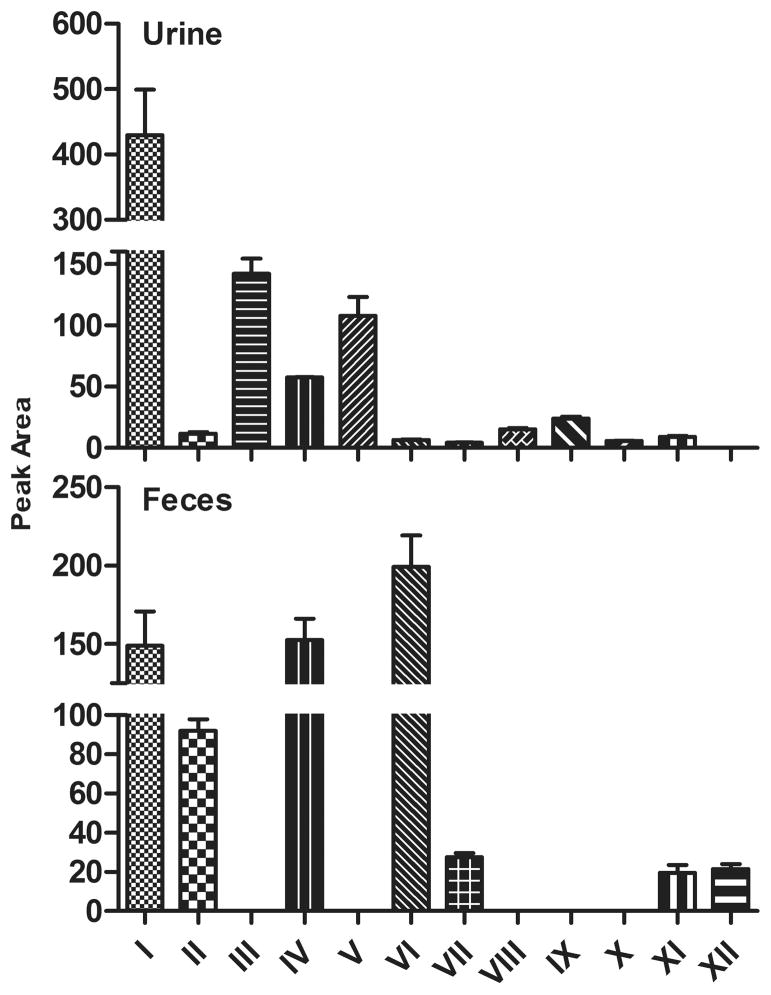
Relative abundance of berberine and its metabolites in mouse urine and feces are displayed in Fig. 2. All the glucuronidated metabolites (III, V, VIII, IX, X) were observed in urine, but not in feces, whereas unconjugated demethylated/demethenylated metabolite XII was detected only in feces and in low abundance. Parent chemical berberine, the most abundant compound in urine, was 1.88-fold more than that in feces. Unconjugated metabolites IV (demethylated metabolite) and VI (demethenylated metabolite) were the two predominant compounds in feces, which were 1.65- and 29.7-fold more abundant than that in urine. Metabolites II (1,3-dioxole ring-opened metabolite) and VII (demethylated/1,3-dioxole ring-opened metabolite) were also detectable in feces, and the amounts of metabolites II and VII in feces were 6.83 and 5.47-fold more than in urine. The amount of sulfated demethenylated metabolite XI in feces was 1.20-fold more than in urine.
Fig. 2. Relative Amount of Berberine and Its Metabolites in Urine and Feces of Mice.
Eleven metabolites were detected in urine and feces of mice, five unconjugated metabolites (metabolites II, IV, VI, VII, and XII) and six conjugated metabolites (metabolites II, V, VIII, IX, X, and XI). All the glucuronidated metabolites (metabolites III, V, VIII, IX, and X) were observed in urine, but not in feces, whereas, unconjugated metabolite XII was detected only in feces and in low abundance. Berberine (I), the parent drug administered, was the most abundant compound in urine, and was 1.88 folds higher in urine than feces. Unconjugated metabolites IV and VI were the two predominant metabolites in feces followed by metabolites II and VII, and the amount of each in feces was 1.65, 29.7, 6.83, and 5.47 folds more than that in urine, respectively. The amount of the sulfated metabolite XI in feces was 1.2 folds more than that in urine.
Berberine Metabolism in MLM and HLM
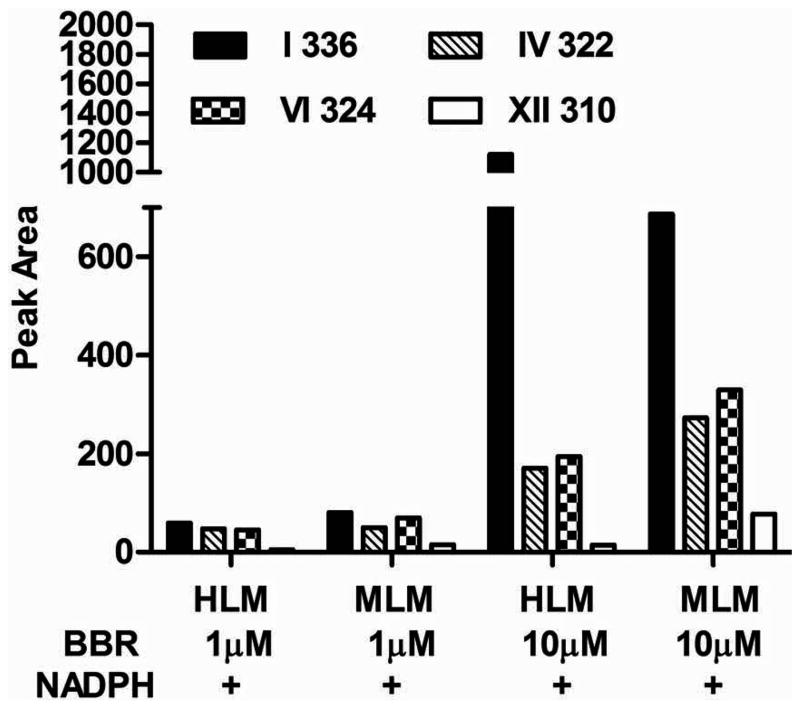
To determine whether CYPs were the enzymes that generate the metabolites mentioned above, berberine was incubated with MLM and HLM. Three of 5 unconjugated metabolites in urine and feces, namely demethylated metabolite IV, demethenylated metabolite VI, and demethylated/demethenylated metabolite XII, were detected after berberine was incubated with MLM and HLM in an NADPH-dependent manner (Fig. 3). The peak areas of all these metabolites were higher by MLM than by HLM. Metabolite VI was the major product in MLM. In MLM incubated with 1 μM berberine, the peak area of metabolite VI was 40% higher than that of metabolite IV and 3.67-fold higher than that of metabolite XII, whereas in MLM incubated with 10 μM berberine metabolite VI was 20% higher than that of metabolite IV, and 3.25-fold higher than that of metabolite XII. In HLM incubated with 1 μM berberine, the amount of metabolite VI was similar to metabolite IV and 8-fold more than that of metabolite XII, whereas in the HLM incubated with 10 μM berberine, the amount of metabolite VI was about 14% more than that of metabolite IV and 12-fold more than metabolite XII.
Fig. 3. Berberine Metabolism in MLM and HLM.
Three of five unconjugated metabolites, namely demethylated metabolite IV (m/z 322), demethenylated metabolite VI (m/z 324) and demethylated/demethenylated metabolite XII (m/z 310), were produced by HLM and MLM in an NADPH-dependent manner. With MLM, metabolite VI (m/z 324) was the major product. In the 1 μM berberine incubation system, the peak area of metabolite VI (m/z 324) was 40% higher than that of metabolite IV (m/z 322) and 3.67-fold higher than that of metabolite XII (m/z 310), whereas in the 10 μM berberine incubation system metabolite VI (m/z 324) was 20% higher than that of metabolite IV (m/z 322) and 3.25-fold higher than that of metabolite XII. In HLM incubated with 1 μM berberine, the amount of metabolite VI (m/z 324) was similar to metabolite IV (m/z 322) and 8-fold more than that of metabolite XII (m/z 310), whereas in the 10 μM berberine incubation system, the amount of metabolite VI (m/z 324) was about 14% more than that of metabolite IV (m/z 322) and 12-fold more than metabolite XII (m/z 310).
Berberine Metabolism in Recombinant Human CYPs
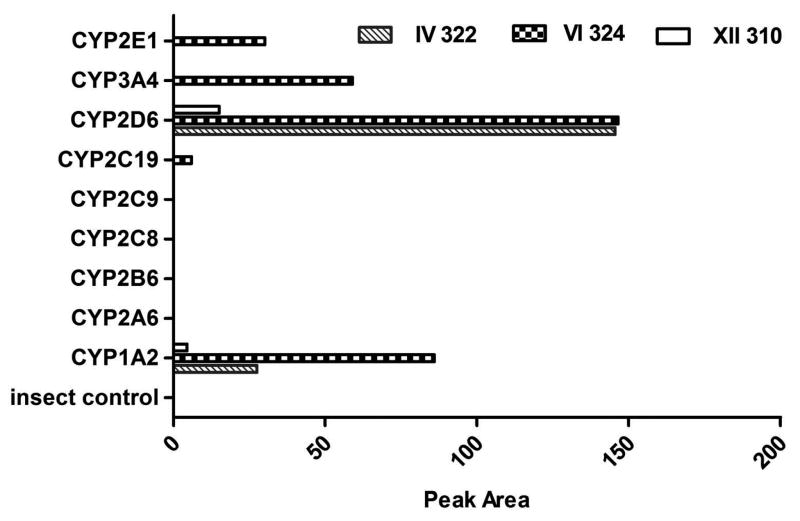
To determine which CYP formed each berberine metabolite, single recombinant human CYP was incubated with berberine. The 3 metabolites formed by liver microsomes were recapitulated in incubation systems of berberine with a panel of recombinant human CYPs (Fig. 4). Among the 9 recombinant human CYP enzymes, CYP2D6 contributed to the formation of all 3 unconjugated metabolites (demethylated metabolite IV, demethenylated metabolite VI, demethylated/demethenylated metabolite XII). CYP1A2 also produced these 3 metabolites, but less than did CYP2D6. CYP3A4, 2E1 and 2C19 catalyzed only the synthesis of metabolite VI with a lower formation rate than CYP2D6 and 1A2.
Fig. 4. Berberine Metabolism in Recombinant Human CYPs.
Those three unconjugated metabolites of berberine observed in liver microsomes incubated with parent compound (metabolite IV, m/z 322; metabolite VI, m/z 324; metabolite XII, m/z 310) were also produced by a panel of recombinant human CYPs. Among the nine recombinant human CYP enzymes, CYP2D6 contributed to the formation of all three unconjugated metabolites. CYP1A2 also produced these three metabolites, but less than by CYP2D6. CYP3A4, 2E1 and 2C19 catalyzed only the synthesis of metabolite VI (m/z 324).
Berberine Metabolism in MLM and HLM Pre-incubated with Specific CYP Inhibitors
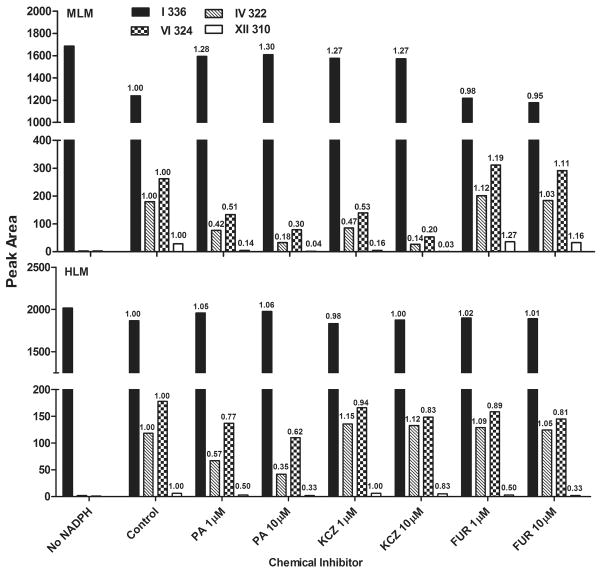
To verify results obtained from the recombinant CYPs, effects of known CYP inhibitors, namely paroxetine, an inhibitor of CYP2D6 (Lam et al., 2002); ketoconazole, an inhibitor of CYP3A4 (Fukuda et al., 1997); and furafylline, an inhibitor of CYP1A2 (Kunze and Trager, 1993), on the biotransformation of berberine in MLM and HLM were examined. An increase in the amount of parent compound remaining in the incubation system or a decrease in metabolites generated was interpreted as inhibition of biotransformation (Fig. 5).
Fig. 5. Berberine Metabolism in MLM and HLM Pre-incubated with Specific CYP Inhibitors.
The effect of paroxetine (PA), furafylline (FUR), ketoconazole (KCZ), which are inhibitors for CYP2D6, CYP1A2 and CYP3A4, on berberine (I, m/z 336) biotransformation in MLM and HLM showed that the metabolism of berberine mediated by various CYPs was inhibited in mice and humans, and formation of metabolite IV (m/z 322), metabolite VI (m/z 324) and metabolite XII (m/z 310) was decreased. The number on top of each bar is test/control. Paroxetine is abbreviated as PA, furafylline is abbreviated as FUR, ketoconazole is abbreviated as KCZ.
In MLM, pre-incubation with paroxetine and ketoconazole increased the amount of berberine remaining and decreased the formation of all metabolites in a concentration-dependent manner, whereas furafylline had little effect on the metabolism of berberine.
In HLM, paroxetine decreased the formation of demethylated metabolite IV, whereas ketoconazole and furafylline had little effect on this process, supporting the hypothesis that CYP2D6 is the most important enzyme in the formation of metabolite IV. The generation of demethenylated metabolite VI by HLM was inhibited the most by paroxetine, intermediately by furafylline, and the least by ketoconazole, indicating that CYP2D6 plays the largest role, CYP1A2 intermediate, and CYP3A4 the least in forming metabolite VI. The production of demethylated/demethenylated metabolite XII was inhibited by paroxetine and furafylline, whereas ketoconazole had little effect on this process, implying that CYP2D6 and 1A2 catalyzed the production of metabolite XII. These results from HLM are similar to that observed with recombinant human CYPs.
The proposed profile of berberine metabolites in mice and humans as well as biotransformation pathways for the three major metabolites in liver are summarized in Fig. 6 based on the previous and current studies. Five unconjugated metabolites of berberine and their corresponding conjugated metabolites are noted. The metabolism of berberine by human recombinant CYPs indicates that CYP2D6 is the primary enzyme involved in the formation of the three berberine metabolites mentioned above (IV, VI and XII), and CYP1A2 produces the same metabolites, but at a slower rate. In addition, CYP3A4, 2E1 and 2C19 also participate in the formation of the demethylenated metabolite VI.
Fig. 6. Proposed Biotransformation Pathways of Berberine.
Berberine biotransformation pathways were proposed based on the previous reports (from Pan et al., 2002; Zuo et al., 2006; and Qiu et al., 2008) and the current study. New metabolites were marked by asterisks. Five unconjugated metabolites of berberine and their corresponding conjugated metabolites are noted. The metabolism of berberine (I)by human recombinant CYPs shows that CYP2D6 is the primary enzyme involved in the formation of three berberine metabolites (IV, VI and XII), whereas CYP1A2 produced the same metabolites but with lower amount. CYP3A4, 2E1 and 2C19 also participate in the formation of demethylenation metabolite VI.
Discussion
In this study, eleven metabolites of berberine were detected in urine and feces of mice, including 5 unconjugated metabolites (II, IV, VI, VII, XII) and 6 conjugated metabolites (III, V, VIII, IX, X, XI). Three of these berberine metabolites were not reported previously, namely metabolite VII, IX and XII. All the glucuronidated metabolites were excreted in urine, but not in feces, whereas, metabolite XII was only detected in feces. Three of the 5 unconjugated metabolites (IV, VI and XII) were produced by MLM, HLM, and recombinant human CYPs incubated with berberine. Moreover, CYP2D6 was identified to be the primary enzyme metabolizing berberine, but other CYPs are also involved.
UPLC-TOFMS combined with multivariate data analysis (Li et al., 2010) were used for structural elucidation and screening of metabolites in urine and feces of mice (supporting Fig. 3) in the present study. Several groups have identified metabolites of berberine in human urine as well as liver, plasma, bile, and urine of rat (Pan et al., 2002; Tsai and Tsai, 2004; Zuo et al., 2006; Qiu et al., 2008). The metabolites identified in urine and feces of mice are similar to that in urine of humans and rats, except three additional metabolites (VII, IX, and XII) were detected (Fig. 1B–1D). Based on MS/MS spectra (Fig. 1 and Supporting Figs 1A–1H), metabolite II is a 1,3-dioxole ring-opened product, and may correspond to jatrorrhizine; metabolite III is a mono-glucuronidated product of metabolite II, which may be a compound having the same structure as jatrorrhizine-3-O-β-D–glucuronide or columbamin-2-O-β-D–glucuronide; metabolite IV, a demethylated metabolite, could be berberrubine or thalifendine; metabolite V is a mono-glucuronidated product of metabolite IV, which may be identical to berberrubine-9-O-β-D-glucuronide or thalifendine-10-O-β-D-glucuronide; metabolite VI is a demethenylated product, and may correspond to demethyleneberberine; metabolite VIII (might be demethyleneberberine glucuronide) is the mono-glucuronidated conjugate of metabolite VI; metabolite X (might be demethyleneberberine-2,3-di-O-β-D-glucuronide) and metabolite XI (might be demethyleneberberine-2-O-sulfate) are a di-glucuronidated product and a sulfated product of metabolite VI, respectively; metabolite VII is a demethylated/1,3-dioxole ring-opened product, a compound detected first in the current study, which might be 3,10-demethyl-palmatine (deduced from conjugated metabolites mentioned in prior studies), and its mono-glucuronidated metabolite is metabolite IX; and metabolite XII, a demethylated/demethenylated compound, is another novel unconjugated metabolite detected in the current study in mouse feces as well as MLM and HLM incubated with berberine, and the detailed structure and function of metabolite XII needs further investigation (Hoshi et al., 1976; Kobayashi et al., 1995; Krishnan and Bastow, 2000; Qiu et al., 2008). Four (metabolites II, VI, VII and XII) of the 5 unconjugated metabolites in mice observed in this study were apparently not detected in urine from rats and humans given berberine in previous reports (Qiu et al., 2008). Only one sulfate metabolite was detected in the present study in urine of mice, which was one of 3 sulfate metabolites reported previously in urine of rats and humans (Pan et al., 2002; Qiu et al., 2008).
According to previous reports and the present study, jatrorrhizine (might be metabolite II) and columbamine, berberrubine (might be metabolite IV) and thalifendine, demethyleneberberine (mighte be metabolite VI) and 3,10-demethyl-palmatine (might be metabolite VII), with each pair being isomers, are metabolites of berberine shared by humans and rats, except for columbamine, which is apparently a berberine metabolite in humans but not in rodents (Qiu et al., 2008). Some of these metabolites have been shown to be effective for their antitumor and antioxidative pharmacological activities in vitro, and are reported to be more potent than the parent berberine (Zuo et al., 2006).
In the present study, all glucuronide metabolites of berberine were detected in mouse urine, but not in feces (Fig. 2). This is probably due to extensive deconjugation of the glucuronide metabolites of berberine in the intestine, active efflux of these metabolites back to blood by liver or intestine, and/or a special transport pattern in kidneys.
Many studies have suggested that CYPs might metabolize berberine, but only until recently CYP2D6, 1A2 and CYP3A4 are reported to metabolize berberine (15 or 20 μM) to form metabolites IV and VI (Li et al., 2011). The present study shows that the new unconjated metabolite XII can also be formed by MLM and HLM as well as recombinant human CYPs. In addition, the formation of metabolite XII can be decreased dramatically by specific CYP2D6 and 1A2 inhibitors. Most drug processing genes, such as CYPs, have homologues in mice that possess high DNA and amino acid identity to that of humans, and function similarly as their human homologs. For example, human CYP1A1, 1A2, 1B1, 2C19, 2D6, and 3A4 have mouse homologues of Cyp1a1, 1a2, 1b1, 2c37, 2d22, and 3a11, respectively (http://www.ncbi.nlm.nih.gov/homologene/). In the present study, the metabolites produced by HLM and MLM were the same (Fig. 3), which suggests similar pathways involved in berberine metabolism in mice and humans. Moreover, the metabolism of berberine by MLM appears to occur at a higher rate than that by HLM. Three unconjugated metabolites (IV, VI, and XII) of berberine were produced in liver microsomes and recombinant enzyme systems, but 2 other unconjugated metabolites (II, VII) detected in feces and urine were not generated in these systems. Given that the amount of metabolite II was abundant in feces but was not formed by liver microsomes, it might be produced by enzymes other than CYPs in livers or intestine, which were not examined in this study. Metabolite VII may not have been detected because only very small amounts were produced.
Using the major human CYP recombinant enzymes (Fig. 4), CYP2D6 was determined to be the primary enzyme catalyzing the biotransformation of berberine. If one considers only the results from the recombinant enzyme study, metabolite VI might be regarded as the most abundant metabolite; however, when liver microsomes were incubated with a lower concentration of berberine (1 μM), which is probably more similar to the in vivo situation, the amount of metabolite VI was higher in MLM, but similar to the amount of metabolite IV formed by HLM. Pre-incubation with a CYP3A4 inhibitor (Fig. 5) had little influence on the overall biotransformation of berberine by HLM. These data reveal that CYP2D6 is most likely the CYP involved in berberine biotransformation in liver of humans at low berberine concentrations, whereas other enzymes might be important at higher concentrations of berberine.
Results from the inhibition studies performed with MLM and HLM (Fig. 5) indicate that metabolite compensation exists, that is, as the formation of one metabolite decreases, the other metabolites increases. The usage of each CYP inhibitor was based on the most comparable inhibition pattern of mice with humans (Bogaards et al., 2000). paroxetine at 1 and 10 μM decreased berberine biotransformation dramatically in HLM and MLM, which indicates that CYP2D is a most important enzyme catalyzing berberine in liver of mice and humans. Although CYP1A2 inhibition by furafylline in liver microsomes is reported to be more potent in humans than mice, it does inhibit mouse Cyp1a2. The present study indicated that 1 and 10 μM furafylline had little influence on the overall metabolism of berberine in MLM, but significantly decreased the formation of berberine metabolites in HLM, which might suggest CYP1A is relatively more important in berberine metabolism in humans than in mice. ketoconazole inhibits CYP3A substrates similarly in mice and humans (Bogaards et al., 2000), and pre-incubation with ketoconazole decreased berberine metabolism in MLM, more than in HLM, which suggests that Cyp3a might be more important for berberine biotransformation in mice than humans.
Of the 18 CYP gene families in humans, CYP1, 2 and 3 families are responsible for the biotransformation of most xenobiotics. CYP2D6, a highly polymorphic enzyme, metabolizes 20–25% of clinically used drugs. There are about 40% Caucasians, 50% Asians, and 30% Africans that have an enzymatically hypo-active CYP2D6. Based on CYP2D6 enzyme activity, humans can be divided into 4 different groups, namely ultra-rapid metabolizers, extensive metabolizers, intermediate metabolizers, and poor metabolizers. Thus, drugs metabolized by CYP2D6, such as berberine, may vary markedly in regard to their pharmacological activities in these four groups (Molanaei et al., 2010). Berberine is metabolized by CYP1A2, which also metabolizes caffeine, and can be induced by polycyclic aromatic hydrocarbons (Deroussent et al., 2010). Therefore, whether berberine affects the biotransformation of CYP1A2 substrates requires further investigation. CYP3A4 accounts for 30% of CYPs in liver (Shimada et al., 1994) and 80% of the CYPs in small intestine (Watkins et al., 1987). Over 50% of drugs in clinical use are metabolized by CYP3A (Thummel and Wilkinson, 1998). The current study suggests that recombinant human CYP3A4 is also responsible for formation of the major metabolite of berberine, therefore, potential drug-drug interactions should be considered when berberine is administered.
The present study demonstrates for the first time the metabolic profile of berberine in urine and feces of mice, and identified 3 novel metabolites. The metabolism of berberine in liver of mice and humans, as well as the specific CYP producing each metabolite were characterized in this study, which shows that CYP2D plays a very important role in berberine biotransformation in mice and humans. CYP1A and CYP3A also metabolize berberine. Therefore, it is concluded that CYP2D6 and other CYPs are involved in berberine metabolism, and that genetic polymorphisms of CYP2D6 as well as potential drug interactions should be considered when berberine or berberine-containing products are used.
Supplementary Material
Acknowledgments
The authors thank all the members of Dr. Klaassen’s laboratory for collecting tissue and reviewing the manuscript. This work was supported by the following grants:
National Institute of Health (ES-009649, ES-019487, DK-081461, and RR021940) to Curtis D. Klaassen, PhD.
National Scientific Foundation of China (No.30801421, No.81072706)
Hunan Provincial Innovation Foundation For Postgraduate (No. 2009bsxt020)
Huge Project to Boost Chinese Drug Development (No.2009ZX09501-032)
863 Projects (No.2009AA022710, 2009AA022703, 2009AA022704)
Abbreviations
- CYP
Cytochrome P450
- HLM
Human liver microsomes
- UPLC-MS/MS
ultra-performance liquid chromatography-tandem mass spectrometry
- MLM
mouse liver microsomes
- OPLS-DA
Orthogonal projection to latent structures-discriminant analysis
- PBS
phosphate buffered saline
- TOFMS
Time-of-flight mass spectrometry
Footnotes
Declaration of interest The authors report no declarations of interest.
References
- Abourashed EA, Khan IA. High-performance liquid chromatography determination of hydrastine and berberine in dietary supplements containing goldenseal. J Pharm Sci. 2001;90:817–822. doi: 10.1002/jps.1035. [DOI] [PubMed] [Google Scholar]
- Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1–23. [PubMed] [Google Scholar]
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, Walther B. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Deroussent A, Re M, Hoellinger H, Cresteil T. Metabolism of sanguinarine in human and in rat: characterization of oxidative metabolites produced by human CYP1A1 and CYP1A2 and rat liver microsomes using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:391–397. doi: 10.1016/j.jpba.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Ohta T, Oshima Y, Ohashi N, Yoshikawa M, Yamazoe Y. Specific CYP3A4 inhibitors in grapefruit juice: furocoumarin dimers as components of drug interaction. Pharmacogenetics. 1997;7:391–396. doi: 10.1097/00008571-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Ikekawa T, Ikeda Y, Shirakawa S, Iigo M. Antitumor activity of berberrubine derivatives. Gann. 1976;67:321–325. [PubMed] [Google Scholar]
- Hua W, Ding L, Chen Y, Gong B, He J, Xu G. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J Pharm Biomed Anal. 2007;44:931–937. doi: 10.1016/j.jpba.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yamashita Y, Fujii N, Takaboshi K, Kawakami T, Kawamura M, Mizukami T, Nakano H. Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med. 1995;61:414–418. doi: 10.1055/s-2006-958127. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Bastow KF. The 9-position in berberine analogs is an important determinant of DNA topoisomerase II inhibition. Anticancer Drug Des. 2000;15:255–264. [PubMed] [Google Scholar]
- Kunze KL, Trager WF. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem Res Toxicol. 1993;6:649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- Lahiri SC, Dutta NK. Berberine and chloramphenicol in the treatment of cholera and severe diarrhoea. J Indian Med Assoc. 1967;48:1–11. [PubMed] [Google Scholar]
- Lam YW, Gaedigk A, Ereshefsky L, Alfaro CL, Simpson J. CYP2D6 inhibition by selective serotonin reuptake inhibitors: analysis of achievable steady-state plasma concentrations and the effect of ultrarapid metabolism at CYP2D6. Pharmacotherapy. 2002;22:1001–1006. doi: 10.1592/phco.22.12.1001.33603. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- Li F, Wang L, Guo GL, Ma X. Metabolism-mediated drug interactions associated with ritonavir-boosted tipranavir in mice. Drug Metab Dispos. 2010;38:871–878. doi: 10.1124/dmd.109.030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ren G, Wang YX, Kong WJ, Yang P, Wang YM, Li YH, Yi H, Li ZR, Song DQ, Jiang JD. Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J Transl Med. 2011;9:62. doi: 10.1186/1479-5876-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu CX, Wang GJ. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos. 2010;38:1779–1784. doi: 10.1124/dmd.110.033936. [DOI] [PubMed] [Google Scholar]
- Molanaei H, Carrero JJ, Heimburger O, Nordfors L, Lindholm B, Stenvinkel P, Odar-Cederlof I, Bertilsson L. Influence of the CYP2D6 polymorphism and hemodialysis on codeine disposition in patients with end-stage renal disease. Eur J Clin Pharmacol. 2010;66:269–273. doi: 10.1007/s00228-009-0759-8. [DOI] [PubMed] [Google Scholar]
- Pan JF, Yu C, Zhu DY, Zhang H, Zeng JF, Jiang SH, Ren JY. Identification of three sulfate-conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol Sin. 2002;23:77–82. [PubMed] [Google Scholar]
- Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab Dispos. 2008;36:2159–2165. doi: 10.1124/dmd.108.021659. [DOI] [PubMed] [Google Scholar]
- Sandson N. Drug-drug interactions: the silent epidemic. Psychiatr Serv. 2005;56:22–24. doi: 10.1176/appi.ps.56.1.22. [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- Tsai PL, Tsai TH. Hepatobiliary excretion of berberine. Drug Metab Dispos. 2004;32:405–412. doi: 10.1124/dmd.32.4.405. [DOI] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19:1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu D, Cao P, Lecker S, Hu Z. Atrogin-1 affects muscle protein synthesis and degradation when energy metabolism is impaired by the antidiabetes drug berberine. Diabetes. 2010;59:1879–1889. doi: 10.2337/db10-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest. 1987;80:1029–1036. doi: 10.1172/JCI113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos. 2006;34:2064–2072. doi: 10.1124/dmd.106.011361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.