Abstract
Background
We investigated whether a multi-locus genetic risk scores (GRS) was associated with presence and progression of abdominal aortic aneurysm (AAA) in a case - control study.
Methods and Results
The study comprised of 1124 patients with AAA (74 ± 8 years, 83% men, 52% of them with a maximal AAA size ≤ 5 cm) and 6524 non-cases (67 ± 11 years, 58% men) from the Mayo Vascular Disease Biorepository. AAA was defined as infrarenal abdominal aorta diameter ≥3.0 cm or history of AAA repair. Non-cases were participants without known AAA. A GRS was calculated using 4 SNPs associated with AAA at genome-wide significance (P ≤ 10−8). The GRS was associated with the presence of AAA after adjustment for age, sex, cardiovascular risk factors, atherosclerotic cardiovascular diseases and family history of aortic aneurysm: odds ratio (OR, 95% confidence interval, CI) 1.06 (1.04 –1.09, p < 0.001). Adding GRS to conventional risk factors improved the association of presence of AAA (net reclassification index 14%, p < 0.001). In a subset of patients with AAA who had ≥2 imaging studies (n = 651, mean (SE) growth rate 2.47 (0.11) mm/year during a mean time interval of 5.41years), GRS, baseline size, diabetes and family history were each associated with aneurysm growth rate in univariate association (all p < 0.05). The estimated mean aneurysm growth rate was 0.50 mm/year higher in those with GRS > median (5.78) than those with GRS ≤ median (p = 0.01), after adjustment for baseline size (p < 0.001), diabetes (p = 0.046) and family history of aortic aneurysm (p = 0.02).
Conclusions
A multi-locus GRS was associated with presence of AAA and greater aneurysm expansion.
Keywords: Abdominal aortic aneurysm, Genetics, Genetic risk score
Abdominal aortic aneurysm (AAA) is conventionally defined as a transverse aortic diameter ≥3.0 cm [1]. The prevalence of AAA increases with age and is about 12.8% and 4.1% in men and women >65 years old, respectively [2]. Acute rupture is a devastating outcome that is associated with a high mortality of nearly 80% [3]. No pharmacological treatment is available to effectively limit disease progression. Early identification through ultrasound screening followed by elective aneurysm repair has been shown to decrease aneurysm-related mortality [4]. Given the significant disease burden and paucity of treatment options, there is a need to identify biomarkers of AAA that may enable individualized screening.
AAA is a multifactorial disease with a heritable component [5]. Genome-wide association studies (GWAS) have found several common single nucleotide polymorphisms (SNPs) to be associated with AAA [6–11]. Whether such variants can improve prediction of presence of AAA beyond conventional risk factors is unknown. The risk of rupture largely depends on aneurysm size and growth rate. Factors that influence aneurysm expansion remain unclear. In particular genetic factors that relate to aneurysm growth are largely unknown. A study of participants in the UK small aneurysm trial found that the 9p21 locus which is associated with atherosclerosis and presence of AAA, was not associated with aneurysm expansion [12]. Whether genetic predisposition to AAA expansion is due to the additive effect of multiple susceptibility alleles is unknown.
We hypothesized that a multi-locus GRS based on SNPs associated with AAA in GWAS may improve disease prediction beyond conventional risk factors and would be associated with aneurysm growth. To test these hypotheses, we performed genotyping using Illumina Human 610 and 660W Quad-v1in participants in the Mayo Clinic Vascular Disease Biorepository [13].
1. Methods
1.1. Study participants
The VDB at Mayo Clinic consists of patients referred for noninvasive vascular evaluation in the Gonda Vascular Center and stress electrocardiographic laboratory, and was initiated in 2008. The design and selection criteria have been reported previously [13]. Briefly, the purpose of this registry is to identify novel biomarkers, including genetic susceptibility markers for common and rare vascular diseases. Until August 2013, more than 11,814 adults have been recruited. Blood samples of participants were drawn at the recruitment. High-density genotyping data were available in 8062 (68%) participants. For the purpose of the current study, we included 7648 (9594.7%) patients, including 1124 with AAA as cases and 6524 non-cases who have ASCVD or were referred for cardiovascular risk assessment but without ASCVD. Demographic information, conventional risk factors and comorbidities were ascertained by previously validated algorithms using ICD-9-CM diagnosis codes, procedure codes, medication use and laboratory data from the institutional electronic health records (EHR). A questionnaire on physical activity, lifestyle and family history was given to each participant at the time of consent and scanned into the database after completion. All participants gave informed consent. The study protocol was approved by the Institutional Review Board of the Mayo Clinic.
1.2. Ascertainment of cases and non-cases of AAA
We sampled subjects based on their AAA status. AAA cases were defined as having 1) an infrarenal abdominal aortic diameter ≥3 cm, or 2) a history of open or endovascular AAA repair. Patients with AAA often have similar risk profiles as those with atherosclerotic cardiovascular disease (ASCVD) or have ASCVD concomitantly. To test whether a GRS for AAA can differentiate patients with AAA from those who may have ASCVD, participants not known to have AAA (including lack of billing codes for aortic aneurysm) were selected as non-cases. Such non-cases could have ASCVD in different arterial locations. We manually reviewed 100 non-cases with any abdominal imaging study in the EHR. None of them had AAA mentioned in the radiology report.
AAA cases were manually reviewed to confirm the maximal aneurysm size (either anteroposterior or transverse diameter). Radiology reports used to screen included abdominal ultrasound, computerized tomography, magnetic resonance imaging and angiography. To assess AAA progression, the latest or the pre-operation measure of AAA size in the EHRs was collected for all AAA cases. Based on previous reports that >85% of adults with ectasia of abdominal aorta will progress to a size ≥3.0 cm [14], and that infrarenal aortic diameter ≥ 2.5 cm was associated with significantly increased risk of cardiovascular events and mortality compared to those with a diameter < 2.5 cm [15], we included aortic size ≥ 2.5 cm as baseline measure if subsequent measure reaches or exceeds 3 cm. Growth rate was used to assess aneurysm expansion, defined as (latest/pre-operation minus first diameter)/ time interval (mm/year). Time interval was calculated in years. We required the shortest follow-up time be at least 3 months for analyses of aneurysm growth.
1.3. Genotyping and calculation of GRS
Genomic DNA was extracted from whole blood samples drawn at the recruitment. Genotyping was performed in Mayo Clinic core lab according to standard protocols using Illumina Infinium Human core Exome Array, and Illumina Human 610 and 660W Quad-v1. Sample call rates were all >95%. Four SNPs had been genotyped for all participants. rs599839 was imputed using the cosmopolitan 1000Genomes Project reference panel using SHAPEIT2 for phasing and IMPUTE2 software for imputation. The IMPUTE 2 information score for this SNP was 0.94. All SNPs followed Hardy–Weinberg equilibrium (all p > 0.05). We used logistic regression to estimate the effect in our data set of five SNPs from independent loci (linkage disequilibrium = 0) that were associated with AAA at a P-value ≤10−8 (Table 1). To be conservative in the analyses, we used Z-tests to assess whether the risk estimates of SNPs in our dataset were substantially different from that in the published literature. Except for LDLR (rs6511720G, P = 0.008 for Z test), risk estimates for four SNPs were not significantly different from that in previous studies (all P > 0.05). Therefore, we excluded rs651172G in the calculation for GRS. We assumed an additive genetic model to construct GRS for each individual by summing the number of risk alleles for each of four SNPs weighted by estimated effect sizes in the GWAS catalog or from the largest meta-analysis and then rescaled by the number of SNPs divided by summed effect size of each SNP, as reported previously [16].
Table 1.
Associations of single nucleotide polymorphisms with presence of AAA.
| Locus | Gene | SNPs | Effect allele | In previous publications
|
In VDB
|
Z test | ||
|---|---|---|---|---|---|---|---|---|
| MAF | OR(95% CI) | MAF | OR(95% CI) | |||||
|
|
|
|
||||||
| P-value | P-value | P-value | ||||||
| 19p13.2 | LDLR | rs6511720 | G | 0.901 | 1.32 (1.20–1.43) [7] | 0.9 | 1.04 (0.89–1.21) | 0.009 |
| Bradley DT, 2013 [7] | 2 × 10^−10 | 0.66 | ||||||
| 12q13.3 | LRP1 | rs1466535 | C | 0.58 | 1.15 (1.10–1.21) [6] | 0.65 | 1.04 (0.94–1.15) | 0.08 |
| Bown MJ, 2011 [6] | 5 × 10^−10 | 0.4 | ||||||
| 9p33.2 | DAB2IP | rs7025846 | A | 0.23 | 1.21 (1.14–1.28) [9] | 0.27 | 1.17 (1.05–1.29) | 0.54 |
| Gretarsdotti, S. 2010 [9] | 5 × 10^−10 | 0.004 | ||||||
| 9p21 | CDKN2A-2B | rs2383207 | T | 0.49 | 1.27 [9] | 0.52 | 1.22 (1.11–1.34) | 0.51 |
| Gretarsdottir S. 2010 [9] | 2 × 10^−8 | 0.0001 | ||||||
| 1p13.3 | SORT1 | rs599839 | G | 0.22 | 0.81 (0.76–0.85) [11] | 0.23 | 0.90 (0.80–1.00) | 0.14 |
| Jones GT, 2013 [11] | 7.2 × 10^−14 | 0.06 | ||||||
MAF: risk allele frequency; OR: odds ratio; CI = confidence interval; LDLR = low density lipoprotein receptor; LRP1 = low density lipoprotein receptor-related protein 1; DAB2IP = DAB2 interacting protein; CDKN2A-2B = Cyclin-dependent kinase inhibitor 2A-2B; SORT1 = Sortilin 1.
1.4. Ascertainment of cardiovascular risk factors and ASCVD
Demographic information was abstracted from the EHR as structured data and conventional cardiovascular risk factors (hypertension, diabetes and dyslipidemia) and ASCVD were ascertained by previously validated algorithms using ICD-9 billing codes and natural language processing [17]. Family history of aortic aneurysm in first-degree relatives and smoking status were ascertained from the study questionnaire. Participants were considered smokers if they had smoked more than 100 cigarettes in the past [18,19]. ASCVD was defined as a history of having any of coronary heart disease, stroke, carotid arterial stenosis or peripheral arterial disease.
1.5. Statistical methods
Descriptive statistics were used to compare demographic information and conventional cardiovascular risk factors between cases and non-cases. Continuous variables were presented as mean (standard deviation) and dichotomous variables as numbers (percentages). Comparisons were performed after adjustment for age and sex. To assess the association of GRS with AAA, logistic regression analysis was performed 1) without adjustment; 2) with adjustment for age and sex; and 3) additionally adjusting for body-mass index, hypertension, diabetes, smoking, dyslipidemia, ASCVD and family history. To assess whether GRS can improve disease identification beyond conventional risk factors, the C-statistic, net reclassification index and integrated discrimination improvement were estimated. The association of GRS with aneurysm growth rate in a linear regression model violated homoscedasticity assumption when both were used as continuous variables. Therefore, we dichotomized GRS based on the median of 651 cases with at least two size measures at an interval ≥3 months. Logistic regression analysis was performed after adjustment for baseline size and other covariates associated with aneurysm growth rate in the univariate analysis. The association of age, sex and conventional risk factors with aneurysm expansion and interaction with GRS were also assessed. Two sub-analyses were performed to assess: 1) whether GRS can improve disease identification beyond age, sex and smoking history-main factors considered in initiating screening; and 2) whether GRS was associated with clinically high-risk aneurysm expansion defined as either with an aneurysm growth rate ≥10 mm/year or with unstable features requiring urgent intervention (rupture or penetrating ulcers). Analyses were performed using the R statistical package (version 2.13) and JMP 11.0 (SAS Institute, Cary, NC) software.
2. Results
Candidate SNPs associated with AAA and results of z-test are shown in Table 1. We constructed GRS using 4 SNPs after excluding rs6511720 (G). Patient characteristics are shown in Table 2. Patients with AAA had higher prevalence of conventional risk factors and ASCVD than non-cases after adjustment for age and sex; 73 out of 1124 patients with AAA had history of AAA repair. In the remaining patients, the maximal AAA size (mean, SE) was 4.69 (0.04) cm and in 52% AAA size was equal or below 5 cm.
Table 2.
Patient characteristics.
| AAA (n = 1124) | Non-AAA (n = 6524) | |
|---|---|---|
| Age, years | 74 (8) | 67 (11) |
| Men | 915 (83) | 4039 (62) |
| Body mass index, kg/m2 | 29.3 (4.9) | 29.1 (5.6) |
| * Smoking (ever) | 952 (87) | 3800 (58) |
| * Hypertension | 908 (82) | 4203 (64) |
| Type 2 diabetes | 342 (25) | 1819 (22) |
| * Dyslipidemia | 959 (87) | 4931 (75) |
| * ASCVD | 966 (88) | 4552 (70) |
| * Family history of aortic aneurysm | 178 (16) | 471 (7) |
| * GRS | 5.34 (2.74) | 4.89 (2.86) |
Values expressed as mean (SD) or number (%).
P-value < 0.05 for comparisons in cases vs. non-cases adjusted for age and sex.
Abbreviations: AAA = abdominal aortic aneurysm; ASCVD = atherosclerotic cardiovascular disease; GRS = genetic risk score.
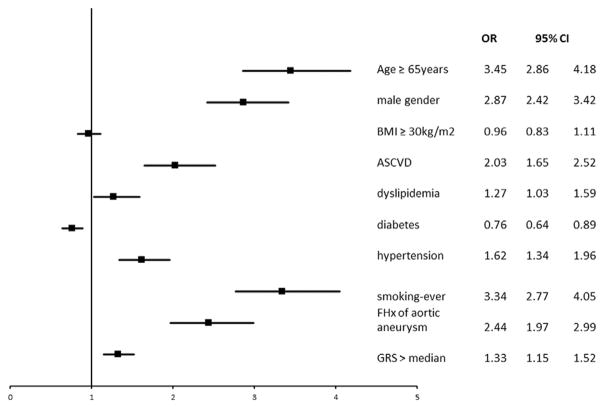
The univariate associations of conventional risk factors, ASCVD, family history and GRS with AAA are shown in Table 3. Associations of GRS and covariates with presence of AAA in multivariable logistic regression model are shown in Fig. 1. The GRS was associated with presence of AAA (unadjusted odds ratio, OR, per weighted allele, 95% confidence interval, CI: 1.06, 1.03–1.08, p < 0.001). The association remained significant after adjustment for age and sex: adjusted OR (95%CI), 1.06 (1.04–1.08), p < 0.001 and further adjustment for body-mass index, hypertension, diabetes, dyslipidemia, smoking, ASCVD and family history: adjusted OR 1.06 (95% CI: 1.04–1.09, p < 0.001). We did not find the presence of family history or male sex to alter the association of genetic risk for AAA (P for interaction term P = 0.3 for family history*GRS and 0.1 for sex*GRS). Adding GRS to conventional risk factors increased c-statistics from 0.789 (95%CI: 0.776–0.802) to 0.791 (95% CI: 0.777–0.803), a marginal improvement (Δ = 0.002, p = 0.049). Adding GRS resulted in better disease discrimination manifested by net reclassification index (NRI = 0.14, p < 0.001).
Table 3.
Univariate association of covariates and GRS with AAA.
| Term | Odds ratio | 95%CI | P-value |
|---|---|---|---|
| Age ≥65 year | 1.68 | 1.48–1.91 | <0.0001 |
| Men | 3.59 | 3.05–4.25 | <0.0001 |
| Body-mass index ≥30 kg/m2 | 1.03 | 0.91–1.18 | 0.6 |
| ASCVD | 3.19 | 2.65–3.87 | <0.0001 |
| Dyslipidemia | 2.25 | 1.87–2.72 | <0.0001 |
| Type 2 diabetes | 1.15 | 0.99–1.35 | 0.06 |
| Hypertension | 2.65 | 2.26–3.14 | <0.0001 |
| Smoking (ever) | 2.60 | 2.09–3.28 | <0.0001 |
| Family history of aortic aneurysm | 2.41 | 2.00–2.91 | <0.0001 |
| GRS | 1.06 | 1.03–1.08 | <0.0001 |
Abbreviations: AAA = abdominal aortic aneurysm; CI = confidence interval; ASCVD = atherosclerotic cardiovascular disease; GRS = genetic risk score.
Fig. 1.
Association of GRS and covariates with presence of AAA in multivariable logistic regression model.
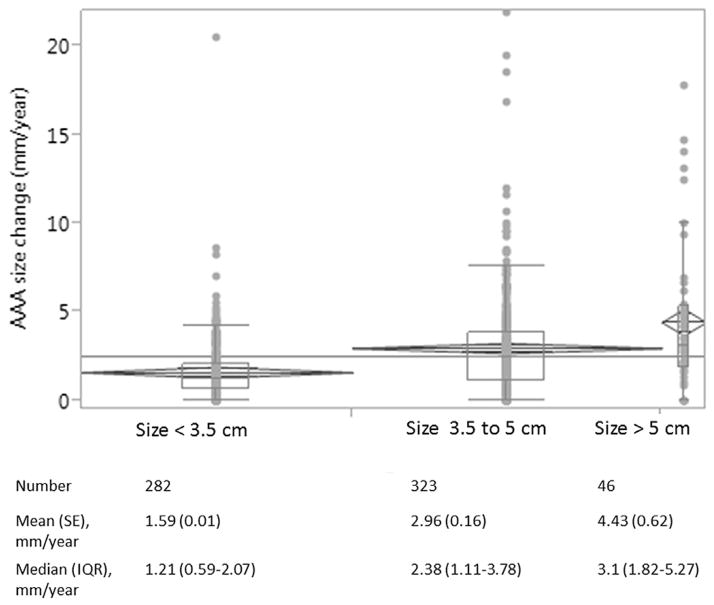
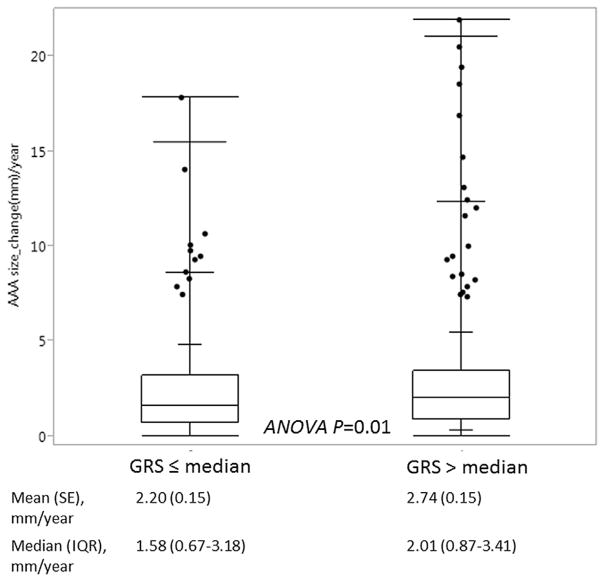
We performed analyses of aneurysm expansion in 651 cases with ≥2 measures of AAA size (pre-aneurysm repair). We compared patient characteristics in cases included versus those not included in the analyses (Supplemental Table 1). Briefly, patients included in the analysis were older, more likely to have hypertension, dyslipidemia than those not included, but no difference in mean GRS. The mean (SE) baseline AAA size of 3.69 (0.03) cm and mean (SE) growth rate was 2.47(0.11) mm/year. The mean time interval between two measures was 5.41 ± 3.56 years. The aneurysm growth rate based on baseline size is shown in Fig. 2. GRS (dichotomized by median), baseline size, diabetes and family history were each associated with aneurysm growth rate in univariate analysis (Supplement Table 2). Associations of GRS and covariates with aneurysm growth rate in a multivariable linear regression model are shown in Table 4. The estimated mean aneurysm growth rate was 0.50 mm/year greater in patients with GRS > median than those with GRS ≤ median after adjustment for covariates (Fig. 3).
Fig. 2.
Aneurysm growth rate based on baseline aneurysm size.
Table 4.
Associations of variables with aneurysm growth rate in a multivariable linear regression model.
| Regression coefficient | Std error | P-value | |
|---|---|---|---|
| Baseline aneurysm size, mm | 1.25 | 0.13 | <0.001 |
| GRS > median | 0.50 | 0.20 | 0.01 |
| Type 2 diabetes | −0.23 | 0.11 | 0.046 |
| Family history of aortic aneurysm | 0.34 | 0.14 | 0.02 |
Fig. 3.
Aneurysm growth rate in patients with GRS > median versus those ≤ median.
In sub-analysis, adding GRS to a model of age, sex and smoking history improved the c-statistic from 0.770 (95% CI: 0.756–0.784) to 0.773 (95% CI: 0.756–0.786) with significant increase in c-statistics (Δ = 0.003, p = 0.02) and improvement in risk discrimination manifested by NRI 14% (p < 0.001).
23 out of 651 patients could be classified as having high-risk aneurysm expansion. GRS, BMI and baseline aneurysm size were associated with presence of high-risk aneurysm expansion in univariate association while other covariates were not (Supplement Table 3). A higher GRS was associated with 25% greater risk of having high-risk aneurysm expansion (OR, 95% CI: 1.25, 1.06–1.47, p = 0.007). The association remained significant after adjustment for BMI and baseline size (adjusted OR, 95% CI: 1.29, 1.08–1.55, p = 0.004).
3. Discussion
The major findings of our study are: 1) a multi-locus GRS based on 4 susceptibility SNPs was associated with presence of AAA independent of conventional risk factors and family history; 2) a higher GRS was associated with greater aneurysm growth rate independent of baseline abdominal aortic size.
Age, male sex, family history and smoking are major risk factors that are considered when deciding about ultrasound screening for AAA. Such screening has decreased aneurysm-related mortality in men older than 65 years [4,20]. Although women are less likely to have AAA compared to men, women with AAA are at higher risk of aneurysm rupture, higher AAA-related mortality than men [21,22], which may due to delayed detection of the disease. Kent et al. analyzed risk factors for AAA in a population of >3 million, reporting about 50% of the patients with AAA were not eligible for screening based on the current criteria [23]. How to initiate tailored screening and improve disease identification for AAA in a cost- effective manner remains a challenge. We found that adding GRS to conventional risk factors could reclassify 132 patients as cases and 160 as non-cases resulting in a NRI of 14%. In addition, NPV of GRS alone was 0.87, while NPV of age, sex and smoking history was 0.70. Our results suggest a potential clinical application of GRS as a screening tool to improve disease detection or to rule out patients with low likelihood of having AAA before initiating imaging studies. Studies of larger samples or with longitudinal follow-up are needed to validate our results.
The GRS was associated with dyslipidemia and ASCVD, but not with hypertension, diabetes and smoking (Supplemental Table 4). No effect modification of dyslipidemia on the associated of GRS with presence of AAA was noted (p for interaction term = 0.7). This is not surprising since SNPs used to generate the GRS have been shown to be associated with lipid traits (LRP1, SORT1) and ASCVD (SORT1, DAB2IP, CDKN2A-2B), indicating pleiotropic effects of these risk variants. However, the association of GRS with AAA remained significant after adjustment for covariates and ASCVD, suggesting genetic susceptibility to AAA and both overlapping and unique mechanisms underlying two traits. We admit that unknown confounders, interaction of environmental factors (such as life-exposure to cholesterol) with susceptibility genetic variants may affect our results. More comprehensive analysis, such as pathway-based analysis may provide additional information, but this is beyond the scope of our study.
Disease-specific GRSs have been reported to improve risk prediction for different cardiovascular diseases [24]. Van’t Hof et al. [25] demonstrated that GRSs for lipid traits and coronary heart disease were associated with presence of AAA, suggesting shared genetic background of lipid levels, ASCVD and AAA. To the best of our knowledge, our study is the first to demonstrate that a disease-specific GRS is associated with AAA independent of conventional risk factors and ASCVD. Adding GRS to conventional risk factors marginally increase the c statistic, but improved risk reclassification significantly. In addition, the OR (1.31, 95%CI 1.14–1.50) of GRS > median for AAA did not significantly change after adjustment for age, sex, BMI, hypertension, type 2 diabetes, smoking, dyslipidemia and ASCVD. Our result suggests that one-time genetic profiling may identify individuals at increased risk for AAA who may benefit from aggressive treatment or life style counseling for modifiable risk factors before genetic and environmental risk factors merge to initiate development of AAA.
The risk for aneurysm rupture is mainly determined by size and growth rate. The impact of conventional risk factors on aneurysm growth is debatable. The SMART [26] study reported initial size as the only predictor of aneurysm expansion and lack of associations of other risk factors including hypertension, dyslipidemia or ASCVD. A meta-analysis of 18 studies found a higher growth rate in current smokers versus ex/non-smokers [27], whereas the UK Small Aneurysm Trial did not find an association of nicotine level with aneurysm growth [28]. One GWAS [10] reported an association of the 9p21 locus with AAA but not with aneurysm growth. We found patients with higher GRS (above median) have a higher growth rate after adjustment for baseline size, family history and diabetes, whereas individual SNPs were not associated with aneurysm growth rate after adjustment for baseline size except for DAP2IP (rs7025486) (Supplemental Table 2). In addition, a higher GRS was associated with higher likelihood of having clinically high-risk aneurysm expansion independent of baseline size.
DAB2IP is associated with endothelial cell proliferation and survival, regulating cell survival through PI3K-Akt and RAS pathways. Our results suggest that 1) cumulative effects of genetic variants at multiple loci at least partially account for aneurysm expansion; and 2) greater aneurysm growth increases wall stress (stretch) that activate DAP2IP protein, accelerating cell apoptosis; or vice versa, pro-apoptotic effect of DAP2IP accelerate aneurysm expansion. Our exploratory results suggest that patients with AAA might benefit from tailored monitoring based on the genetic profile.
Several limitations need to be mentioned. First, all subjects were from a referral population at a tertiary medical center, and the majority were of European ancestry. Hence, the generalizability to other populations or ethnicities is unclear. Second, only 60% of cases were included in the sub-analysis of aneurysm growth rate. We compared characteristics in cases included in the progression analysis versus those not included (Supplemental Table 1). There was no statistically significant difference between two groups. Third, not all controls were screened for AAA. We compared those with any abdominal imaging studies and those without (Supplement Table 6). Patients without any abdominal imaging study were less likely to have risk factors, family history, and ASCVD, but there was no statistical difference in GRS. Lastly, we used an additive model to build GRS based on the assumption that the risk for AAA is proportional to the number of risk alleles and that there is no interaction among the loci (we did not find evidence for interaction among SNPs). Genetic variants might have a dominant or recessive effect on AAA, and assuming additivity may be an over-simplification of the true biological mechanism underlying the disease. However, this is the method employed by the majority studies to assess the association of GRS based on common variants with disease of interest, and to date seems to well approximate the genetic risk for most common diseases.
In conclusion, we demonstrated that a multi-locus GRS was associated with presence of AAA and with aneurysm growth. There is an increasing interest in incorporating findings of common disease risk alleles in the clinical setting [29,30]. Our study suggests the potential of translating results from previous GWAS for AAA for use in the clinical setting, to improve disease identification and risk stratification.
Supplementary Material
Acknowledgments
All authors have approved submission of the manuscript. We thank the staff of the Medical Genome Facility Genotyping Core (GTC) at the Mayo Clinic for carrying out the genotyping for this study. The GTC is supported in part by the NCI Cancer Center Support Grant P30 CA 15083. Dr. Kullo was supported by grant U01 HG-06379 from the National Human Genome Research Institute. The publication was made possible by Center for translational Science Activities Grant UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Authors have nothing to disclose and have no relationships to industry.
Abbreviations
- AAA
abdominal aortic aneurysm
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- CI
confidence interval
- EHR
electronic health record
- GWAS
Genome-wide association studies
- OR
odds ratio
- SNP
Single nucleotide polymorphism
- T2D
Type 2 diabetes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2015.12.031.
Footnotes
Disclosure
The authors have nothing to disclose and have no relationships to industry.
Conflict of interest
None of the authors have any conflict of interest to report.
References
- 1.Go AS, et al. Heart disease and stroke statistics–2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pande RL, Beckman JA. Abdominal aortic aneurysm: populations at risk and how to screen. J Vasc Interv Radiol. 2008;19:S2–8. doi: 10.1016/j.jvir.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 4.Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the u.S. Preventive services task force. Ann Intern Med. 2014;160:321–329. doi: 10.7326/M13-1844. [DOI] [PubMed] [Google Scholar]
- 5.Powell JT, Greenhalgh RM. Multifactorial inheritance of abdominal aortic aneurysm. Eur J Vasc Surg. 1987;1:29–31. doi: 10.1016/s0950-821x(87)80020-8. [DOI] [PubMed] [Google Scholar]
- 6.Bown MJ, et al. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. Am J Hum Genet. 2011;89:619–627. doi: 10.1016/j.ajhg.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley DT, et al. A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet. 2013;6:498–504. doi: 10.1161/CIRCGENETICS.113.000165. [DOI] [PubMed] [Google Scholar]
- 8.Elmore JR, et al. Identification of a genetic variant associated with abdominal aortic aneurysms on chromosome 3p12.3 by genome wide association. J Vasc Surg. 2009;49:1525–1531. doi: 10.1016/j.jvs.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Gretarsdottir S, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–697. doi: 10.1038/ng.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helgadottir A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 11.Jones GT, et al. A sequence variant associated with sortilin-1 (sort1) on 1p13.3 is independently associated with abdominal aortic aneurysm. Hum Mol Genet. 2013;22:2941–2947. doi: 10.1093/hmg/ddt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tragante V, et al. The impact of susceptibility loci for coronary artery disease on other vascular domains and recurrence risk. Eur Heart J. 2013;34:2896–2904. doi: 10.1093/eurheartj/eht222. [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Kalloo FS, Dalenberg AK, Kullo IJ. An electronic medical record-linked biorepository to identify novel biomarkers for atherosclerotic cardiovascular disease. Glob Cardiol Sci Pract. 2013;2013:82–90. doi: 10.5339/gcsp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devaraj S, Dodds SR. Ultrasound surveillance of ectatic abdominal aortas. Ann R Coll Surg Engl. 2008;90:477–482. doi: 10.1308/003588408X301064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freiberg MS, et al. Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events: 10-year follow-up data from the cardiovascular health study. Circulation. 2008;117:1010–1017. doi: 10.1161/CIRCULATIONAHA.107.720219. [DOI] [PubMed] [Google Scholar]
- 16.Ding K, Bailey KR, Kullo IJ. Genotype-informed estimation of risk of coronary heart disease based on genome-wide association data linked to the electronic medical record. BMC Cardiovasc Disord. 2011;11:66. doi: 10.1186/1471-2261-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullo IJ, et al. Leveraging informatics for genetic studies: use of the electronic medical record to enable a genome-wide association study of peripheral arterial disease. J Am Med Inf Assoc. 2010;17:568–574. doi: 10.1136/jamia.2010.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.State-specific Secondhand Smoke Exposure and Current Cigarette Smoking Among Adults – United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1232–1235. [PubMed] [Google Scholar]
- 19.LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive services task force recommendation statement. Ann Intern Med. 2014;161:281–290. doi: 10.7326/M14-1204. [DOI] [PubMed] [Google Scholar]
- 20.Sidloff D, et al. Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation. 2014;129:747–753. doi: 10.1161/CIRCULATIONAHA.113.005457. [DOI] [PubMed] [Google Scholar]
- 21.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the united states, 2001–2004. J Vasc Surg. 2007;45:891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Mureebe L, Egorova N, McKinsey JF, Kent KC. Gender trends in the repair of ruptured abdominal aortic aneurysms and outcomes. J Vasc Surg. 2010;51:9S–13S. doi: 10.1016/j.jvs.2009.10.129. [DOI] [PubMed] [Google Scholar]
- 23.Kent KC, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 24.Mega JL, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015 Jun 6;385(9984):2264–2271. doi: 10.1016/S0140-6736(14)61730-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van ’tHof FN, et al. Impact of inherited genetic variants associated with lipid profile, hypertension, and coronary artery disease on the risk of intracranial and abdominal aortic aneurysms. Circ Cardiovasc Genet. 2013;6:264–270. doi: 10.1161/CIRCGENETICS.113.000022. [DOI] [PubMed] [Google Scholar]
- 26.Schlosser FJ, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47:1127–1133. doi: 10.1016/j.jvs.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 27.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 28.Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 29.Kullo IJ, et al. Return of results in the genomic medicine projects of the emerge network. Front Genet. 2014;5:50. doi: 10.3389/fgene.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullo IJ, Jarvik GP, Manolio TA, Williams MS, Roden DM. Leveraging the electronic health record to implement genomic medicine. Genet Med. 2013;15:270–271. doi: 10.1038/gim.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.