Abstract
Purpose/Objectives
Adjuvant endocrine therapy (AET) has been shown to improve survival in hormone receptor–positive breast cancer survivors, but as many as half do not complete recommended treatment. Management of medication-related side effects and engagement with providers are two potentially modifiable factors, but their associations with adherence are not well understood. The aims were to build on survey results to qualitatively explore survivors’ experiences with prescribed AET to (a) describe appraisal and management of AET side effects and (b) deconstruct decisions to initiate, discontinue, or maintain AET.
Research Approach
The authors used a mixed-methods explanatory sequence research design with a qualitative emphasis.
Setting
Survivors were recruited from a clinical cancer registry maintained at the University of Texas Southwestern Medical Center, which includes the Harold C. Simmons Comprehensive Cancer Center (National Cancer Institute–designated), in Dallas.
Participants
452 survivors completed a survey, and 30 took part in telephone interviews.
Methodologic Approach
Qualitative methods were used in which the authors recorded and transcribed interviews for analysis and used open coding to reduce data into themes.
Findings
Among adherent survivors, the themes of tolerance of side effects and perseverance were strong. Nonadherent survivors expressed more difficulty managing side effects and perceived fewer benefits when side effects were bothersome. The most common side effects mentioned by all survivors were menopausal symptoms and joint pain; less common side effects were cognitive decline and cardiac distress. Some sought advice from their oncology team. Nonadherent survivors appeared initially motivated to maintain AET but identified a tolerance limit for side effects after which a provider’s recommendation was less influential in their decision to maintain or discontinue AET.
Interpretation
This study elucidated adherence as a complex continuum of behaviors, appraisals, and decision points. These insights may be particularly useful in counseling survivors taking AET and promoting timely delivery of clinical interventions to enhance adherence.
Implications for Nursing
Nurses should be involved in the planning and implementation of clinical interventions to manage side effects and other barriers to AET adherence.
Keywords: breast cancer survivors, side effects, adjuvant endocrine therapy, survivorship care, symptoms
Adjuvant endocrine therapy (AET) (including tamoxifen [Nolvadex®] and aromatase inhibitors [AIs]) is widely recognized as a critical component of breast cancer treatment for women with hormone receptor–positive disease (Chlebowski & Geller, 2006; Chlebowski, Kim, & Haque, 2014). Several randomized, controlled trials have demonstrated significant reductions for recurrence risk and mortality in women treated with tamoxifen (Early Breast Cancer Trialists’ Collaborative Group, 2005, 2011), and similar results have been found with AIs (Dowsett et al., 2010). Clinical guidelines have historically recommended AET to women with hormone receptor–positive disease for five years following primary treatment (Burstein et al., 2010). Updated guidelines now recommend as many as 10 years of continuous therapy (Burstein et al., 2014; Burstein, Lacchetti, & Griggs, 2016) in light of emerging data demonstrating increased survival benefits for a longer period of treatment (Davies et al., 2013; Gray et al., 2013; Regan, 2015). Despite the evidence of benefits, as many as 50% of eligible women do not initiate AET or do not complete the recommended duration of therapy (Chlebowski et al., 2014; Hershman et al., 2010). This estimate suggests greater nonadherence than for other medications prescribed for older adults with chronic diseases (DiMatteo, 2004). Comparatively, a quantitative review of 569 empirical studies reported mean medication adherence rates of 68% for diabetes, 77% for cardiovascular diseases, and 81% for arthritis medications among older adults (DiMatteo, 2004).
Some studies have suggested that modifiable factors, such as patient–provider communication and improved management of medication-related side effects, may promote adherence to these therapies (Arriola et al., 2014; Lin, Zhang, & Manson, 2011), but the full range and complexity of factors influencing adherence are not well understood (Murphy, Bartholomew, Carpentier, Bluethmann, & Vernon, 2012). Among women prescribed AET, one survey estimated that 39%–46% of users required additional support (typically medical interventions, such as nonsteroidal anti-inflammatory drugs or sleep medication) to manage medication-related side effects, including hot flashes, insomnia, and joint pain (Garreau, Delamelena, Walts, Karamlou, & Johnson, 2006). However, few effective interventions are designed to help survivors address these side effects, and efforts to improve adherence have had limited success (Haynes, Ackloo, Sahota, McDonald, & Yao, 2008; Mathes, Antoine, Pieper, & Eikermann, 2014; Touchette & Shapiro, 2008). In addition, few interventions address the roles of the patient and provider in adherence. Given the longer period of treatment now recommended, a deeper understanding of the experience from the patient perspective is needed to develop appropriate clinical interventions to support patients as they initiate and maintain their treatment course.
Information is lacking about the process survivors use when making decisions about whether to initiate, maintain, or discontinue AET. The purpose of this mixed-methods study was to explore the range of survivors’ experiences with prescribed AET by purposively sampling survivors who are adherent and nonadherent for interviews. The aims were to build on results from a previously conducted survey to qualitatively (a) describe survivors’ reported appraisal and management of medication-related side effects and (b) deconstruct survivors’ decisions to initiate, discontinue, or maintain AET.
Methods
To characterize the breast cancer survivor experience with AET, the authors chose a mixed-methods explanatory sequence research design with a qualitative emphasis (Creswell, 2013). Mixed-methods designs provide an opportunity for researchers to systematically combine the strengths of quantitative data with qualitative data to produce richer insights about complex research questions than is possible with either method alone (Creswell & Tashakkori, 2007; Dures, Rumsey, Morris, & Gleeson, 2011). For the quantitative component, the authors used a clinical cancer registry to invite 1,510 cancer survivors to participate in a survey on survivorship care (Carpentier et al., 2013; Hamann et al., 2013). The target population for the qualitative interviews was the subset of respondents (n = 204) who indicated that a physician had recommended AET and who were willing to be contacted for further research. The authors then used a purposive sampling technique to recruit 30 women, a number recommended to achieve theoretical saturation in qualitative designs (Bowen, 2008), to create two approximately equal groups of participants (i.e., 17 adherent and 13 nonadherent survivors). The authors used survey responses to characterize survivors as adherent if they reported completing or were currently taking medication as prescribed, and as nonadherent if medication use was discontinued at some point before the end of the prescribed period. To recruit participants, the authors first mailed invitation letters to 68 eligible survivors, inviting participation in 30-minute telephone interviews and providing a toll-free telephone number for additional information or to opt out. After two weeks, the authors telephoned survivors who did not opt out to further describe the study and determine interest in participating. Survivors who completed interviews received a $10 gift card as compensation for their time. The study was approved by the institutional review boards of the University of Texas Health Science Center at Houston and the University of Texas Southwestern Medical Center in Dallas.
Three interviewers conducted all interviews by telephone; they were recorded and transcribed verbatim for analysis. All interviewers had similar training in behavioral research and experience in conducting telephone-based interviews. Two of the interviewers subsequently served as primary coders, and their insight from having conducted the original interviews was a strength in interpreting comments in transcripts.
All interviews were 30–60 minutes in length and were completed within an eight-week timeframe (from January to March 2013). During interviews, participants were first asked to describe the type of AET they were prescribed (i.e., tamoxifen or AI) and whether they were currently taking, had completed, or had discontinued therapy. Interviews followed a semistructured format with open-ended questions and a series of follow-up questions (see Appendix A for a telephone interview question guide). Informed by grounded theory approaches (Strauss & Corbin, 1990), two primary coders used an open coding procedure to develop a code book with examples and specific coding criteria (MacQueen, McLellan, Kay, & Milstein, 1998). To evaluate inter-rater reliability, the authors used a percent agreement exercise recommended for qualitative researchers (MacQueen et al., 1998) in which they randomly selected one interview that they independently coded. Results demonstrated strong agreement (81%) in coding procedures.
The authors analyzed the 30 interviews in three batches of 10 interviews each, updating codes and resolving coding disagreements with assistance from coauthors until consensus was reached. They examined overall frequency of codes and specific similarities and differences between adherent and nonadherent participants. The authors also triangulated their survey results with relevant qualitative data to confirm emerging themes. All authors reviewed and were in agreement with the subthemes and general themes from the analysis.
Findings
Most interview participants (N = 30) were Caucasian, married, and college educated, with a mean age of 57 years (range = 49–86 years) (see Table 1). Time since diagnosis was less than five years for most survivors, and most were diagnosed at disease stages I or II. In addition, most survivors reported that their oncologist was their primary provider. The mean reported time on therapy was 2.6 years (SD = 1.99) for all survivors (adherent and nonadherent). Based on interviews, most participants reported taking AIs at initiation and some reported switching medications. Survivors who participated in interviews did not differ from survey respondents on sociodemographic variables. Survivors who were not eligible to participate in interviews (i.e., those who did not receive a doctor’s recommendation for AET or were not willing to be contacted for research) were more likely to be aged older than 65 years, be unmarried, have in situ disease, or have less than a high school education, but did not differ significantly from eligible survivors in terms of race/ethnicity, time since diagnosis, insurance status, or access to care.
TABLE 1.
Comparison of Sociodemographics and Medical History of Survey Respondents and Interview Participants
| Survey Respondents Recruited From Registry (N = 452) |
Subset Eligible for Interview (N = 204) |
All Interviewees (N = 30) |
Adherent Interviewees (N = 17) |
Nonadherent Interviewees (N = 13) |
||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||
| Variable | n | % | n | % | n | n | n | pa |
| Sociodemographics | ||||||||
|
| ||||||||
| Age (years) | 0.203 | |||||||
| 49 or younger | 63 | 14 | 27 | 13 | 1 | 1 | – | |
| 50–64 | 228 | 50 | 118 | 58 | 18 | 9 | 9 | |
| 65 and older | 161 | 36 | 59 | 29 | 11 | 7 | 4 | |
| Race or ethnicity | 0.181 | |||||||
| Non-Hispanic White | 336 | 74 | 160 | 78 | 27 | 15 | 12 | |
| Non-Hispanic Black | 65 | 14 | 22 | 11 | 1 | – | 1 | |
| Hispanic | 37 | 8 | 15 | 7 | 2 | 2 | – | |
| Other or unknown | 14 | 3 | 7 | 3 | – | – | – | |
| Marital statusb | 0.453 | |||||||
| Married or partnered | 300 | 66 | 148 | 73 | 22 | 14 | 8 | |
| Other | 147 | 33 | 55 | 27 | 8 | 3 | 5 | |
| Missing data | 5 | 1 | 1 | 1 | – | – | – | |
| Educationb | 0.096 | |||||||
| High school diploma | 78 | 17 | 23 | 11 | 4 | 2 | 2 | |
| Some college or technical school | 140 | 31 | 58 | 28 | 5 | 3 | 2 | |
| Bachelor’s degree | 138 | 31 | 71 | 35 | 15 | 9 | 6 | |
| Graduate degree | 92 | 20 | 52 | 25 | 6 | 3 | 3 | |
| Missing data | 4 | 1 | – | – | – | – | – | |
|
| ||||||||
| Medical history | ||||||||
|
| ||||||||
| Tumor stagec | 0.174 | |||||||
| 0 | 81 | 18 | 25 | 12 | 5 | 4 | 1 | |
| I | 137 | 30 | 63 | 31 | 13 | 5 | 8 | |
| II | 114 | 25 | 58 | 28 | 3 | 3 | – | |
| III | 33 | 7 | 17 | 8 | 2 | 2 | – | |
| Missing data | 87 | 19 | 41 | 20 | 7 | 3 | 4 | |
| Years since diagnosis | 0.616 | |||||||
| Fewer than five | 313 | 69 | 140 | 69 | 22 | 13 | 8 | |
| Five or more | 139 | 31 | 64 | 31 | 8 | 4 | 5 | |
| Doctor recommendation for taking AET | – | |||||||
| Yes | 279 | 62 | 204 | 100 | 30 | 17 | 13 | |
| No | 159 | 35 | – | – | – | – | – | |
| Missing data | 14 | 3 | – | – | – | – | – | |
| AET at initiation (self-report)d | – | |||||||
| Tamoxifen or raloxifene | – | – | – | – | 10 | – | – | |
| Aromatase inhibitors | – | – | – | – | 20 | – | – | |
| Switched medication | – | |||||||
| Yes | – | – | – | – | 8 | – | – | |
| No | – | – | – | – | 22 | – | – | |
|
| ||||||||
| Access and use | ||||||||
|
| ||||||||
| Received care in 2010–2011 | – | |||||||
| Yes | 403 | 89 | 21 | 10 | 27 | 14 | 13 | |
| No | 49 | 11 | 183 | 90 | 3 | 3 | – | |
| Provider seenb | 0.812 | |||||||
| None | 48 | 11 | 21 | 10 | 3 | 2 | 1 | |
| PCP | 12 | 3 | 4 | 2 | – | – | – | |
| Oncologist | 313 | 69 | 148 | 73 | 22 | 11 | 11 | |
| PCP and oncologist | 79 | 17 | 31 | 15 | 5 | 4 | 1 | |
| Overall healthb | 0.801 | |||||||
| Excellent | 61 | 13 | 31 | 15 | 5 | 3 | 3 | |
| Very good | 167 | 37 | 75 | 37 | 12 | 7 | 2 | |
| Good | 159 | 35 | 74 | 36 | 8 | 5 | 7 | |
| Fair or poor | 57 | 13 | 21 | 10 | 4 | 1 | 2 | |
| Missing data | 8 | 2 | 3 | 1 | 1 | 1 | – | |
|
| ||||||||
| Cancer concernse | ||||||||
|
| ||||||||
| Cancer worry | – | |||||||
| High | 243 | 54 | 107 | 52 | 17 | 8 | 9 | |
| Low | 201 | 44 | 94 | 46 | 12 | 8 | 4 | |
| Missing data | 8 | 3 | 3 | 1 | 1 | 1 | – | |
| Outcome expectations | – | |||||||
| High | 82 | 18 | 28 | 14 | 3 | 1 | 2 | |
| Low | 358 | 79 | 173 | 85 | 26 | 15 | 11 | |
| Missing data | 12 | 3 | 3 | 1 | 1 | 1 | – | |
The p value reflects comparisons between survey respondents and interview participants on variables of interest.
These variables were assessed in the survey only.
Tumor stage was only collected consistently in registry data beginning in 2006.
These data were not available in the registry and were obtained by self-report in interviews.
Participants reported cancer concerns in the survey using a five-point Likert-type scale from 1 (strongly disagree) to 5 (strongly agree). Responses for cancer worry and outcome expectations were subsequently dichotomized (high = greater than 3, low = 3 or less).
AET—adjuvant endocrine therapy; PCP—primary care provider
Note. Because of rounding, percentages may not total 100.
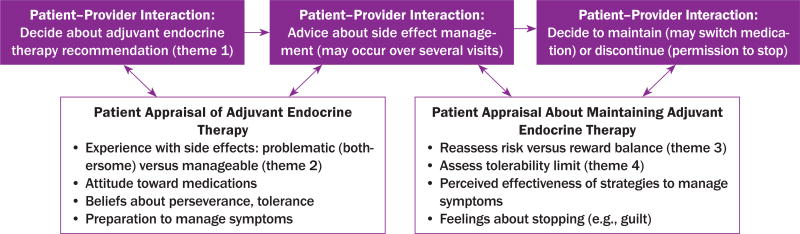
From the interviews, the authors identified four major themes that described the range of AET experiences for adherent and nonadherent survivors and guided their decision-making processes to initiate and maintain therapy (see Figure 1). The themes influencing decisions were (a) initial acceptance of the provider recommendation for AET, (b) variable experiences with side effects, (c) risk versus reward of maintaining AET, and (d) ability to tolerate side effects. The authors also identified that challenges in patient–provider communication were a cross-cutting issue across all themes.
FIGURE 1.
General Themes and Supporting Quotes
Theme 1: Initial Acceptance of the Provider Recommendation for Adjuvant Endocrine Therapy
Adherent and nonadherent survivors reported being receptive to taking AET when it was recommended by their doctor, the first in a sequence of patient–provider interactions related to adherence decisions. For example, some said they were “at least willing to give [AET] a try” simply based on their doctor’s recommendation. Other survivors viewed AET as a part of treatment they were “obligated” to do, in some cases saying that “it was not an option” to forego the therapy. Many participants expressed that the relationship with their oncologist (many survivors continued seeing their oncologist as their primary doctor) influenced their trust in the doctor’s recommendation and their willingness to take AET as part of their ongoing breast cancer care. One woman described it this way: “I believe in my doctors, so if they tell me [taking AET] is the right thing to do, that’s what I’ve got to do.”
Theme 2: Variable Experiences With Side Effects
Adherent and nonadherent survivors reported experiencing side effects with AET (see Figure 2), which prompted self-appraisal of side effects and effectiveness of management strategies. The most common side effects reported were menopausal symptoms (e.g., facial flushing, hot flashes) and joint pain (e.g., arthralgia, bone pain). However, survivors also reported less common side effects that were problematic, including cardiac distress (e.g., chest pain), fractures, blood clots, and cognitive changes. In some cases, women reported that they felt blindsided by the side effects. Describing her cognitive changes after initiating AET, one survivor said, “I didn’t even know my body was going to go through that. It hit me like a boom.” The authors also noted that the severity of these side effects for survivors was highly variable, particularly for older women and those who reported other comorbidities, such as cardiac or musculoskeletal ailments. Consistent with other studies (Garreau et al., 2006), the authors noted that survivors who reported taking AIs more frequently described bone- or joint-related symptoms. As one said, “I already had osteoporosis to start with, and the Femara® certainly did not help.”
FIGURE 2.
Patient–Provider Interactions About Adjuvant Endocrine Therapy Decisions and Patient Appraisals
Among adherent survivors, subthemes of tolerance of side effects and perseverance were strong, particularly when they were able to partner with their providers to resolve symptoms. Many adherent survivors expressed that side effects were “no big deal” in their everyday lives and were typically more manageable (e.g., brittle fingernails) than other common side effects. Others described a positive attitude as part of perseverance and their strategy to adhere to treatment. For example, one survivor said, “If you have an issue … you attack it and resolve the problem first. Then, you keep a really positive attitude about the outcome.”
Nonadherent survivors also reported greater difficulty resolving their symptoms, particularly when they were unexpected or unusual. One woman said,
[My doctor] told me I would probably have night sweats and hot flashes, but that’s all I really expected. I didn’t expect the [severe side effects] I had. … It started with pain in my shoulders, and then it moved to my jaw. Eventually, it moved to every joint in my body.
Several survivors expressed that they did not feel prepared to manage side effects, often sharing that they received little or almost nothing in terms of information about expected side effects or management strategies from their provider. This was particularly true when the side effects were nonspecific—in other words, when survivors said they did not feel well or did not feel like themselves on the medication, a subtheme many nonadherent survivors characterized as persistent and unpleasant. For example, one woman said, “I just don’t feel exactly like myself [on Arimidex®]. I don’t feel real clear-headed, and I feel groggy a lot of the time. If you’re not sleeping well, you don’t know if one thing causes the other.”
The two most commonly reported medication-related side effects were menopausal symptoms and joint pain, but differences were seen in how problematic these symptoms were for survivors interviewed. The authors noted that 13 survivors mentioned menopausal symptoms (including vaginal dryness, hot flashes, and facial flushing) as part of their interviews and that this was equivalent between adherent and nonadherent participants. However, more than half of those women also described their menopausal symptoms as manageable and did not characterize these as problematic side effects of medication. In contrast, joint pain was often described as bothersome or in the context of an extreme or debilitating side effect, particularly by nonadherent survivors. As the interviews conveyed, these experiences were quite variable across survivors taking the medications.
Theme 3: Risk Versus Reward
The prospect of preventing breast cancer recurrence was generally a compelling AET benefit described by adherent and nonadherent survivors in weighing risks and rewards of maintaining AET in the face of challenging side effects. One survivor said, “I just felt like this was what I had to do to keep the cancer from coming back, and I’ll do what I have to do.” Adherent survivors more often described coping strategies to persevere in the face of side effects, even when providers did not give advice to help manage medication-related symptoms. Of her bothersome hot flashes, one survivor said, “I mentioned it to [my doctor], but I knew it was just one of those things I would have to cope with, so I just did.” However, for survivors who experienced extreme or debilitating side effects, the benefits of maintaining AET in the face of quality-of-life challenges were less clear. One survivor said, “I called my doctor and told her I was going to stop taking Femara, that it was affecting me adversely, and that my quality of life was more important.” In consulting with clinicians, survivors sought doctor-recommended strategies for symptom management but were frequently dissatisfied with the results or consultation. One survivor said,
Nothing really worked for my [extreme joint pain]. [My doctor] told me to change the timing, taking it in the evening. … We tried all kinds of things. I took Tylenol® … all kinds of stuff … but it didn’t work. … I felt like a 90-year-old woman, so I said, “This is not worth it, and I should stop.”
Participants first reported their cancer concerns in the survey (e.g., cancer worry, outcome expectations) using a validated cancer scale (Crespi, Ganz, Petersen, Castillo, & Caan, 2008). In comparing these responses to qualitative data from these same participants, the authors observed that a slightly larger proportion of adherent survivors (n = 15) reported greater outcome expectations than nonadherent survivors (n = 11). Similarly, a greater proportion of adherent survivors (n = 8) reported greater cancer worry (e.g., fear of recurrence) than nonadherent survivors (n = 4). For the participants, these cancer concerns were relevant factors in making decisions about adherence.
Theme 4: Ability to Tolerate Side Effects
Though generally motivated to maintain AET, many nonadherent survivors identified a limit to which they were willing to tolerate negative, unresolved side effects, after which the provider’s recommendation to continue therapy was less influential. For nonadherent survivors, some mentioned that stopping medication (on their own or in consultation with providers) offered immediate relief. One participant said, “I was walking with crutches and canes for support, but after I stopped taking the tamoxifen, the pain subsided.” Because of extreme physical or emotional distress, some survivors reported that the possibility of facing breast cancer again was preferable to continuing AET. According to one survivor,
I took myself off the medicine. I went to my primary care physician and told him what I had done. He almost had a heart attack … and I said, “I’ve had tamoxifen, and I’ve had breast cancer. I would rather have breast cancer.”
Although some survivors expressed emotional conflict (e.g., guilt, worry) over the decision to discontinue treatment, the authors also noted a descriptive subtheme that nonadherent survivors found that AET simply was not suitable for everyone. As one survivor said, “I’m not totally against the medication. I think it just wasn’t for me.”
Some survivors who chose to discontinue therapy did so without the provider’s approval, but adherent and nonadherent survivors expressed a preference to engage in joint decision making regarding their treatment decisions. One adherent survivor said, “Talking to my oncologist and my history of the cancer coming back, we talked about the tamoxifen and going on it. That’s when I decided to go ahead and get back on.” Joint decision making was more commonly mentioned by nonadherent survivors, some of whom reported finding that open discussions with their providers eased anxiety about making the right decision for them. For example,
[My oncologist] pulled out my chart … all my history, and we sat down. She said, “You have a very low risk of the disease coming back, and you are very sensitive to drugs. [Given the odds], it would be OK if you went off the drug.” She said, “I have no problem if you make that decision.” So, that’s what I did.
Discussion
This mixed-methods study (emphasizing qualitative insights) highlighted a range of physical and emotional experiences of survivors taking AET, punctuated by interactions with the medical team and changing appraisals about its importance in reducing cancer and mortality risk. Although the decision to initiate AET appeared to be straightforward for most survivors, the overall diversity of experiences implies that no single strategy supports survivors throughout endocrine therapy. However, collectively, the findings underscore the value of patient-centered care from a multilevel perspective (Zapka, Taplin, Ganz, Grunfeld, & Sterba, 2012) in understanding the challenges of cancer recovery outside of primary treatment.
The current study elucidated AET as a complex continuum of behaviors, appraisals, and decision points at the initiation of therapy, during the course of treatment (including onset of side effects), and following success or lack of success in coping efforts. These behaviors may include changing daily habits (e.g., increasing exercise), seeking support from family and friends, and assessing whether coping efforts were effective in reducing any unpleasant symptoms. Many survivors described that timely advice from providers about side effect management was an important element of their appraisal and ultimate decision to maintain or discontinue therapy. However, many survivors reported what could be construed as missed opportunities to provide information or advice for symptom management during medical encounters. In some cases, survivors felt exceptionally unprepared or blindsided by side effects, diminishing their willingness to continue treatment or receive medical counseling. In a few cases, survivors experienced side effects that were so debilitating that the fear of recurrence seemed preferable to enduring ongoing therapy. Although this was not the case for most participants, it does highlight the potentially avoidable distress even common side effects can cause survivors when not addressed in a timely manner. More effective strategies for supporting survivors and resolving their concerns are urgently needed to minimize this distress.
Strengths and Limitations
The current study was strong in several ways. To the authors’ knowledge, this was one of the first studies to (a) investigate the role of AET adherence through a mixed-methods design (i.e., used quantitative and qualitative data), (b) use registry data to identify adherent versus nonadherent survivors, and (c) compare these groups to understand factors influencing adherence decisions from the perspective of adherent and nonadherent survivors. Many studies of adherence use prescription claims data only. Although administrative data provide researchers with an objective measure of adherence, they do not contain information on modifiable factors associated with long-term adherence. This is an advantage in using patient experiences described in qualitative interviews. Although qualitative data added richness to the interpretation of quantitative data, they are by nature not necessarily representative or generalizable, a limitation in comparing these results to those of other survivors. However, qualitative and quantitative studies have had similar results (Garreau et al., 2006; Mathes et al., 2014), suggesting that the burden of complications needs to be assessed and addressed via the recommendation of management and coping strategies to prevent survivors from deciding to discontinue therapy.
Implications for Nursing and Intervention Opportunities
The results of the current study provide direct implications for nurses, who should be involved in the planning and implementation of interventions to facilitate adherence. Few interventions have been found to be effective for managing side effects for the duration of therapy. Some evidence suggests that tailored patient counseling, particularly for older adult survivors, may be initially beneficial to promoting adherence (Hugtenburg, Timmers, Elders, Vervloet, & van Dijk, 2013); however, in general, clinical interventions for medication adherence have not included behavioral approaches beyond reminder systems (Easthall, Song, & Bhattacharya, 2013; Hurtado-de-Mendoza, Cabling, Lobo, Dash, & Sheppard, 2016). Increasing knowledge of side effects, communicating the benefits of the prescribed medication to improve adherence motivation, and identifying opportunities to resolve barriers to medication use (such as side effects) have been highlighted in reviews as critical components in a long-term adherence model in clinical practice (Accordino & Hershman, 2013; DiMatteo, Haskard-Zolnierek, & Martin, 2012). Oncology nurses can play an important role in informing patients about expected risk and side effects, which could prevent early disruptions in adherence (Heisig et al., 2015) and facilitate shared decision making. Additional tools, for use by physicians and other members of the oncology team (Taplin, Foster, & Shortell, 2013), are also needed to specifically assess and monitor symptoms before symptom burden becomes intolerable. For example, quality-of-life assessment may promote identification of appropriate management strategies for survivors who experience side effects (Cella & Fallowfield, 2008). Nurses can play an important role in discussing coping strategies, including exercise, which may mitigate symptom burden in cancer survivors (Bluethmann et al., 2015). Older adult survivors and survivors with preexisting comorbidities (Bluethmann, Mariotto, & Rowland, 2016), may also be at increased risk for polypharmacy and related medication complications that may diminish AET tolerability and, in turn, benefits for cancer survival (Fontein et al., 2013; Puts et al., 2014).
Conclusion
As providers begin to implement the new American Society of Clinical Oncology guidelines that recommend a longer duration of AET in clinical practice, the patient experiences described may also provide useful direction in following recommendations for quality survivorship care (McCabe et al., 2013). Among these considerations, physicians should recognize that AET may not be feasible for all breast cancer survivors with hormone receptive–positive disease without support from their healthcare team. From the current study, this may be particularly true for older survivors, those with comorbidities, and those who experience extreme or debilitating side effects. Early assessment and management of symptoms may improve opportunities to maintain therapy as recommended.
Knowledge Translation.
Most survivors made the decision to initiate endocrine therapy when recommended, but information and support are necessary for survivors to maintain a long-term medical regimen.
Early assessment and management of medication-related side effects may improve opportunities to maintain endocrine therapy as recommended.
Endocrine therapy may not be feasible for every survivor, particularly for older survivors or those with comorbidities or debilitating treatment side effects.
Acknowledgments
Bluethmann and Mollica were supported through the Cancer Prevention Fellowship at the National Cancer Institute. Bluethmann also received funding through the Cancer Education and Career Development Program at the School of Public Health, University of Texas Health Science Center at Houston (R25CA57712). The findings and conclusions are those of the authors and do not necessarily represent the official positions of the National Cancer Institute/National Institutes of Health. Mention of specific products and opinions related to those products do not indicate or imply endorsement by the Oncology Nursing Society.
Bluethmann, Murphy, Tiro, and Bartholomew contributed to the conceptualization and design. Bluethmann, Murphy, and Tiro led analysis and interpretation of data. Murphy provided statistical support.
Appendix A
Telephone Interview Questions Guide
GENERAL QUESTIONS
When were you diagnosed with breast cancer?
Did your doctor recommend you take endocrine therapy as part of your breast cancer treatment?
Which form of therapy were you prescribed?
What kind of information on endocrine therapies did your doctor provide you?
How long were you supposed to take the medication? How many years?
Were you pre- or postmenopausal when you started taking the medication?
Are you currently taking the medication?
If no:
When did you stop (about how long did you take the medication)?
Tell me about your experience with the medication.
Tell me about the reasons for stopping (probes: any other reasons, side effects, concerns about the medication?).
Who did you talk to about stopping the medication?
What advice/help did you receive try to resolve problems with the medication?
What information were you given about possible side effects when you started the medication? What strategies did you try to cope with or reduce the side effects? How did side effects interfere with your everyday routine?
How do you think your experience compares to other women?
How does your experience compare to your expectations before starting treatment?
If yes (still taking):
Please tell me about your experience with the medication.
What is your daily routine for remembering to take the medication?
What aspects of taking the medication have you found most difficult? Easy?
What side effects have you had? If had side effects, what role does your doctor play in managing side effects? What advice/help did you receive to try to resolve problems with the medication? How did side effects interfere with your everyday routine?
How do you think your experience compares to other women?
How does your experience compare to your expectations before starting treatment?
Describe your motivation for continuing the medication.
SURVIVORSHIP QUESTIONS
Since completing your breast cancer treatment, have you made any other changes to your life (probe: change eating habits, start exercising)?
If yes, what motivated you to make those changes?
What tools/strategies do you use to manage maintaining a healthy lifestyle and/or keeping up with appointments?
How often do you see your oncologist now? What do you discuss during your appointment?
Footnotes
All of the authors approved final analysis and contributed to the manuscript preparation.
References
- Accordino MK, Hershman DL. Disparities and challenges in adherence to oral antineoplastic agents. American Society of Clinical Oncology Educational Book. 2013:271–276. doi: 10.14694/EdBook_AM.2013.33.271. [DOI] [PubMed] [Google Scholar]
- Arriola KR, Mason TA, Bannon KA, Holmes C, Powell CL, Horne K, O’Regan R. Modifiable risk factors for adherence to adjuvant endocrine therapy among breast cancer patients. Patient Education and Counseling. 2014;95:98–103. doi: 10.1016/j.pec.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, Demark-Wahnefried W. Grasping the ‘teachable moment’: Time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psycho-Oncology. 2015;24:1250–1257. doi: 10.1002/pon.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiology, Biomarkers and Prevention. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen GA. Naturalistic inquiry and the saturation concept: A research note. Qualitative Research. 2008;8:137–152. [Google Scholar]
- Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression summary. Journal of Oncology Practice. 2016;12:390–393. doi: 10.1200/JOP.2016.011239. [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. Journal of Clinical Oncology. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. Journal of Clinical Oncology. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier MY, Tiro JA, Savas LS, Bartholomew LK, Melhado TV, Coan SP, Vernon SW. Are cancer registries a viable tool for cancer survivor outreach? A feasibility study. Journal of Cancer Survivorship. 2013;7:155–163. doi: 10.1007/s11764-012-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Research and Treatment. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Kim J, Haque R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prevention Research. 2014;7:378–387. doi: 10.1158/1940-6207.CAPR-13-0389. [DOI] [PubMed] [Google Scholar]
- Crespi CM, Ganz PA, Petersen L, Castillo A, Caan B. Refinement and psychometric evaluation of the impact of cancer scale. Journal of the National Cancer Institute. 2008;100:1530–1541. doi: 10.1093/jnci/djn340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JW. Research design: Qualitative, quantitative, and mixed methods approaches. Thousand Oaks, CA: Sage; 2013. [Google Scholar]
- Creswell JW, Tashakkori A. Differing perspectives on mixed methods research. Journal of Mixed Methods Research. 2007;1:303–308. [Google Scholar]
- Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Peto R. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Medical Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Haskard-Zolnierek KB, Martin LR. Improving patient adherence: A three-factor model to guide practice. Health Psychology Review. 2012;6:74–91. [Google Scholar]
- Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. Journal of Clinical Oncology. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- Dures E, Rumsey N, Morris M, Gleeson K. Mixed methods in health psychology: Theoretical and practical considerations of the third paradigm. Journal of Health Psychology. 2011;16:332–341. doi: 10.1177/1359105310377537. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive-based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3(8):e002749. doi: 10.1136/bmjopen-2013-002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontein DB, Seynaeve C, Hadji P, Hille ET, van de Water W, Putter H, van de Velde CJ. Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: An international tamoxifen exemestane adjuvant multinational trial analysis. Journal of Clinical Oncology. 2013;31:2257–2264. doi: 10.1200/JCO.2012.45.3068. [DOI] [PubMed] [Google Scholar]
- Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: The patients’ perspective. American Journal of Surgery. 2006;192:496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, Dewar JA. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer [Abstract 05] Journal of Clinical Oncology. 2013;31(18 Suppl) [Google Scholar]
- Hamann HA, Tiro JA, Sanders JM, Melhado TV, Funk RK, Carpentier MY, Vernon SW. Validity of self-reported genetic counseling and genetic testing use among breast cancer survivors. Journal of Cancer Survivorship. 2013;7:624–629. doi: 10.1007/s11764-013-0301-y. [DOI] [PubMed] [Google Scholar]
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2008;2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- Heisig SR, Shedden-Mora MC, von Blanckenburg P, Schuricht F, Rief W, Albert US, Nestoriuc Y. Informing women with breast cancer about endocrine therapy: Effects on knowledge and adherence. Psycho-Oncology. 2015;24:130–137. doi: 10.1002/pon.3611. [DOI] [PubMed] [Google Scholar]
- Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. Journal of Clinical Oncology. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugtenburg JG, Timmers L, Elders PJ, Vervloet M, van Dijk L. Definitions, variants, and causes of nonadherence with medication: A challenge for tailored interventions. Patient Preference and Adherence. 2013;7:675–682. doi: 10.2147/PPA.S29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-de-Mendoza A, Cabling ML, Lobo T, Dash C, Sheppard VB. Behavioral interventions to enhance adherence to hormone therapy in breast cancer survivors: A systematic literature review. Clinical Breast Cancer. 2016;16(4):247–255.e3. doi: 10.1016/j.clbc.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prevention Research. 2011;4:1360–1365. doi: 10.1158/1940-6207.CAPR-11-0380. [DOI] [PubMed] [Google Scholar]
- MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. Cultural Anthropology Methods. 1998;10(2):31–36. [Google Scholar]
- Mathes T, Antoine S, Pieper D, Eikermann M. Adherence enhancing interventions for oral anticancer agents: A systematic review. Cancer Treatment Reviews. 2014;40:102–108. doi: 10.1016/j.ctrv.2013.07.004. [DOI] [PubMed] [Google Scholar]
- McCabe MS, Bhatia S, Oeffinger KC, Reaman GH, Tyne C, Wollins DS, Hudson MM. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. Journal of Clinical Oncology. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Research and Treatment. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts MT, Santos B, Hardt J, Monette J, Girre V, Atenafu EG, Alibhai SM. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Annals of Oncology. 2014;25:307–315. doi: 10.1093/annonc/mdt386. [DOI] [PubMed] [Google Scholar]
- Regan MM. Predicting benefit of endocrine therapy for early breast cancer. Breast. 2015;24(Suppl. 2):S129–S131. doi: 10.1016/j.breast.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A, Corbin JM. Basics of qualitative research: Grounded theory procedures and techniques. 2. Thousand Oaks, CA: Sage; 1990. [Google Scholar]
- Taplin SH, Foster MK, Shortell SM. Organizational leadership for building effective health care teams. Annals of Family Medicine. 2013;11:279–281. doi: 10.1370/afm.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette DR, Shapiro NL. Medication compliance, adherence, and persistence: Current status of behavioral and educational interventions to improve outcomes. Journal of Managed Care Pharmacy. 2008;14(6 Suppl. D):S2–S10. [Google Scholar]
- Zapka J, Taplin SH, Ganz P, Grunfeld E, Sterba K. Multilevel factors affecting quality: Examples from the cancer care continuum. Journal of the National Cancer Institute. Monographs. 2012;44:11–19. doi: 10.1093/jncimonographs/lgs005. [DOI] [PMC free article] [PubMed] [Google Scholar]