Abstract
The interaction of oviductal epithelial cells (OECs) with the spermatozoa has beneficial effects on the sperm functions. The aim of this study is to evaluate the in vitro fertilizing capacity of incubating spermatozoa previously selected by density gradient in OEC and determinate some sperm characteristics that could explain the results obtained. In this study, we assessed in vitro fertilization (IVF), tyrosine phosphorylation, phosphatidylserine translocation, nuclear DNA fragmentation, and chromatin decondensation. Three experimental sperm groups, previously selected by Percoll gradient, were established according to the origin of the sperm used for IVF: (i) W30 group: spermatozoa were incubated with oocytes in the absence of OEC; (ii) NB group: after sperm incubation in OEC, the unbound spermatozoa were incubated with oocytes, in the absence of OEC; and (iii) B group: after sperm incubation with OEC, the bound spermatozoa were incubated with oocytes in the OEC plates. The results showed that sperm from the NB group led to a lower IVF yield, accompanied by low penetration rates (NB: 19.6%, B: 94.9%, and W30: 62.9%; P < 0.001) and problems of nuclear decondensation. Moreover, higher levels of tyrosine phosphorylation were observed in the NB group compared with the W30 and B groups (NB: 58.7%, B: 2.5%, and W30: 4.5%; P < 0.01). A similar trend was observed in phosphatidylserine translocation (NB: 93.7%, B: 5.7%, and W30: 44.2%; P < 0.01). These results demonstrate that the OEC exerts a rigorous degree of sperm selection, even within an already highly selected population of spermatozoa, and can capture the best functional spermatozoa for fertilization.
Keywords: DNA status, in vitro fertilization, oviductal epithelial cells, phosphatidylserine translocation, sperm selection, tyrosine phosphorylation
INTRODUCTION
Once deposited in the female genital tract, boar sperm undergo a selection process that starts in the lower part of the uterus (cervix), continues in the uterus horns, and finishes in the upper part of the female genital tract, in the oviduct, where fertilization takes place. Sperm reaching the oviduct are selected according to their morphological intactness,1 maturity,2 uncapacitated status,3,4 and high-quality chromatin.5 When sperm come into contact with oviductal epithelial cells (OECs) that line the female tract and its secretions, some spermatozoa are stored,6 allowing the selection of sperm with certain qualities.7 A highly fertile subpopulation of the original ejaculate will bind to epithelial cells, mainly in the spermatic reservoir.8 Therefore, two functionally distinct sperm subpopulations with the ability to reach and enter into the oviduct can be discerned: (i) those bound to the epithelium, which are considered to have been selected because of their high-quality7 and found at the bottom of the crypts of the oviductal folds, where they form a reservoir; (ii) those found in the lumen, not bound to the epithelium, and showing membrane modifications or poor vitality.9,10,11,12 The binding of sperm to oviductal cells is mediated by carbohydrates on the oviductal cell apical membranes and lectin-like molecules on the sperm rostral surface.13 During the capacitation of spermatozoa, these lectin-like molecules may be released from the sensitive acrosomal region of the sperm head, allowing the sperm to abandon the epithelium14 in a sequential process after ovulation15 and swim into the ampulla region, where fertilization occurs.3
The interaction of OECs with the spermatozoa has beneficial effects on the sperm functions. Many authors have shown that the coincubation of spermatozoa with OECs or their conditioned media maintains sperm viability and motility,16,17,18,19,20 low levels of capacitation,4 enhances the fertilization potential by protecting them from oxidative stress21 and, even in some species, stabilizes the sperm chromatin structure.22
Numerous in vitro techniques such as swim-up, Percoll discontinuous gradient, other colloid gradients, and Sephadex gel filtration among others have been developed for sperm selection and capacitation in an attempt to obtain the best spermatozoa for fertilization (viable and motile).23,24,25 All these systems are based on their selection on sperm motility and morphology and have certainly improved the yields of in vitro fertilization (IVF). However, despite the progress of assisted reproduction technologies (ARTs), the techniques for sperm selection are still insufficient to identify the most suitable spermatozoa for fertilization.26,27
All the above led as to believe that if an initial selection of sperm could be made in a physical medium such as a Percoll gradient (mimicking the uterus), subsequent selection by means of a biological system such as oviductal cell culture (mimicking the oviduct's action) might provide a more suitable sperm subpopulation for use in ART, particularly when semen quality is poor or when intracytoplasmic sperm injection (ICSI) is to be performed. To develop this hypothesis, IVF was carried out using sperm bound and unbound to oviductal cells. Besides, to determine putative differences between these kinds of spermatozoa, sperm functionality parameters were determined: to assess the level of sperm capacitation, protein tyrosine phosphorylation (TP) was evaluated because it is well documented that is a crucial event involved in capacitation and that the sperm areas that show tyrosine phosphorylation depend on their capacitation status.28 The phosphatidylserine translocation (PS) was considered the tool of election to assess the destabilization of sperm membrane which is more intensely if spermatozoa are capacitated.29 Percoll techniques can enrich the sperm population by separating out those with nicked DNA and with poorly condensed chromatin;30 to know if OEC can improve the sperm selection done previously with Percoll gradient we use the TUNEL and PI stain techniques to determine DNA fragmentation and chromatin condensation.
MATERIALS AND METHODS
Culture media
Unless otherwise indicated, all the chemicals used in this study were purchased from Sigma-Aldrich Química S.A. (Madrid, Spain). The medium used for oocyte maturation was NCSU-37 supplemented with 0.57 mmol l−1 cysteine, 1 mmol l−1 dibutyryl cAMP, 5 μg ml−1 insulin, 50 μmol l−1β-mercaptoethanol, 1 mM glutamine, 10 IU ml−1 eCG (Folligon, Intervet International B.V., Boxmeer, Holland), 10 IU ml−1 hCG (Chorulon, Intervet International B.V., Boxmeer, Holland), 10 ng ml−1 EGF, and 10% (v/v) porcine follicular fluid.23 The fertilization medium was modified TALP supplemented with 3 mg ml−1 fatty acid-free BSA and 1.10 mM Na-pyruvate.23 Oviductal epithelial cells were cultured in TCM 199 with Earle's salts, l-glutamine and NaHCO3 supplemented with 13% (v/v) of fetal calf serum, 150 IU ml−1 penicillin, and 100 μg ml−1 streptomycin (Gibco BRL, Paisley, UK).
Ethics
The study was carried out in strict accordance with the recommendations in the Guiding Principles for the Care and Use of Animals (DHEW Publication, NIH, 80–23) and was performed following approval by the Veterinary Ethical Committee of University of Murcia. The care and the use of animals were performed in accordance with the Spanish Policy for Animal Protection RD1201/05, which meets the European Union Directive 86/609 on the protection of animals used for experimental and other specifics purposes.
Culture of porcine oviductal epithelial cells (OECs)
The procedure used to culture OEC was described previously by Ouhibi et al.31 with the following minor modifications. Oviducts from commercial cycling gilts were recovered from slaughterhouse material. They were rinsed once in saline and twice in phosphate buffered saline (PBS) at 37°C in the laboratory. In a Petri dish, fat pads and connective tissues were mechanically removed from the oviducts with sterile forceps and fine scissors. The oviducts were closed at one end with a clip, filled with a trypsin-EDTA solution for endothelial cell culture (500 BAAE units of porcine trypsin and 180 μg EDTA), before closed and incubated for 45 min at 38.5°C.32
After incubation, the wall of the oviduct was gently squeezed and its contents flushed into a Petri dish containing preequilibrated cell culture medium. The epithelial cell clusters were dissociated by gentle, repeated pipetting followed by centrifugation at 700 g for 5 min. The supernatant was discarded, and the pellet resuspended with fresh cell culture medium and seeded at a final concentration of approximately 1 × 105 cells ml−1 into Nunc plates (Nunc, Roskilde, Denmark) and cultured at 38.5°C under 5% CO2. The medium was changed 72 h after the beginning of the incubation and every 2 days until cell confluence was observed (5–7 days after initial seeding). The purity of cultures was established by testing for indirect immunocytochemistry using anti-cytokeratin monoclonal antibody 8.13, obtaining in all cases purity above 95%.
Preparation of spermatozoa
Semen was collected from mature fertile boars (2–4-year-old) in an artificial insemination center using the manual method and a dummy.33 The sperm-rich fraction was collected in a prewarmed thermo while the gel fraction was held on a gauze tissue covering the thermos opening. The semen was then diluted 1:2 with isothermal Beltsville Thawing Solution extender34 and transported to the laboratory. Only high-quality semen samples were used: more than 80% motility, <10% abnormal morphology, and <5% damaged acrosomes. Once in the laboratory, sperm cells were selected by centrifugation on a Percoll (Pharmacia, Uppsala, Sweden) discontinuous gradient (45%–90%) in a 12 ml conic centrifuge tube, with 2 ml of 45% Percoll layered on top of 2 ml of 90% Percoll. Finally, 0.5 ml diluted semen was added, taking care to avoid mixing the solutions,23 and centrifuged at 700 g for 30 min. The sperm pellet was resuspended in 10 ml TALP medium35 previously preequilibrated at 38.5°C in 5% CO2 in 100% humidified air and later washed by centrifugation at 700 g for 10 min. Finally, the sperm concentration was adjusted to 1 × 106 spermatozoa ml−1.
Coincubation of spermatozoa with porcine OEC
Nunc plates, with OEC monolayers cultured for 7 days, were washed twice with culture medium. Then, the culture medium was replaced by 2 ml TALP medium, and 1 × 106 washed sperm cells ml−1 were added. Washed spermatozoa were co-incubated with OEC monolayers at 38.5°C and 5% CO2 in air for 30 min. After coincubation, Nunc plates were washed several times with TALP medium to retrieve all the sperm in the supernatant, not bound to OEC (NB), leaving only those sperm bound to OEC (B). The medium was replaced with fresh TALP medium.
The number of spermatozoa from the NB group was calculated using a counting chamber. The difference between added and collected sperm was used to estimate the number of sperm remaining bound to the OEC (B group) and was subsequently used to calculate the sperm concentration for IVF. Among the different replicates, the number of sperm bound to OEC (B group) ranged 50%–60%.
In vitro maturation and fertilization
Ovaries of prepubertal gilts were transported at 37°C from a local abattoir to the laboratory in physiological saline containing 100 mg ml−1 kanamycin. Cumulus-oocyte complexes were collected from follicles (3-6 mm in diameter) by aspiration and washed twice in modified PBS supplemented with 4 mg ml−1 polyvinyl alcohol. Oocytes with evenly granulated cytoplasm and several layers of cumulus oophorus cells were selected and rinsed twice in maturation medium previously equilibrated for at least 3 h under 5% CO2 in maximally humidified air at 38.5°C. Groups of 50 oocytes were matured in 500 μl maturation medium for 22 h under 5% CO2 in air at 38.5°C. After the maturation period, the oocytes were washed 3 times and transferred to hormone-free maturation medium for another 22 h.23
After maturation, oocytes were mechanically stripped of cumulus by gentle aspiration with a pipette. Denuded oocytes were washed 3 times in TALP medium and groups of 25–30 oocytes were transferred to a Nunc plate containing 2 ml TALP medium previously equilibrated at 38.5°C under 5% CO223,36 with or without OEC according to the experiment. Among different replicates, the concentration ranged from 5 × 105 to 6 × 105 cells ml−1, depending on the number of sperm bound to OEC in each one. At 18–20 h postinsemination, putative zygotes were fixed for 30 min (0.5% glutaraldehyde in PBS), stained for 15 min (1% Hoechst 33342 in PBS), washed in PBS containing 1 mg ml−1 polyvinylpyrrolidone, mounted on glass slides and examined under an epifluorescence microscope at ×400 magnification for evidence of sperm penetration.
Tyrosine phosphorylation (TP) evaluated by indirect immunofluorescence and Western blot
An immunofluorescence technique was used to determine the localization of proteins phosphorylated in tyrosine residues.4,25,37 Sperm from the different groups were washed with PBS and centrifuged at 270 g for 10 min and then fixed in 2% formaldehyde solution for 60 min at 4°C. Spermatozoa were washed once in PBS and blocked with 2% (w/v) BSA-PBS and incubated overnight at 4°C before addition of the primary antibody. The sperm were washed and resuspended in PBS, smeared onto a microscope slide, and allowed to dry in air. Slides were then incubated for 1 h with anti-phosphotyrosine monoclonal antibody at 4°C (clone 4G10, 1:200, Millipore, Madrid, Spain) rinsed with PBS, and incubated for an additional hour with fluorescein-conjugated goat anti-mouse antibody (1:400, Bio-Rad Laboratories, Madrid, Spain). After rinsing with PBS, samples were mounted on the slides with 90% glycerol/PBS (v/v). Sperm were observed with a microscope equipped with fluorescent optics (Leica DMLS, Barcelona, Spain) for antibody detection. After removing the supernatant, the Petri dish samples were processed following a similar protocol (PBS, formaldehyde, and primary antibody and secondary antibody added in an amount sufficient to cover the plate and under continuous stirring).
For Western blot, samples were resuspended in lysis buffer and boiled for 5 min. Protein extracts equivalent to 1 × 106 sperm were loaded per lane38 and resolved on a 10% SDS-PAGE gel and transferred to a PVDF membrane (Millipore, CA, USA). Nonspecific binding sites were blocked by incubation in TBS with 0.1% Tween 20 (TBS-T) and 5% BSA (A-9647, Sigma-Aldrich, Madrid, Spain) for 1 h, washed in TBS-T and then incubated with anti-phosphotyrosine antibody (4G10, Millipore, Madrid, Spain, 1:10 000) at 4°C overnight. Then, the membrane was washed in TBS-T and incubated with peroxidase conjugate secondary antibody (1721011, Bio-Rad Laboratories, CA, USA, 1:10 000) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence detection kit (ECL Plus, Amersham, GE Healthcare) according to the manufacturer's instructions. The Precision Plus Protein™ Dual Color Standards (Bio-Rad Laboratories, CA, USA) was used as a molecular weight standard.
Evaluation of membrane phosphatidylserine translocation (PS)
Translocation of PS residues to the outer leaflet of the plasma membrane was detected by Annexin V-Cy3™ Apoptosis Detection Kit (Sigma, Madrid, Spain). For this assay, 1 μl Annexin V with 5 μl CFDA in 450 μl of binding buffer (commercial kit) was mixed with 50 μl of each sperm sample. After 10 min of incubation in the dark, at room temperature, samples were fixed with 10 μl formaldehyde (10% in PBS).39
Evaluation of sperm DNA fragmentation by TUNEL
Terminal deoxynucleotidyl transferase-mediated BrdUTP nick-end labeling (TUNEL) was used to determine sperm DNA fragmentation, following the method described previously.25 In brief, the cells were concentrated by centrifugation, fixed in a solution of ethanol and PBS (70/30, v/v) for 30 min to induce sperm membrane permeabilization, and stored at −20°C until analysis. Cells (approximately 1 × 106) were washed twice with PBS and resuspended in 50 μl of DNA-labeling solution containing 10 μl of reaction buffer, 0.75 μl TdT enzyme, 8.0 μl of BrdUTP (5-Bromo-2’- deoxyuridine 5’- triphosphate) (APO-BrdU™ TUNEL Assay Kit, Invitrogen SA, Barcelona, Spain), and 31.25 μl of dH2O. The cells were incubated in the DNA-labeling solution for 120 min at 37°C. At the end of incubation, 1 ml of rinse buffer was added, and the mixture was centrifuged twice. Finally, the pellet was incubated with anti-BrdU antibody for 30 min at 37°C in a temperature-controlled bath. Negative controls were incubated in the absence of enzyme terminal transferase. The samples were measured by flow cytometry (Epics XL, Beckman Coulter, L’Hospitalet de Llobregat, Barcelona, Spain). Green fluorescence was collected with an FL1 sensor using a 525 nm band-pass filter, determining two populations.
Determination of chromatin condensation
Sperm chromatin was stained with propidium iodide (PI) to determine sperm chromatin condensation.25 Seminal samples were centrifuged (1200 g, 3 min) and the pellet was resuspended in a solution of ethanol and PBS (70/30, v/v) for 30 min to induce sperm membrane permeabilization. The samples were then centrifuged, the supernatant was discarded, and the pellet was resuspended in a PI solution (10 mg ml−1) in PBS. The samples were maintained in darkness for 1 h before flow cytometric analysis. Red PI fluorescence was collected with an FL3 sensor using a 650 nm band-pass filter.
Experimental design
A semen sample (ejaculated spermatozoa) was washed by centrifugation through discontinuous Percoll gradients and incubated in fertilization medium (TALP) with or without OEC for 30 min (Figure 1a). The spermatozoa incubated without OEC were denominated W30 group. From the plates incubated with OEC, we obtained the supernatant with sperm cells unbound to OEC (NB group) and spermatozoa bound to OEC (B group).
Figure 1.
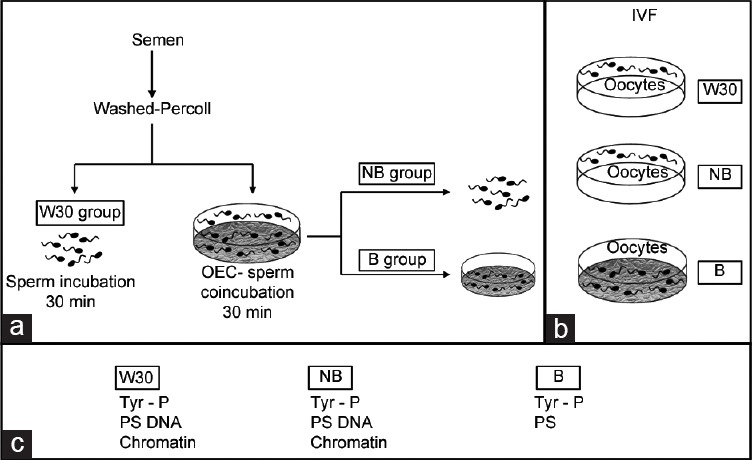
Graphical representation of the experimental design. The shaded plates indicate those with OEC monolayers. (a) Different experimental groups of spermatozoa. Ejaculated sperm was washed through a Percoll gradient. An aliquot of sperm was incubated in TALP medium without OEC for 30 min (W30 group). A second sperm aliquot was co-incubated with OEC for 30 min, and two sperm populations were obtained: Sperm bound to OEC (B group) and sperm from the supernatant not bound to OEC (NB group). (b) IVF experiment. Sperm from W30 (W30 group) and sperm unbound to OEC (NB group) were co-incubated with oocytes in the absence of OEC. In addition, oocytes were co-incubated with sperm bound to OEC (B group) (the sperm-oocyte incubation was performed in the presence of OEC). After 18 h of coincubation, IVF parameters were evaluated. (c) Functionality sperm assays.
IVF with sperm recovered under different conditions of incubation (experiment 1): three different experimental groups (W30, NB, and B) were established according to the origin of the sperm used for IVF (Figure 1b). For the three experimental groups, sperm were previously selected by Percoll gradient: (i) W30 group: spermatozoa were incubated with oocytes in the absence of oviductal epithelial cells. (ii) NB group: after sperm incubation in OEC, the unbound spermatozoa were incubated with oocytes, in the absence of OEC. (iii) B group: after sperm incubation with OEC, the bound spermatozoa were incubated with oocytes in the OEC plates (IVF carried out on OEC). Oocytes were collected 18 h postinsemination and were fixed for subsequent evaluation of the IVF parameters. The penetration rate, the number of spermatozoa bound to the zona pellucida, the number of spermatozoa per penetrated oocyte, the number of swollen spermatozoa, and male pronuclear formation were assessed for each group.23 A total of five repetitions were performed, using 689 oocytes.
Assessment of sperm functionality selected by OEC (experiment 2): tyrosine protein phosphorylation (TP), plasma membrane phosphatidylserine translocation (PS), sperm DNA fragmentation and chromatin condensation were evaluated in the three experimental sperm groups (W30, NB, and B) before carrying out the IVF (Figure 1c). For the sperm groups in suspension (W30 and NB), protein TP (by indirect immunofluorescence and Western blot), PS translocation, DNA fragmentation, and chromatin condensation were studied. In the case of sperm from the B group, due to the impossibility of unbinding them, phosphorylation by indirect immunofluorescence (Supplementary Figure 1 (524.8KB, tif) ) and PS translocation (Supplementary Figure 2 (257.3KB, tif) ) were evaluated in situ, but it was not possible to perform the rest of the assays.
Evaluation of tyrosine phosphorylation (TP) for sperm that remained bound to OEC (B group). The left panels show captures fluorescence, and right panels show the corresponding bright filed.
Evaluation of membrane phosphatidylserine translocation (PS) for sperm that remained bound to OEC (B group). (a) Rhodamine filter to evaluate the exposure of PS. (b) Fluorescein filter to evaluate functional esterases.
TP of the sperm proteins evaluated by indirect immunofluorescence was classified and grouped into three different categories (Figure 2) according to the patterns given by Luño et al.,4 following a hierarchical sequence of capacitation events, which includes phosphorylation in the different regions of the sperm (acrosome, equatorial subsegment, and flagellum). Pattern I include spermatozoa with no fluorescence signal in the equatorial subsegment (Figure 2a), with phosphorylated acrosome (Figure 2b), with phosphorylated tail (Figure 2c), or with both acrosome and tail phosphorylated (Figure 2d). Pattern II includes spermatozoa with a signal in the equatorial subsegment, no signal in the acrosome area and with (Figure 2e) or without (Figure 2f) a signal in the flagellum. Finally, pattern III includes spermatozoa with signal in the equatorial subsegment and acrosome area and with (Figure 2g) or without (Figure 2h) signal in the flagellum. Four repetitions were performed using 1650 spermatozoa. In the evaluation of TP proteins by Western blot, eight repetitions were performed.
Figure 2.
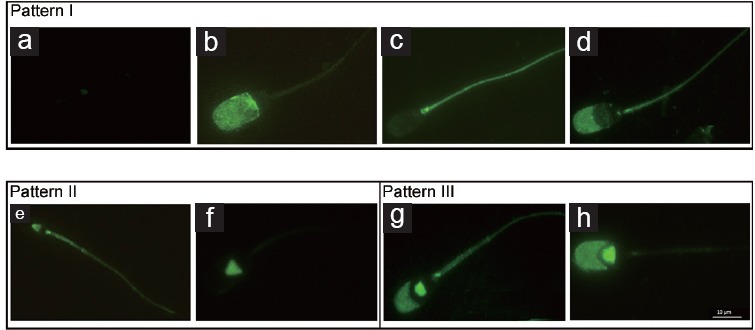
Tyrosine phosphorylation (TP) patterns in boar spermatozoa. Monoclonal anti-phosphotyrosine antibody and FITC-conjugated goat anti-mouse IgG were used to detect TP sperm patterns by indirect immunofluorescence. Pattern I includes spermatozoa without fluorescence (a), with phosphorylated acrosome (b), with phosphorylated tail (c) and with phosphorylated acrosome and tail (d). Pattern II includes spermatozoa with fluorescence in equatorial subsegment with (e) or without (f) the presence of a signal in the flagellum. Pattern III includes spermatozoa with a signal in the equatorial subsegment and acrosome area and with (g) or without (h) the presence of signal in the flagellum.
Dual sperm staining was used for PS and the samples were examined by epifluorescence microscopy. Sperm with functional esterases (CFDA+) were visualized in green fluorescence (fluorescein filter) and sperm with PS exposed (Annexin +) in red fluorescence (rhodamine filter)39 (Supplementary Figure 3 (916.5KB, tif) ). Five repetitions were performed using 4800 spermatozoa.
Patterns shown by sperm evaluated with the Annexin V-Cy3™ Apoptosis Detection Kit. (a1) Sperm with PS exposed (Annexin +). (a2) Sperm without PS exposed (Annexin −). (b1) Sperm with functional esterases (CFDA+). (b2) Sperm without functional esterases (CFDA−).
For the DNA fragmentation assay, cells were classified into two categories according to the intensity of fluorescence: low green fluorescence (low DNA damage) and high green fluorescence (high DNA damage). In addition, measurements were expressed as the mean green intensity fluorescence units, which were used as an index of the DNA fragmentation.25 A total of eight repetitions were performed.
For the chromatin condensation assay, measurements were expressed as the mean red intensity fluorescence units, which were used as index of the state of the chromatin condensation, as this is directly related to the PI uptake by DNA. A total of eight repetitions were performed.
Statistical analysis
Data are expressed as the mean ± s.e.m. and analyzed by ANOVA, considering the specific sperm treatment as the main variable. When ANOVA revealed a significant effect, values were compared by the least significant difference pairwise multiple comparison post hoc test (Tukey). Differences were considered statistically significant at P < 0.05.
RESULTS
Experiment 1 – IVF with sperm recovered under different conditions of incubation
IVF provides information on gamete interaction and is the ultimate goal for many reproductive studies. For this reason, IVF was performed using sperm from different incubation conditions (Figure 1b).
The studied IVF parameters changed depending on the origin of the sperm used (W30, NB, or B) (Figure 3). The penetration rate (PEN) statistically differed among the three study groups, being highest for the sperm bound to oviductal cells (B group: 94.9%), followed by the W30 group (62.9%) and finally the NB group (19.6%) (Figure 3a; P < 0.001).
Figure 3.
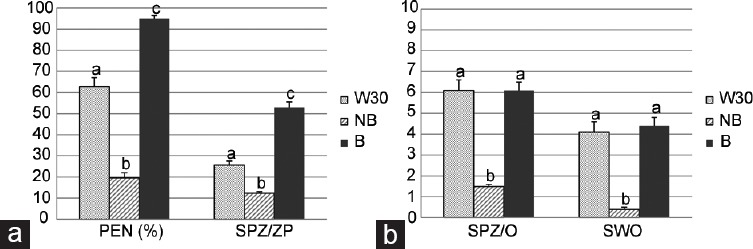
IVF outcomes using different sperm sources. For W30 and NB groups IVF was performed in the absence of OEC; B group: IVF was performed in the presence of OEC. (a) Rate of penetration (PEN%) and mean number of sperm bound to zona pellucida (SPZ/ZP). (b) Mean number of spermatozoa per penetrated oocyte (SPZ/O) based on penetrated oocytes and swollen spermatozoa (SWO) based on penetrated oocytes. Data showed mean ± s.e.m., and the letters a, b, and c in different bars denote significant differences (P < 0.001).
The number of spermatozoa bound to the zona pellucida (SPZ/ZP) followed the same trend (B group: 52.9%, W30: 25.7%, and NB: 12.4%; Figure 3a; P < 0.001). However, although binding to the zona pellucida was greatest in the group B, the number of spermatozoa per penetrated oocyte (SPZ/O) was similar to that observed in the washed spermatozoa (W30), and higher than in the NB group (B group: 6.1, W30: 6.1, and NB: 1.5; Figure 3b; P < 0.001). Although male pronuclear formation was observed in all groups studied, the number of swollen spermatozoa (SWO) was lower in the NB group than in the others (B group: 4.4, W30: 4.1, and NB: 0.4; Figure 3b; P < 0.001).
Experiment 2 – assessment of sperm functionality selected by OEC
A great difference was found in the phosphorylation patterns (patterns II and III) (measured by indirect immunofluorescence) between the sperm subpopulations of the bound (B) and unbound (NB) groups (Figure 4a) (P < 0.01).
Figure 4.
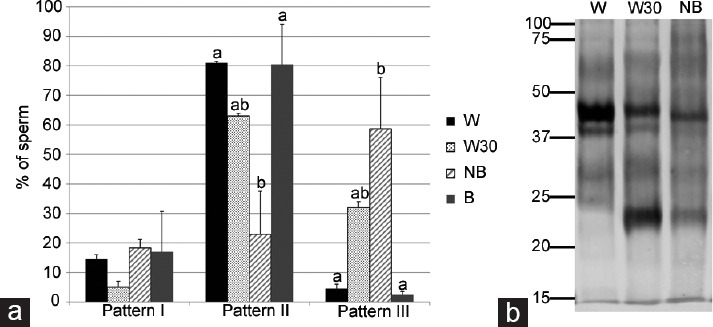
Protein tyrosine phosphorylation (TP) in isolated sperm. (a) Immunolocation by indirect immunofluorescence. Data showed mean ± s.e.m., and the letters a, b in different bars denote significant differences (P < 0.02). (b) Western blotting detection. Molecular weight is expressed in kDa. W: Washed spermatozoa without incubation. W30: Washed spermatozoa after 30 min of incubation. NB: Spermatozoa not bound to OEC after 30 min of coincubation.
When sperm were incubated in the absence of OEC (W30), the principal pattern exhibited was pattern II, followed by pattern III. However, pattern II was much more prevalent and pattern III much less prevalent in B than in NB and W30. The unbound sperm group (NB) showed a high level of TP in pattern III. This pattern III was reduced (P < 0.01) when sperm cells bound to OEC (group B). The localization of phosphorylated proteins showed that coincubation with OEC modifies the sperm staining pattern. No differences were observed in pattern I among the experimental groups.
Besides location, we identified the molecular weights of the phosphorylated proteins in the different sperm experimental groups by Western blot (Figure 4b), since a single location may contain proteins of different molecular weights. As occurred with phosphorylated proteins evaluated by immunocytochemistry, bands with different signal patterns between the experimental groups were observed by Western blot. Note that a band of approximately 45 kDa had a strong signal in the W group (line 1) but faded after 30 min of incubation (W30, line 2) and was even weaker in the unbound sperm group (NB, line 3). Something similar occurred in another band of 40 kDa. Furthermore, after 30 min, washed sperm (W30, line 2) showed a phosphorylated protein band with a molecular weight of about 23 kDa, which was absent before incubation (W, line 1). This phosphorylation band also appeared in the NB sperm (line 3), but with much less intensity. Overall, the unbound sperm group (NB, line 3) showed lower intensity/signal bands than the other groups.
When the translocation of PS, a marker of sperm capacitation and/or apoptosis, was analyzed for the different sperm sources, significant differences were observed (Figure 5). The levels of PS reached 44.2% in the W30 group and 93.8% in the unbound sperm group (NB). Conversely, sperm that remained bound to OEC (B) had a PS translocation of 5.8%, which is lower than the other sperm groups (P < 0.05). In addition, B group shows the most number of sperm with functional esterases (CFDA+) relative to the total of annexin positive sperm.
Figure 5.
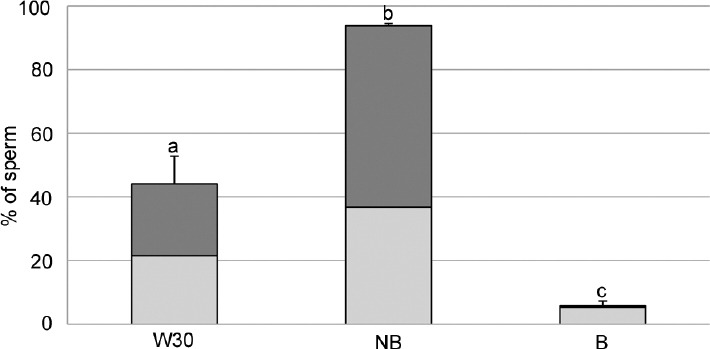
Phosphatidylserine translocation of sperm labeled with Annexin V-Cy3. The complete bars show percentage of annexin positive sperm. The light grey color corresponds to the CFDA positive (sperm with functional esterases) and dark grey correspond to CFDA negative (sperm without functional esterases). W30: Washed spermatozoa after 30 min of incubation. NB: Spermatozoa not bound to OEC after 30 min of coincubation. B: Spermatozoa bound to OEC after 30 min of coincubation. Data showed mean ± s.e.m. and the letters a, b, and c in different bars denote significant differences (P < 0.01).
The degree of chromatin condensation measured by PI staining was higher in the W30 than in unbound group (NB) (23.3 ± 0.8 vs 17.8 ± 0.9; P < 0.05), and inversely related with PI uptake by DNA. When DNA damage was measured by TUNEL in these groups, no differences were found (5.60 ± 0.69 vs 4.46 ± 0.75, respectively).
DISCUSSION
The insemination of large numbers of sperm translates into a selection of a few highly fertile sperm able to reach and interact with the oviduct and subsequently with oocytes. However, although some factors have been identified as fundamental for spermatozoa to reach the sperm reservoir,40 how exactly oviductal cells select the best sperm for successful fertilization remains unknown.
Various investigators have characterized some aspects of the superior sperm quality associated with oviductal binding,41 concluding that sperm-oviduct binding plays a key role in the maintenance of sperm fertilizing ability. Hence, an IVF system based on OEC incubation and further collection of the sperm bound to OEC should be of great use for selecting the best spermatozoa for ICSI, an ART technique widely used in human infertility clinics. In this study, we aim to evaluate the fertilizing capacity of spermatozoa, previously selected by Percoll gradient, incubated in OEC.
The IVF results show that sperm bound to the OEC (B group) were able to fertilize oocytes to a greater extent than those not in contact with the OEC (W30 group). This was expected because other authors had already mentioned similar results.32 However, we did not expect the unbound sperm group (NB) to have such low levels of fertilization. We believe that sperm not bound to OEC are less capable of fertilizing due to the high levels of capacitation, as seen in experiment 2, and subsequent apoptosis.42 The number of spermatozoa bound to the zona pellucida showed the same trend as the penetration rate (highest values in the B group). The ability of sperm to bind to the zona pellucida is one of the most important characteristics of the sperm fertilizing capacity.43
Although male pronuclear formation was evident in all the groups studied, the number of swollen spermatozoa (SWO) was lower in the NB group than in the others. Despite problems with binding to the zona pellucida and penetrating oocytes, some NB sperm can penetrate oocytes and can form male pronucleus because the oocyte can repair preexisting damage in the sperm.44 However, in some cases, this oocyte repair capacity may be insufficient to repair highly damaged sperm, resulting in nonswollen spermatozoa. The IVF results obtained support the hypothesis that OEC selects the best spermatozoa for fertilization although many of the qualities which spermatozoa must present to be selected by the OEC remain unknown.
An important factor in sperm selection by the OEC is the capacitation status of the sperm population, as mentioned by other authors.3,4,45 The sperm population bound to the epithelial cell population showed a degree of fluorescence in the equatorial segment but no fluorescence in the acrosomal area. However, in sperm unbound to the cells (NB group), this pattern was reversed, i.e., they showed fluorescence in the acrosomal area. In human spermatozoa, it has been established that a high degree of TP is considered a prerequisite for fertilization.46 In this way, sperm must exhibit the highest degree of phosphorylation at the moment before contact with the zona pellucida of the oocyte to activate the acrosome reaction. Conversely, if these levels are reached earlier, far from the oocyte, sperm initiate the apoptosis pathway. In this manuscript, the sperm with the highest level of protein TP (pattern III) were those in the NB group which, nevertheless, was the group with the lowest percentage of penetration, precisely because they reached a high level of phosphorylation very early.
On the one hand, during the capacitation process, there is a redistribution of tyrosine-phosphorylated proteins localized in the acrosomal region of boar sperm47 and, on the other hand, the oviductal epithelium secretes proteins capable of reversing sperm capacitation.48 This redistribution is also evident in the Western blot where the incubation of washed spermatozoa leads to the appearance of new bands due to increased levels of phosphorylation of the principal sperm pieces,49 mainly the equatorial subsegment. The same bands were also found in the NB group, indicating that phosphorylation levels were also increased. This protein redistribution may help explain the change in the intensity of the observed bands. However, we cannot affirm this because only localization patterns were analyzed without distinguishing the intensities of fluorescence, so the amount of phosphorylated protein in each sperm principal pieces was not ascertained. We suggest that the changes observed in sperm phosphorylation after coincubation with OEC are not only due to the redistribution of proteins but also due to modifications in phosphorylation-dephosphorylation in the proteins.50 It is still unknown whether OECs select the sperm bound to them based on other criteria and then adjust their levels of phosphorylation, or whether only sperm with a low capacitation pattern is bound.
It is still a matter of controversy too, whether PS translocation is due to capacitation,51,52,53,54 the apoptosis process55 or both.42 Our results showed that OECs exercise a selective function and that binding does not occur randomly. It is not surprising that after 30 min of incubation, the percentage of spermatozoa with PS translocation increased56 due to an increase in the capacitated sperm population in the sample, which leads to destabilization of the membrane and thus the translocation of PS.57 Accordingly, if OEC binds mainly uncapacitated spermatozoa,3 this population in our study would correspond to spermatozoa without PS translocation (annexin −), while sperm with PS translocation (annexin +) showed not be able to bind to OEC, remaining in the supernatant after coincubation, because they are capacitated or apoptotic. OEC would also be able to discriminate between annexin negative spermatozoa, with their morphologically superior quality, and annexin positive sperm.58 Moreover, PS translocation is associated with a decrease in the adenosine triphosphate (ATP) level of spermatozoa59 and with a lower mitochondrial potential,60 and so the OECs do not bind these spermatozoa because they have insufficient energy reserves to remain in the sperm reservoir and perform fertilization. Another explanation is that phospholipids (including PS) are incorporated into the sperm membrane by OEC61 as a selection mechanism. This mechanism would result in a negative selection, similar to that reported previously,62 for detecting early phases of disturbed membrane functions and marking for elimination those sperm which probably have a reduced life span,63,64 and hence are less suitable for storage in the oviduct because they have ceased to be functional. This could explain the increase in PS translocation observed in NB sperm. Nevertheless, the mechanisms that lead OEC to select and bind with live spermatozoa without PS translocation in the plasma membrane (annexin −) remains unknown.
When ART are used, it is necessary to select sperm in an attempt to eliminate sperm with poor quality and seminal plasma despite the prejudicial effects this may have. In this sense, it has been shown that during centrifugation to remove seminal plasma, ROS are generated and these, in turn, induce capacitation, apoptosis and DNA damage.65 In this study, we observed that washing sperm through a Percoll gradient induced chromatin decondensation. After 30 min of incubating sperm in a medium with OEC, the sperm that had not been bound to the OEC had the same chromatin condensation as ejaculated spermatozoa (S) even though the sperm had been selected through a Percoll gradient, leaving only sperm of the highest quality.25 It has been shown that during sperm capacitation chromatin decondensation occurs, which prepares the sperm for the subsequent formation of pronucleus after fertilization.66 The reason for the greater compactness of the unbound sperm is unknown although it has been attributed to defects in the disulfide bonds of proteins to prevent chromatin decondensation. This hypothesis is reinforced by the IVF results since the NB sperm showed the lowest level of nucleus decondensation in oocyte (SWO). In this regard, it has been shown that alterations in the protamine packing of sperm DNA may contribute to a delay in the initiation of the zygotic S-phase,67 increase the length of the zygotic G2-phase,68 and block blastocyst formation.69 On the other hand, Ardón et al.5 suggested that chromatin instability may be associated with plasma membrane characteristics that hinder the binding of such sperm to the oviduct.
Another important factor related to the nucleus is DNA fragmentation and its effect on fertility. Although there is still controversy regarding this, it has been shown that DNA fragmentation in sperm and poor quality samples increases and is correlated with failed fertilization in ICSI.70 However, in normozoospermic samples, fertility failure has been observed caused by spermatozoa with damaged DNA.71 The results presented in this study show that spermatozoa selected by Percoll gradient showed less DNA fragmentation. However, after 30 min of incubation with or without OEC, DNA fragmentation was in fact unaffected, so selection with Percoll could be efficient because the selective effect by the OEC was not evident in the case of DNA fragmentation.30
Although sperm selection by density gradient initially selects the best spermatozoa from a sperm population, such selection is insufficient to identify the most suitable spermatozoon for the subsequent fertilization process. This study shows that OECs exert a more rigorous second selection. We conclude that OECs are responsible for real sperm selection. Such selection by OEC could in the future represent a solution to obtaining the best spermatozoa for fertilization and embryo development. Using a noninvasive technique that does not damage or impair, the sperm for later use would avoid the subjective selection that currently exists in conventional sperm selection techniques.
AUTHOR CONTRIBUTIONS
RL-U conceived and designed the experiments, performed the experiments, analyzed the data, and wrote the manuscript. FAG-V wrote the manuscript. JG performed the experiments and analyzed the data. CM conceived and designed the experiments, performed the experiments, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared that they have no competing interests.
ACKNOWLEDGMENTS
The authors would like to thank Virginia Reig for her assistance at the start of the experiments. We also thank Rodrigo Diego for his technical support with the use of Annexin V-Cy3™ and Juan Carvajal for technical assistance. This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the European Regional Development Fund (FEDER), Grants AGL2012-40180-C03-01 and AGL2015-66341-R.
Supplementary information is linked to the online version of the study on the Asian Journal of Andrology website.
REFERENCES
- 1.Waberski D, Magnus F, Ardon F, Petrunkina A, Weitze K, et al. Binding of boar spermatozoa to oviductal epithelium in vitro in relation to sperm morphology and storage time. Reproduction. 2006;131:311–8. doi: 10.1530/rep.1.00814. [DOI] [PubMed] [Google Scholar]
- 2.Petrunkina A, Gehlhaar R, Drommer W, Waberski D, Töpfer-Petersen E. Selective sperm binding to pig oviductal epithelium in vitro. Reproduction. 2001;121:889–96. [PubMed] [Google Scholar]
- 3.Fazeli A, Duncan A, Watson P, Holt W. Sperm-oviduct interaction: induction of capacitation and preferential binding of uncapacitated spermatozoa to oviductal epithelial cells in porcine species. Biol Reprod. 1999;60:879–86. doi: 10.1095/biolreprod60.4.879. [DOI] [PubMed] [Google Scholar]
- 4.Luño V, López-Úbeda R, García-Vázquez FA, Gil L, Matás C. Boar sperm tyrosine phosphorylation patterns in the presence of oviductal epithelial cells: in vitro, ex vivo, and in vivo models. Reproduction. 2013;146:315–24. doi: 10.1530/REP-13-0159. [DOI] [PubMed] [Google Scholar]
- 5.Ardón F, Helms D, Sahin E, Bollwein H, Töpfer-Petersen E, et al. Chromatin-unstable boar spermatozoa have little chance of reaching oocytes in vivo. Reproduction. 2008;135:461–70. doi: 10.1530/REP-07-0333. [DOI] [PubMed] [Google Scholar]
- 6.Hunter R. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J Reprod Fertil. 1981;63:109–17. doi: 10.1530/jrf.0.0630109. [DOI] [PubMed] [Google Scholar]
- 7.Talevi R, Gualtieri R. Molecules involved in sperm-oviduct adhesion and release. Theriogenology. 2010;73:796–801. doi: 10.1016/j.theriogenology.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Gualtieri R, Talevi R. Selection of highly fertilization-competent bovine spermatozoa through adhesion to the fallopian tube epithelium in vitro. Reproduction. 2003;125:251–8. [PubMed] [Google Scholar]
- 9.Mburu J, Einarsson S, Lundeheim N, Rodriguez-Martinez H. Distribution, number and membrane integrity of spermatozoa in the pig oviduct in relation to spontaneous ovulation. Anim Reprod Sci. 1996;45:109–21. doi: 10.1016/s0378-4320(96)01566-7. [DOI] [PubMed] [Google Scholar]
- 10.Mburu J, Rodriguez-Martinez H, Einarsson S. Changes in sperm ultrastructure and localisation in the porcine oviduct around ovulation. Anim Reprod Sci. 1997;47:137–48. doi: 10.1016/s0378-4320(96)01631-4. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Martinez H, Tienthai P, Suzuki K, Funahashi H, Ekwall H, et al. Involvement of oviduct in sperm capacitation and oocyte development in pigs. Reprod Suppl. 2001;58:129–45. [PubMed] [Google Scholar]
- 12.Tienthai P, Johannisson A, Rodriguez-Martinez H. Sperm capacitation in the porcine oviduct. Anim Reprod Sci. 2004;80:131–46. doi: 10.1016/S0378-4320(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 13.Gualtieri R, Talevi R. In vitro-cultured bovine oviductal cells bind acrosome-intact sperm and retain this ability upon sperm release. Biol Reprod. 2000;62:1754–62. doi: 10.1095/biolreprod62.6.1754. [DOI] [PubMed] [Google Scholar]
- 14.Töpfer-Petersen E, Wagner A, Friedrich J, Petrunkina A, Ekhlasi-Hundrieser M, et al. Function of the mammalian oviductal sperm reservoir. J Exp Zool. 2002;292:210–5. doi: 10.1002/jez.1157. [DOI] [PubMed] [Google Scholar]
- 15.Brüssow KP, Ratky J, Rodriguez-Martinez H. Fertilization and early embryonic development in the porcine fallopian tube. Reprod Domest Anim. 2008;43:245–51. doi: 10.1111/j.1439-0531.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, Barratt C, Lippes J, Pacey A, Cooke I. The sequential effects of human cervical mucus, oviductal fluid, and follicular fluid on sperm function. Fertil Steril. 1994;61:1129–35. doi: 10.1016/s0015-0282(16)56768-5. [DOI] [PubMed] [Google Scholar]
- 17.Kervancioglu ME, Saridogan E, Aitken RJ, Djahanbakhch O. Importance of sperm-to-epithelial cell contact for the capacitation of human spermatozoa in fallopian tube epithelial cell cocultures. Fertil Steril. 2000;74:780–4. doi: 10.1016/s0015-0282(00)01514-4. [DOI] [PubMed] [Google Scholar]
- 18.Quintero I, Ghersevich S, Caille A, Munuce MJ, Daniele S, et al. Effects of human oviductal in vitro secretion on spermatozoa and search of sperm-oviductal proteins interactions. Int J Androl. 2005;28:137–43. doi: 10.1111/j.1365-2605.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 19.Munuce MJ, Serravalle A, Caille AM, Zumoffen C, Botti G, et al. Human tubal secretion can modify the affinity of human spermatozoa for the zona pellucida. Fertil Steril. 2009;91:407–13. doi: 10.1016/j.fertnstert.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 20.Zumoffen C, Caille A, Munuce M, Cabada M, Ghersevich S. Proteins from human oviductal tissue-conditioned medium modulate sperm capacitation. Hum Reprod. 2010;25:1504–12. doi: 10.1093/humrep/deq063. [DOI] [PubMed] [Google Scholar]
- 21.Huang VW, Zhao W, Lee CL, Lee CY, Lam KK, et al. Cell membrane proteins from oviductal epithelial cell line protect human spermatozoa from oxidative damage. Fertil Steril. 2013;99:1444–52.e3. doi: 10.1016/j.fertnstert.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 22.Ellington JE, Evenson DP, Fleming JE, Brisbois RS, Hiss GA, et al. Coculture of human sperm with bovine oviduct epithelial cells decreases sperm chromatin structural changes seen during culture in media alone. Fertil Steril. 1998;69:643–9. doi: 10.1016/s0015-0282(98)00023-5. [DOI] [PubMed] [Google Scholar]
- 23.Matás C, Coy P, Romar R, Marco M, Gadea J, et al. Effect of sperm preparation method on in vitro fertilization in pigs. Reproduction. 2003;125:133–41. doi: 10.1530/rep.0.1250133. [DOI] [PubMed] [Google Scholar]
- 24.Buffone M, Doncel G, Briggiler CM, Vazquez-Levin M, Calamera J. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Hum Reprod. 2004;19:139–46. doi: 10.1093/humrep/deh040. [DOI] [PubMed] [Google Scholar]
- 25.Matás C, Vieira L, García-Vázquez FA, Avilés-López K, López-Úbeda R, et al. Effects of centrifugation through three different discontinuous Percoll gradients on boar sperm function. Anim Reprod Sci. 2011;127:62–72. doi: 10.1016/j.anireprosci.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Mortimer D. Sperm preparation methods. J Androl. 2000;21:357–66. [PubMed] [Google Scholar]
- 27.McDowell S, Kroon B, Ford E, Hook Y, Glujovsky D, et al. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2014;10:CD010461. doi: 10.1002/14651858.CD010461.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, et al. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–37. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 29.Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, et al. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci. 2001;114:3543–55. doi: 10.1242/jcs.114.19.3543. [DOI] [PubMed] [Google Scholar]
- 30.Amiri I, Ghorbani M, Heshmati S. Comparison of the DNA fragmentation and the sperm parameters after processing by the density gradient and the swim up methods. J Clin Diagn Res. 2012;6:1451–3. doi: 10.7860/JCDR/2012/4198.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouhibi N, Benet G, Menezo Y. Fetal bovine oviduct epithelial cell monolayers: method of culture and identification. J Tissue Cult Methods. 1991;13:289–94. [Google Scholar]
- 32.Romar R, Coy P, Gadea J, Rath D. Effect of oviductal and cumulus cells on zona pellucida and cortical granules of porcine oocytes fertilized in vitro with epididymal spermatozoa. Anim Reprod Sci. 2005;85:287–300. doi: 10.1016/j.anireprosci.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 33.King G, Macpherson J. A comparison of two methods for boar semen collection. J Anim Sci. 1973;36:563–5. doi: 10.2527/jas1973.363563x. [DOI] [PubMed] [Google Scholar]
- 34.Pursel V, Johnson L. Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J Anim Sci. 1975;40:99–102. doi: 10.2527/jas1975.40199x. [DOI] [PubMed] [Google Scholar]
- 35.Rath D, Long C, Dobrinsky J, Welch G, Schreier L, et al. In vitro production of sexed embryos for gender preselection: high-speed sorting of X-chromosome-bearing sperm to produce pigs after embryo transfer. J Anim Sci. 1999;77:3346–52. doi: 10.2527/1999.77123346x. [DOI] [PubMed] [Google Scholar]
- 36.Matás C, Sansegundo M, Ruiz S, García-Vázquez FA, Gadea J, et al. Sperm treatment affects capacitation parameters and penetration ability of ejaculated and epididymal boar spermatozoa. Theriogenology. 2010;74:1327–40. doi: 10.1016/j.theriogenology.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Tardif S, Dubé C, Chevalier S, Bailey JL. Capacitation is associated with tyrosine phosphorylation and tyrosine kinase-like activity of pig sperm proteins. Biol Reprod. 2001;65:784–92. doi: 10.1095/biolreprod65.3.784. [DOI] [PubMed] [Google Scholar]
- 38.Jha K, Salicioni A, Arcelay E, Chertihin O, Kumari S, et al. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol Hum Reprod. 2006;12:781–9. doi: 10.1093/molehr/gal085. [DOI] [PubMed] [Google Scholar]
- 39.Marti E, Perez-Pe R, Colas C, Muino-Blanco T, Cebrian-Perez J. Study of apoptosis-related markers in ram spermatozoa. Anim Reprod Sci. 2008;106:113–32. doi: 10.1016/j.anireprosci.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Soriano-Úbeda C, Matás C, García-Vázquez FA. An overview of swine artificial insemination: retrospective, current and prospective aspects. J Exp Appl Anim Sci. 2013;1:67–97. [Google Scholar]
- 41.Holt WV, Fazeli A. The oviduct as a complex mediator of mammalian sperm function and selection. Mol Reprod Dev. 2010;77:934–43. doi: 10.1002/mrd.21234. [DOI] [PubMed] [Google Scholar]
- 42.Aitken RJ. The capacitation-apoptosis highway: oxysterols and mammalian sperm function. Biol Reprod. 2011;85:9–12. doi: 10.1095/biolreprod.111.092528. [DOI] [PubMed] [Google Scholar]
- 43.Oehninger S, Mahony M, Özgür K, Kolm P, Kruger T, et al. Clinical significance of human sperm-zona pellucida binding. Fertil Steril. 1997;67:1121–7. doi: 10.1016/s0015-0282(97)81449-5. [DOI] [PubMed] [Google Scholar]
- 44.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 45.Lefebvre R, Suarez SS. Effect of capacitation on bull sperm binding to homologous oviductal epithelium. Biol Reprod. 1996;54:575–82. doi: 10.1095/biolreprod54.3.575. [DOI] [PubMed] [Google Scholar]
- 46.Sakkas D, Leppens-Luisier G, Lucas H, Chardonnens D, Campana A, et al. Localization of tyrosine phosphorylated proteins in human sperm and relation to capacitation and zona pellucida binding. Biol Reprod. 2003;68:1463–9. doi: 10.1095/biolreprod.102.011023. [DOI] [PubMed] [Google Scholar]
- 47.Flesch FM, Wijnand E, Van de Lest C, Colenbrander B, Van Golde L, et al. Capacitation dependent activation of tyrosine phosphorylation generates two sperm head plasma membrane proteins with high primary binding affinity for the zona pellucida. Mol Reprod Dev. 2001;60:107–15. doi: 10.1002/mrd.1067. [DOI] [PubMed] [Google Scholar]
- 48.Ghersevich S, Massa E, Zumoffen C. Oviductal secretion and gamete interaction. Reproduction. 2015;149:R1–14. doi: 10.1530/REP-14-0145. [DOI] [PubMed] [Google Scholar]
- 49.Harayama H, Muroga M, Miyake M. A cyclic adenosine 3´, 5´-monophosphate-induced tyrosine phosphorylation of syk protein tyrosine kinase in the flagella of boar spermatozoa. Mol Reprod Dev. 2004;69:436–47. doi: 10.1002/mrd.20176. [DOI] [PubMed] [Google Scholar]
- 50.Signorelli JR, Díaz ES, Fara K, Barón L, Morales P. Protein phosphatases decrease their activity during capacitation: a new requirement for this event. PLoS One. 2013;8:e81286. doi: 10.1371/journal.pone.0081286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barroso G, Morshedi M, Oehninger S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum Reprod. 2000;15:1338–44. doi: 10.1093/humrep/15.6.1338. [DOI] [PubMed] [Google Scholar]
- 52.Duru NK, Morshedi MS, Schuffner A, Oehninger S. Cryopreservation-thawing of fractionated human spermatozoa is associated with membrane phosphatidylserine externalization and not DNA fragmentation. J Androl. 2001;22:646–51. [PubMed] [Google Scholar]
- 53.de Vries K, Wiedmer T, Sims P, Gadella B. Caspase-independent exposure of aminophospholipids and tyrosine phosphorylation in bicarbonate responsive human sperm cells. Biol Reprod. 2003;68:2122–34. doi: 10.1095/biolreprod.102.012500. [DOI] [PubMed] [Google Scholar]
- 54.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–68. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]
- 55.Aitken RJ, Baker MA. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int J Dev Biol. 2013;57:265–72. doi: 10.1387/ijdb.130146ja. [DOI] [PubMed] [Google Scholar]
- 56.Kotwicka M, Jendraszak M, Warchoł J. Plasma membrane translocation of phosphatidylserine in human spermatozoa. Folia Histochem Cytobiol. 2002;40:111–2. [PubMed] [Google Scholar]
- 57.Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 58.Hoogendijk CF, Kruger TF, Bouic PJ, Henkel RR. A novel approach for the selection of human sperm using annexin V-binding and flow cytometry. Fertil Steril. 2009;91:1285–92. doi: 10.1016/j.fertnstert.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 59.Kotwicka M, Jendraszak M, Skibinska I, Jedrzejczak P, Pawelczyk L. Decreased motility of human spermatozoa presenting phosphatidylserine membrane translocation-cells selection with the swim-up technique. Hum Cell. 2013;26:28–34. doi: 10.1007/s13577-011-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barroso G, Taylor S, Morshedi M, Manzur F, Gaviño F, et al. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: a comparison of two different sperm subpopulations. Fertil Steril. 2006;85:149–54. doi: 10.1016/j.fertnstert.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 61.Evans RW, Weaver DE, Clegg ED. Diacyl, alkenyl, and alkyl ether phospholipids in ejaculated, in utero-, and in vitro-incubated porcine spermatozoa. J Lipid Res. 1980;21:223–8. [PubMed] [Google Scholar]
- 62.Teijeiro JM, Dapino DG, Marini PE. Porcine oviduct sperm binding glycoprotein and its deleterious effect on sperm: a mechanism for negative selection of sperm? Biol Res. 2011;44:329–37. [PubMed] [Google Scholar]
- 63.Watson P. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev. 1995;7:871–91. doi: 10.1071/rd9950871. [DOI] [PubMed] [Google Scholar]
- 64.Watson P. The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci. 2000;60:481–92. doi: 10.1016/s0378-4320(00)00099-3. [DOI] [PubMed] [Google Scholar]
- 65.Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Lamirande E, Gabriel M, Zini A. Human sperm chromatin undergoes physiological remodeling during in vitro capacitation and acrosome reaction. J Androl. 2012;33:1025–35. doi: 10.2164/jandrol.111.015982. [DOI] [PubMed] [Google Scholar]
- 67.Eid L, Lorton S, Parrish J. Paternal influence on S-phase in the first cell cycle of the bovine embryo. Biol Reprod. 1994;51:1232–7. doi: 10.1095/biolreprod51.6.1232. [DOI] [PubMed] [Google Scholar]
- 68.Eid L, Parrish J. Duration of G2-phase and onset of M-phase during the first cell cycle of the bovine embryo is dependent on bull in vivo fertility. Theriogenology. 1995;43:205. [Google Scholar]
- 69.Fatehi A, Bevers M, Schoevers E, Roelen B, Colenbrander B, et al. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J Androl. 2006;27:176–88. doi: 10.2164/jandrol.04152. [DOI] [PubMed] [Google Scholar]
- 70.Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor-quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69:528–32. doi: 10.1016/s0015-0282(97)00536-0. [DOI] [PubMed] [Google Scholar]
- 71.Aitken RJ, De Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007;14:727–33. doi: 10.1016/s1472-6483(10)60676-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of tyrosine phosphorylation (TP) for sperm that remained bound to OEC (B group). The left panels show captures fluorescence, and right panels show the corresponding bright filed.
Evaluation of membrane phosphatidylserine translocation (PS) for sperm that remained bound to OEC (B group). (a) Rhodamine filter to evaluate the exposure of PS. (b) Fluorescein filter to evaluate functional esterases.
Patterns shown by sperm evaluated with the Annexin V-Cy3™ Apoptosis Detection Kit. (a1) Sperm with PS exposed (Annexin +). (a2) Sperm without PS exposed (Annexin −). (b1) Sperm with functional esterases (CFDA+). (b2) Sperm without functional esterases (CFDA−).


