Abstract
It has been suggested that the energy required for sperm motility is produced by oxidative phosphorylation while glycolysis seems to be an important source for ATP transmission along the flagellum. Some studies have investigated the chemical and kinetic properties of the enzyme glyceraldehyde 3-phosphate dehydrogenase to identify any changes in the regulation of glycolysis and sperm motility. In contrast, there are few studies analyzing the genetic basis of hypokinesis. For this reason, we investigated the glyceraldehyde 3-phosphate dehydrogenase gene in human sperm to evaluate whether asthenozoospermia was correlated with any changes in its expression. Semen examination and glyceraldehyde 3-phosphate dehydrogenase gene expression studies were carried out on 116 semen samples divided into two groups – Group A consisted of 58 normokinetic samples and Group B of 58 hypokinetic samples. Total RNA was extracted from spermatozoa, and real-time PCR quantification of mRNA was carried out using specific primers and probes. The expression profiles for the Groups A and B were very similar. The mean delta Ct was as follows – Group A, 5.79 ± 1.04; Group B, 5.47 ± 1.27. Our study shows that in human sperm, there is no difference in glyceraldehyde 3-phosphate dehydrogenase gene expression between samples with impaired motility and samples with normal kinetics. We believe that this study could help in the understanding of the molecular mechanisms of sperm kinetics, suggesting that hypomotility may be due to a possible posttranscriptional impairment of the control mechanism, such as mRNA splicing, or to posttranslational changes.
Keywords: adenosine-5’-triphosphate, gene expression, sperm glyceraldehyde 3-phosphate dehydrogenase, sperm motility
INTRODUCTION
Sperm produces high levels of adenosine-5’- triphosphate (ATP), which is used mainly for kinetic activity. There are two much-debated pathways for ATP production such as mitochondrial oxidative phosphorylation and glycolysis. ATP is synthesized more efficiently by mitochondrial respiration than by the glycolytic pathway. It has therefore been suggested that the energy required for sperm motility is produced by oxidative phosphorylation while glycolysis seems to be an important source for ATP transmission along the flagellum.1,2
Various studies have found a positive correlation between mitochondrial activity and sperm motility.3,4 Mitochondria are in fact localized in a limited region of the sperm, so it is unlikely that the ATP could rapidly disperse along the flagellum by diffusion alone. Mathematical models based on the study of ATP diffusion and morphometric estimates of the volume of the mouse sperm flagellum revealed that the ATP produced by the mitochondria in the midpiece cannot provide the energy required for the entire flagellum.5 In rodents, for example, which have a very long flagellum, it is unlikely that ATP can reach the far end. For these reasons, various authors have suggested metabolic strategies for the production and delivery of ATP along the flagellum, such as ATP shuttles or ATP formation through glycolysCs.2,6,7 The studies by Travis et al.8 and Mukai and Okuno9 demonstrated that oxidative phosphorylation is not necessary for the flagellum's function. Further confirmation of this theory is provided by Miki et al.10 through the generation of “knockout" mice for the sperm-specific isoenzyme of glyceraldehyde 3-phosphate dehydrogenase (Gapds-/-). The authors found that sperm from Gapds-/- mice had impaired motility and a lower ATP concentration than wild-type mice even though mitochondrial respiration, evaluated by the rate of oxygen uptake, was not affected.
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme of the glycolytic pathway that catalyzes the conversion of glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate. Two isoforms of this enzyme exist in mammals: GAPDH, found in all somatic cells; GAPDS, found only in sperm. GAPDS (GAPD2 in humans) is located in the principal piece of the flagellum, closely bound to the fibrous sheath. Various studies have investigated the chemical and kinetic properties of this enzyme to identify any changes in the regulation of glycolysis and sperm motility. In contrast, there are few studies analyzing the genetic basis of hypokinesis. For this reason, our study investigated the GAPD2 gene in human sperm to evaluate if asthenozoospermia was correlated with any changes in its expression in spermatozoa.
METHODS
Patients
The study was approved by our University Hospital's Institutional Review Board. The written informed consent was obtained from all study participants. We studied 116 semen samples from 116 patients attending the Seminology Laboratory - Sperm Bank, the Department of Experimental Medicine, “La Sapienza” University of Rome for analysis of seminal fluid from 2012 to 2013. The patients had not been medically or surgically treated in the 3 months before the study and did not have any conditions (fever, etc.) that might interfere with the semen analysis. Semen examination and gene expression were performed for all patients.
Semen analysis
Semen samples were collected by masturbation after 3–5 days of abstinence. All samples were allowed to liquefy at 37°C for 60 min and were then assessed according to the World Health Organization guidelines (2010).11 The following variables were taken into consideration: ejaculate volume (ml), sperm concentration (n × 106 ml−1), total sperm number (n × 106 per ejaculate), progressive motility (%), and morphology (% abnormal forms). In addition to raw motility data, absolute values in terms of millions of motile sperm per ejaculate were also calculated (obtained by multiplying the total sperm per ejaculate by the percentage of sperm motility). A sperm viability test was carried out to differentiate cell death from immotility by staining with eosin Y 0.5% in saline solution.
These samples were divided into two groups on the basis of their motility (WHO 2010)11 such as Group A consisted of 58 samples with progressive motility ≥45% (normokinetic samples) and Group B of 58 samples with progressive motility ≤30% (hypokinetic samples).
Gene expression
GAPD2 gene expression studies were carried out on 116 semen samples from patients aged 16–50 years. Semen samples were diluted with PBS to around 10 × 106 sperm ml−1 and underwent osmotic shock to eliminate the nongamete cell component so as to enable the study of both motile and immotile sperm. Aliquots of 1 ml of the diluted samples were centrifuged at 1600 g 10 min, and the pellets were incubated with 1 ml of cell lysis buffer (0.1% SDS, 0.5% Triton X-100 in distilled H2O) for 60 min on ice. After incubation, the absence of any round cells was confirmed under the optical microscope.
Total RNA was extracted from sperm using the SV Total RNA Isolation System RNeasy Mini Kit (PROMEGA, Madison, WI, USA) according to the manufacturer's instructions. Reverse transcription for cDNA synthesis was carried out on 2 μg of RNA extracted from each sample in a final reaction volume of 20 μl according to the manufacturer's instructions (PROMEGA, Madison, WI, USA).
Real-time PCR quantification of mRNA was carried out using gene expression Assay Mix containing unlabeled primers and TaqMan MGB fluorescent probes labeled at the 5’ end with 6-carboxy-fluorescein (FAM, reporter dye) (Applied Biosystems, Carlsbad, CA, USA). GAPD2-specific primers and probes were used, and PRM2 was used as the endogenous gene for sample normalization. In our experience, the expression of this PRM2 gene is constant in both normokinetic and hypokinetic samples.
The reaction was carried out in a 25 μl reaction volume containing 12.5 μl Master Mix 2X, 1.25 μl TaqMan gene expression Assay Mix 20X (Applied Biosystems, Carlsbad, CA, USA), 2.5 μl cDNA 10 ng μl−1, and 8.75 μl nuclease-free H2O. Real-time PCR amplification was carried out in a 48-well plate with STEP ONE (Applied Biosystems, Carlsbad, CA, USA). To improve statistical reliability and reproducibility, each sample was loaded in triplicate onto the reaction plate, and the mean threshold cycle (Ct) value was considered for each gene. To minimize the variability due to the different efficiencies of the reverse transcription reactions, the quantity of GAPD2 cDNA was normalized to the quantity of PRM2.
Statistical analysis
Gene expression was analyzed by ΔΔCt (comparative Ct), where the first ΔCt indicates the normalization of the CT of the target gene versus endogenous gene whereas the second ΔCt, ΔΔCt, indicates the calibration of the target samples in comparison with the control. GAPD2 expression measured by real-time PCR was calculated comparing the samples with the control. The control sample (CTRL) is the mean of the ΔCt of the samples from Group A.
The Kolmogorov–Smirnov test was used to evaluate the distribution of the parameters (ΔCt, total motility). Data were expressed as mean ± standard deviation (s.d.). The Mann–Whitney was used to compare normokinetic and hypokinetic samples. Spearman's correlation between ΔCt and total motility and between ΔCt and millions of motile sperm per ejaculate was calculated.
Data were adjusted for possible confounding factors such as age. All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA). A two-tailed P < 0.05 was considered statistically significant.
RESULTS
Table 1 shows the means and s.d.s for age in years, semen parameters, and sperm vitality for Groups A and B. There were statistically significant differences between Groups A and B for all parameters except semen volume. GAPD2 mRNA expression was evaluated using real-time PCR on normokinetic and hypokinetic samples with the ΔΔCt method. Figure 1 shows the values for GAPD2 expression in Group B versus Group A, assuming Group A as the control, equal to 1 (fold change 1.2 vs 1.0). The difference between the two values was not statistically significant. It can be seen that the expression profiles for the two groups are very similar. The mean ΔCt was as follows – Group A, 5.79 ± 1.04; Group B, 5.47 ± 1.27. The Mann–Whitney analysis of the ΔCt of the normokinetic and hypokinetic samples demonstrated that there was no statistically significant difference (P = 0.19) between the two groups in the expression of the gene of interest for crude and adjusted data (Figure 2). There was no correlation between patient's age and GAPD2 gene expression (r2= 0.005; P = 0.46).
Table 1.
Means and standard deviations for age in years, semen parameters, and sperm vitality for Groups A and B

Figure 1.
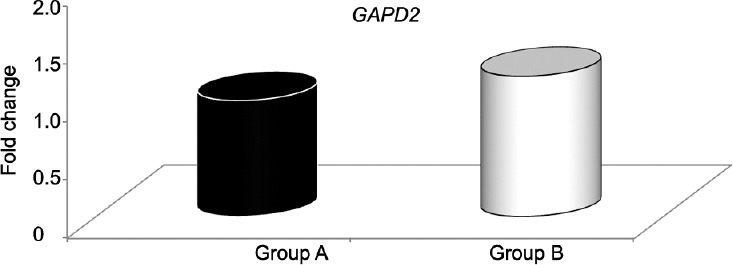
Relative GAPD2 expression in Group B versus A, assuming Group A (control) = 1.
Figure 2.
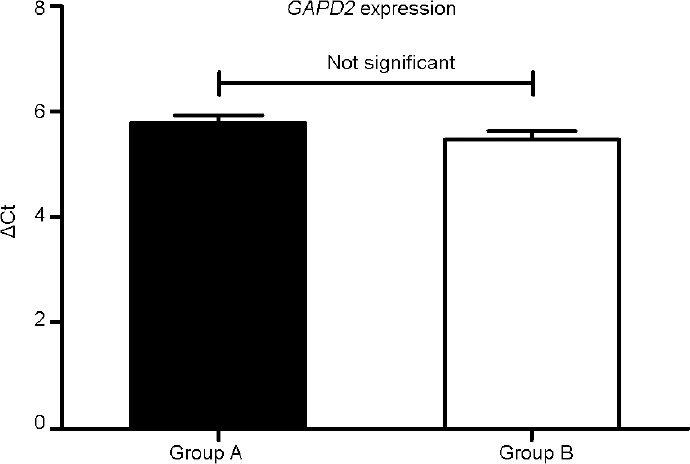
GAPD2 expression in Groups A and B.
Correlation analysis revealed that GAPD2 gene expression (ΔCt) increases with increasing motility in both Groups A and B although this correlation was not statistically significant (P = 0.40; P = 0.22) (Figure 3). There was no correlation between the millions of motile sperm per ejaculate and ΔCt (Figure 4).
Figure 3.
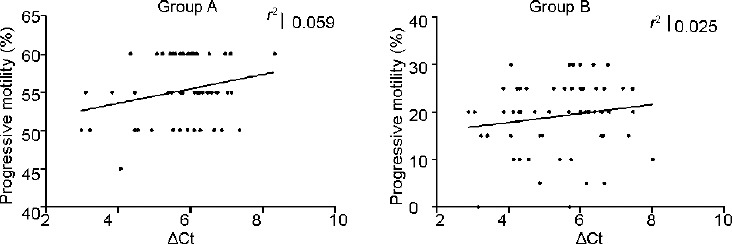
ΔCt versus progressive motility in Groups A and B.
Figure 4.
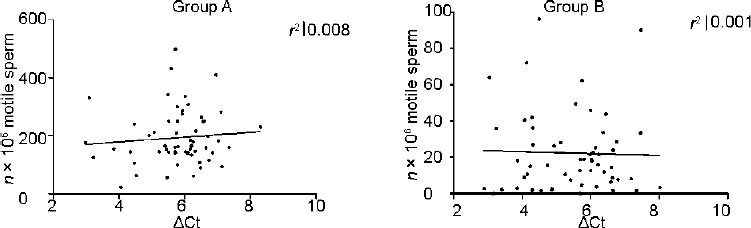
Absolute values in terms of millions of motile sperm per ejaculate versus ΔCt in Groups A and B.
DISCUSSION
Despite numerous studies, the molecular and biochemical events involved in sperm kinetics are still unknown. In all kinetics-related biochemical processes, ATP acts as the molecular motor which generates power for the flagellar movement. Glycolysis seems to be an important source for delivering ATP along the entire flagellum.1 Westhoff and Kamp12 and Welch et al.13 found that glyceraldehyde 3-phosphate dehydrogenase, the NAD-dependent glycolytic enzyme that catalyzes the transformation of glyceraldehyde 3-phosphate (GAP) to 1,3-biphosphoglycerate (1,3 BPG), is bound in large quantities to the fibrous sheath of the sperm of various mammals, including humans. In 2004, Mukai and Okuno9 demonstrated that sperm incubated with mitochondrial respiration inhibitors (carbonyl cyanide m-chlorophenylhydrazone and antimycin A) showed a high beat frequency in the presence of glucose while the same sperm did not retain their motility with pyruvate or lactate, thus indicating that the ATP produced by the glycolytic process was sufficient for flagellar movement. The length of the flagellar structure of mammalian sperm impedes ATP diffusion from the mitochondria in the midpiece to the principal piece and endpiece, which together account for the majority of its structure. For this reason, special catalytic isoenzymes could be the result of a reorganization of the metabolism guided by a structural modification.14 The localization of these enzymes in the fibrous sheaths thus enables the local production of ATP in proximity with the dynein's ATPase along the entire axoneme to the end of the flagellum. It also enables the enzymes to function correctly despite the mechanical perturbations taking place during the flagellar beat.
In sperm, various factors coincide to create a high throughput ATP production system, namely large cell surface in relation to the small volume, abundance of glycolytic enzymes bound to the fibrous sheaths, and ATP use by the ATPase in the dyneins, enabling the reactions to take place in a forward direction.15 Glycolysis thus seems to have an important role in energy production. Evidence of this theory comes from studies of GAPD2 by Elkina et al.16 who demonstrated an enzyme activity 2.5–3 times lower in hypokinetic samples than in normokinetic samples while tissue localization studies17 revealed GAPD2 in the testicles and epididymis. In both human and rat testicles, this enzyme was present mainly in the cytoplasm of all spermatogenic cells while, in the epididymis, it was found in the sperm tail. Liu et al. study17 also demonstrated the localization of the glycolytic enzyme in the principal piece of the sperm flagellum and the ability of anti-GAPD2 antibodies to inhibit sperm motility and penetration into the zona pellucida. A very recent study by Takei et al.18 investigated the correlation between the glycolytic metabolic pathway and flagellar movement in mouse sperm though analysis of motility in relation to beat cross frequency, bend angle, sliding velocity, and local bending. The results of that study revealed that in the presence of substrates of mitochondrial respiration and glycolysis inhibitors, there is a reduction in ATP concentration and kinetic parameters, especially in the distal part of the flagellum. In humans, the sperm isoenzyme glyceraldehyde 3-phosphate dehydrogenase is coded by a GAPD2 paralogs gene, located in chromosome 19q13.12, which contains 11 exons. The length of the primary transcript of this gene is 11 908 nucleotides, and it produces a protein of 408 amino acid residues. The sperm isoenzyme differs from the somatic isoenzyme in that it has 73 residues constituting the N-terminal region, rich in proline, which is needed to bind the fibrous sheaths.
Studies in mice found that Gapds mRNA is initially found in round spermatids, reaches its peak levels in condensed spermatids but is only found later in elongated spermatids,19,20 suggesting late gene expression. In fact, Mezquita et al.21 identified alternative transcription initiation in chicken testes characterized by alternative splicing in 5’ regions of the pre-mRNA, resulting in at least six different types of mRNAs and polyadenylation; all these processes could underlie the various mechanisms for the regulation of Gapds expression.
The presence of Gapds mRNA in the first stages of spermatogenesis and evidence of the enzyme only in later stages also indicates that the translation is controlled by specific mechanisms identified in transline protein or TB RBP (testis brain RNA-binding protein), i.e., proteins that bind DNA or RNA, carrying out different functions. In vitro, mouse transline proteins form a Gapds mRNA complex, necessary for the incorporation of the GAPDS protein in the fibrous sheaths during growth.22 In fact, germ cells use mRNA storage and delayed translation mechanism after the chromatin has already been packaged. Sperm not only contains haploid genetic information but also has an abundance of transcripts, thought to be remnants of mRNA which represent a genetic fingerprint and can be considered a historic record of gene expression during spermatogenesis. It is thus possible that errors during the spermatogenetic process are encapsulated within the repository of spermatozoal RNA.23
Sperm from fertile and infertile men have different quantities of transcripts,24,25 and some studies have found different gene expressions to be correlated with different sperm concentrations, percentage motility and morphology.26 Research on gene expression could, therefore, provide significant information on physiological or pathological processes. Specifically, GAPD2 gene expression could be a good marker for the analysis of spermatogenesis as its transcription and translation products are temporarily separated and form a complex with RNA binding protein, which induces the translation and delays mRNA degradation. This was demonstrated by Dorosh et al.27 through analysis of the expression of the spermatogenesis-related genes MND1, SPATA22, GAPD2, and ACR in 47 testicular biopsies of men with nonobstructive azoospermia. The aim of that study was to identify markers predictive of sperm recovery in such patients. It found a low level of GAPD2 transcripts in these patients, suggesting that changes to the profile of this gene were associated with impaired spermatogenesis. In contrast, Shen et al.28 evaluated gene and protein expression in selected sperm from 30 asthenozoospermic semen samples, with 30 normozoospermic samples used as the control. The proteome study found 15 new proteins expressed at different levels in the cases in comparison with the controls, including the GAPD2 enzyme. The authors carried out a quantitative RT PCR analysis to compare the transcript and protein levels, finding no correlation between gene expression and protein expression. Asthenozoospermic samples showed lower GAPD2 gene expression than normozoospermic samples, but the transcriptional level was not correlated with the protein level. The noncorrespondence between gene transcription and protein translation seems to be the result of a complex network of factors which interact under given conditions to determine a pathological phenotype. Therefore, the role played by GAPD2 in sperm motility currently remains unknown.
No variability in gene expression associated with sperm motility was found in our study. There was no difference in the GAPD2 gene expression profile between normozoospermic and hypokinetic samples, indicating that transcription occurs normally in these two semen phenotypes. A correlation analysis of our data found that GAPD2 gene expression (ΔCt) rises with increasing total motility in both normo and hypokinetic samples although this correlation was not statistically significant. Furthermore, no correlation was found between millions of motile sperm per ejaculate and ΔCt. The latter finding is very interesting as it suggests that hypomotility may be due to a possible posttranscriptional impairment of the control mechanism, such as mRNA splicing, or posttranslational changes, such as phosphorylation, glycosylation, cutting, and posttranslational splicing.
These protein structural changes may provide the basis for diverse functional activities such as the potential role of GAPD2 in sperm/oocyte binding. A recent study, in fact, found GAPD2 localization in the apical part of the sperm head in addition to the principal piece of the flagellum.29 A posttranscriptional regulation of gene expression, possibly related to the generation of piRNAs, could also be postulated.30 Furthermore, glyceraldehyde 3-phosphate dehydrogenase regulates the glycolytic process and seems to be the target of compounds that impair male fertility.31 This could also suggest that environmental factors might influence the chemical and kinetic properties of this enzyme.
One limitation of our study is that we do not know the protein expression levels. It would be interesting to perform a proteomic study to understand the relationship between hypokinesia and sperm protein levels. However, the study also has some strengths: awareness of GAPD2 expression levels in given semen phenotypes adds to our knowledge of molecular sperm energy production and enables us to theorize that changes to the mechanisms of posttranscriptional regulation of mRNA or translational changes may be the basis for impaired kinetics.
CONCLUSION
This study shows that there are no differences in GAPD2 expression between human sperm samples with impaired motility and samples with normal kinetics. This could suggest that GAPD2 has a minor role in sperm motility and demonstrates the need to continue research into protein expression and function. The study of posttranscriptional and translational changes could be an interesting aspect in the evaluation of the molecular pathways of sperm motility and the identification of clinical targets for therapeutic treatment.
AUTHOR CONTRIBUTIONS
LG and DP performed conception and design of the work. MP, MG, and GC carried out the molecular genetic studies. LG and DP drafted the article. LG made final approval of the version to be published. FL and AL revised the paper critically. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was supported by a grant from the Italian Ministry of Education and Research (MIUR-PRIN) and the University oC Rome “La Sapienza”, Faculty of Medicine. The authors would like to thank Marie-Hélène Hayles for her assistance with the English translation.
REFERENCES
- 1.Turner RM. Tales from the tail: what do we really know about sperm motility? J Androl. 2003;24:790–803. doi: 10.1002/j.1939-4640.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 2.Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–74. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- 3.Ferramosca A, Focarelli R, Piomboni P, Coppola L, Zara V. Oxygen uptake by mitochondria in demembranated human spermatozoa: a reliable tool for the evaluation of sperm respiratory efficiency. Int J Androl. 2008;31:337–45. doi: 10.1111/j.1365-2605.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 4.Paoli D, Gallo M, Rizzo F, Baldi E, Francavilla S, et al. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil Steril. 2011;95:2315–9. doi: 10.1016/j.fertnstert.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Du J, Tao J, Kleinhans FW, Mazur P, Critser JK. Water volume and osmotic behaviour of mouse spermatozoa determined by electron paramagnetic resonance. J Reprod Fertil. 1994;101:37–42. doi: 10.1530/jrf.0.1010037. [DOI] [PubMed] [Google Scholar]
- 6.Krisfalusi M, Miki K, Magyar PL, O’Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–8. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, et al. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol. 2007;302:463–76. doi: 10.1016/j.ydbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travis AJ, Foster JA, Rosenbaum NA, Visconti PE, Gerton GL, et al. Targeting of a germ cell-specific type 1 hexokinase lacking a porin-binding domain to the mitochondria as well as to the head and fibrous sheath of murine spermatozoa. Mol Biol Cell. 1998;9:263–76. doi: 10.1091/mbc.9.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–7. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 10.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–6. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 12.Westhoff D, Kamp G. Glyceraldehyde 3-phosphate dehydrogenase is bound to the fibrous sheath of mammalian spermatozoa. J Cell Sci. 1997;110(Pt 15):1821–9. doi: 10.1242/jcs.110.15.1821. [DOI] [PubMed] [Google Scholar]
- 13.Welch JE, Brown PL, O’Brien DA, Magyar PL, Bunch DO, et al. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J Androl. 2000;21:328–38. [PubMed] [Google Scholar]
- 14.Kuravsky ML, Aleshin VV, Frishman D, Muronetz VI. Testis-specific glyceraldehyde-3-phosphate dehydrogenase: origin and evolution. BMC Evol Biol. 2011;11:160. doi: 10.1186/1471-2148-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukai C, Travis AJ. What sperm can teach us about energy production. Reprod Domest Anim. 2012;47:164–9. doi: 10.1111/j.1439-0531.2012.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkina YL, Atroshchenko MM, Bragina EE, Muronetz VI, Schmalhausen EV. Oxidation of glyceraldehyde-3-phosphate dehydrogenase decreases sperm motility. Biochemistry (Mosc) 2011;76:268–7. doi: 10.1134/s0006297911020143. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Sun CM, Zhang CL, Wang X, Li JY. Location and characterization of GAPDS in male reproduction. Urol Int. 2013;90:449–54. doi: 10.1159/000345629. [DOI] [PubMed] [Google Scholar]
- 18.Takei GL, Miyashiro D, Mukai C, Okuno M. Glycolysis plays an important role in energy transfer from the base to the distal end of the flagellum in mouse sperm. J Exp Biol. 2014;217:1876–86. doi: 10.1242/jeb.090985. [DOI] [PubMed] [Google Scholar]
- 19.Bunch DO, Welch JE, Magyar PL, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S protein distribution during mouse spermatogenesis. Biol Reprod. 1998;58:834–41. doi: 10.1095/biolreprod58.3.834. [DOI] [PubMed] [Google Scholar]
- 20.Welch JE, Brown PR, O’Brien DA, Eddy EM. Genomic organization of a mouse glyceraldehyde 3-phosphate dehydrogenase gene (Gapd-s) expressed in post-meiotic spermatogenic cells. Dev Genet. 1995;16:179–89. doi: 10.1002/dvg.1020160210. [DOI] [PubMed] [Google Scholar]
- 21.Mezquita J, Pau M, Mezquita C. Several novel transcripts of glyceraldehyde-3-phosphate dehydrogenase expressed in adult chicken testis. J Cell Biochem. 1998;71:127–39. doi: 10.1002/(sici)1097-4644(19981001)71:1<127::aid-jcb13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 22.Shchutskaya YY, Elkina YL, Kuravsky ML, Bragina EE, Schmalhausen EV. Investigation of glyceraldehyde-3-phosphate dehydrogenase from human sperms. Biochemistry (Mosc) 2008;73:185–91. doi: 10.1134/s0006297908020107. [DOI] [PubMed] [Google Scholar]
- 23.Ostermeier GC, Goodrich RJ, Moldenhauer JS, Diamond MP, Krawetz SA. A suite of novel human spermatozoal RNAs. J Androl. 2005;26:70–4. [PubMed] [Google Scholar]
- 24.Steger K, Wilhelm J, Konrad L, Stalf T, Greb R, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod. 2008;23:11–6. doi: 10.1093/humrep/dem363. [DOI] [PubMed] [Google Scholar]
- 25.Avendaño C, Franchi A, Jones E, Oehninger S. Pregnancy-specific {beta}-1-glycoprotein 1 and human leukocyte antigen-E mRNA in human sperm: differential expression in fertile and infertile men and evidence of a possible functional role during early development. Hum Reprod. 2009;24:270–7. doi: 10.1093/humrep/den381. [DOI] [PubMed] [Google Scholar]
- 26.Bonache S, Mata A, Ramos MD, Bassas L, Larriba S. Sperm gene expression profile is related to pregnancy rate after insemination and is predictive of low fecundity in normozoospermic men. Hum Reprod. 2012;27:1556–67. doi: 10.1093/humrep/des074. [DOI] [PubMed] [Google Scholar]
- 27.Dorosh A, Tepla O, Zatecka E, Ded L, Koci K, et al. Expression analysis of MND1/GAJ, SPATA22, GAPDHS and ACR genes in testicular biopsies from non-obstructive azoospermia (NOA) patients. Reprod Biol Endocrinol. 2013;11:42. doi: 10.1186/1477-7827-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen S, Wang J, Liang J, He D. Comparative proteomic study between human normal motility sperm and idiopathic asthenozoospermia. World J Urol. 2013;31:1395–401. doi: 10.1007/s00345-013-1023-5. [DOI] [PubMed] [Google Scholar]
- 29.Margaryan H, Dorosh A, Capkova J, Manaskova-Postlerova P, Philimonenko A, et al. Characterization and possible function of glyceraldehyde-3-phosphate dehydrogenase-spermatogenic protein GAPDHS in mammalian sperm. Reprod Biol Endocrinol. 2015;13:15. doi: 10.1186/s12958-015-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan H, Cai T, Lin X, Wu Y, Wang X, et al. Integrative proteomic and transcriptomic analyses reveal multiple post-transcriptional regulatory mechanisms of mouse spermatogenesis. Mol Cell Proteomics. 2013;12:1144–57. doi: 10.1074/mcp.M112.020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.du Plessis SS, Agarwal A, Mohanty G, van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl. 2015;17:230–5. doi: 10.4103/1008-682X.135123. [DOI] [PMC free article] [PubMed] [Google Scholar]


