Abstract
Prior studies suggested that the use of androgen deprivation therapy (ADT) in patients with prostate cancer (PC) might cause the impairment of cognitive function which is one of the common symptoms of dementia; however, the association between ADT and cognitive impairment still remains controversial. This retrospective cohort study aimed to investigate the relationship between ADT and subsequent risk of dementia using a population-based dataset. Data for this study were taken from the Taiwan (China)Longitudinal Health Insurance Database 2005. We included 755 PC patients who received ADT in the study cohort and 559 PC patients who did not receive ADT in the comparison cohort. Each patient was individually tracked for a 5-year period to define those who subsequently received a diagnosis of dementia. Results show that the incidence rates of dementia per 100 person-years were 2.35 (95% confidence interval [95% CI]: 1.82–2.98) and 1.85 (95% CI: 1.35–2.48) for PC patients who received ADT and those who did not receive ADT, respectively. The adjusted hazard ratio (HR) for dementia for PC patients who received ADT was 1.21 (95% CI: 0.82–1.78, P = 0.333) compared to those who did not receive ADT. In addition, the adjusted HRs for dementia for PC patients receiving ADT with gonadotropin-releasing hormone (GnRH) agonists and without GnRH agonists were 1.39 (95% CI: 0.80–2.40, P = 0.240) and 1.13 (95% CI: 0.75–1.71, P = 0.564), respectively, compared to PC patients not receiving ADT. We concluded that there was no difference in the risk of subsequent dementia between PC patients who did and those who did not receive ADT.
Keywords: androgen deprivation therapy, dementia, epidemiology, prostate cancer
INTRODUCTION
Prostate cancer (PC) is a prevalent androgen-dependent malignancy.1,2 It was the second major cause of cancer-related mortality in males in the United States and contributed to 1%–2% of deaths in the male population.1,3 Androgen deprivation therapy (ADT) is a current standard for the palliative treatment of metastasized patients or as an adjuvant therapy for intermediate or high-risk PC in combination with radiation.4,5,6,7 It can decrease testosterone levels, suppress the prostate-specific antigen (PSA), stabilize the disease, and possibly prolong survival in PC.8,9 However, the decreasing serum level of endogenous testosterone in PC patients was considered to contribute to some adverse effects and chronic diseases.10,11
Many previous studies reported that males receiving ADT for PC had increased risk of many chronic diseases, such as metabolic syndrome, cardiovascular diseases, fractures, anxiety, and depression compared to males who did not receive ADT.8,11,12,13 Recent studies suggest that ADT use may be associated with structural and functional changes in brain areas relevant to dementia.14,15,16,17 Some studies have suggested that the use of ADT might cause the impairment of cognitive function, which is one of the common symptoms of dementia; however, the association between ADT and cognitive impairment still remains controversial.18,19,20,21,22,23 For instance, a longitudinal study in the United States and a cross-sectional study in China both found that patients receiving ADT were more likely to experience cognitive impairment than the comparison group.19,20 Conversely, longitudinal studies conducted in the United States and Canada showed no evidence that ADT caused adverse effects on cognitive function.21,23 Another cross-sectional study in Canada also found that the ADT did not affect the physical or cognitive function of PC patients.22
To date, to the best of our knowledge, no study has attempted to investigate the relationship between ADT and the subsequent risk of dementia, even though ADT might affect brain function and further contribute to dementia or cognitive impairment.14,15,16,17 In addition, previous studies did not show consistent findings for the association between ADT and cognitive impairment. Therefore, the aim of this retrospective cohort study was to investigate the relationship between ADT and the subsequent risk of dementia using a population-based dataset in Taiwan, China.
MATERIALS AND METHODS
Database
Data for this population-based retrospective cohort study were taken from the Taiwan (China) Longitudinal Health Insurance Database 2005 (LHID2005). The LHID2005 includes medical records and registry data for 1 million individuals randomly selected from all enrollees in the Taiwan National Health Insurance (NHI) (Taiwan, China) program in 2005 (n = 25.68 million). The NHI program was begun in 1995 and provides affordable and comprehensive medical services for all citizens in Taiwan, China. The LHID2005 is provided to researchers in Taiwan (China) for academic purposes. Many studies which used this dataset have been published.24,25
This study was exempt from full review by the Institutional Review Board of the National Defense Medical Center (Taiwan, China) because the LHID2005 consists of de-identified secondary data released to the public for research purposes.
Study sample
This retrospective cohort study initially selected 1481 patients who received a first-time diagnosis of PC (ICD-9-CM code 185, malignant neoplasm of the prostate) in ambulatory care centers from January 1, 2001 to December 31, 2008. We then excluded patients aged <40 years (n = 25) because of the very low prevalence of PC in that age group. The date of the first ambulatory care visit for receiving ADT medications, including gonadotropin-releasing hormone (GnRH) agonists (leuprolide, goserelin, and triptorelin), antiandrogens (cyproterone acetate, flutamide, bicalutamide, and nilutamide), and estrogens, was defined as the index date for PC patients who received ADT treatment. For PC patients who did not receive ADT, the first ambulatory care visit in which they received a PC diagnosis was identified as the index date. Of the remaining selected PC patients, we further excluded patients who had a history of dementia (ICD-9-CM codes 290.0–290.4, 294.1, 331.0–331.2, or 331.82) before their index date (n = 37). We then excluded 105 patients who received an orchiectomy (ICD-9-CM procedure codes 623 or 624) during the 5-year follow-up period starting from their index date because this study only concentrated on medical castration. As a result, a total 1314 PC patients were included in this cohort study. PC patients who received ADT were defined as study cohort (n = 755). Moreover, PC patients who did not receive ADT were identified as comparison cohort (n = 559).
In this study, each patient (n = 1314) was individually tracked for a 5-year period to define those who subsequently received a diagnosis of dementia (ICD-9-CM codes 290.0–290.4, 294.1, 331.0–331.2, or 331.82) following the index date. Mini-mental state examination in combination with CT scan or magnetic resonance imaging were frequently used to diagnose dementia in the LHID2005. To increase the dementia diagnosis validity, we did not include those who had a history of major psychosis or a substance-related disorder (ICD-9-CM codes 291–299 or 303–305), stroke (ICD-9-CM codes 430–438), or traumatic brain injury (ICD-9-CM codes 801–804 or 850–854) prior to the index date.
Statistical analysis
All analyses in this study were conducted with the SAS system (SAS System for Windows, version 9.2, SAS Institute, Cary, NC, USA). Chi-squared tests were used to investigate differences in sociodemographic characteristics (monthly income and the urbanization level and geographic location of the patient's residence) between PC patients who received and those who did not receive ADT.
To estimate the relationship between ADT and the risk of subsequent dementia during the 5-year follow-up period, we further performed Cox proportional hazard regressions. The dependent variable was the time to the first diagnosis of dementia. We also censored patients who died during the 5-year follow-up period (516 of the PC patients died [39.3%] in this study). In addition, adjustments in this study were made for geographical location, monthly income, urbanization level, and age of the selected patients.
In addition, we evaluated the HRs for PC patients receiving ADT with GnRH agonists and without GnRH agonists compared to those not receiving ADT. The conventional two-sided P = 0.05 was used to determine statistical significance in this study.
RESULTS
Table 1 shows the distribution of demographic characteristics of PC patients stratified by whether or not they received ADT. Chi-squared tests showed that there was no difference in urbanization level between PC patients who received ADT and those who did not (P = 0.077). However, there were statistical differences in age (P < 0.001), geographic region (P < 0.001), and monthly income (P < 0.001) between these two cohorts.
Table 1.
Demographic characteristics of patients with prostate cancer, stratified by whether or not patients received ADT (n=1314)
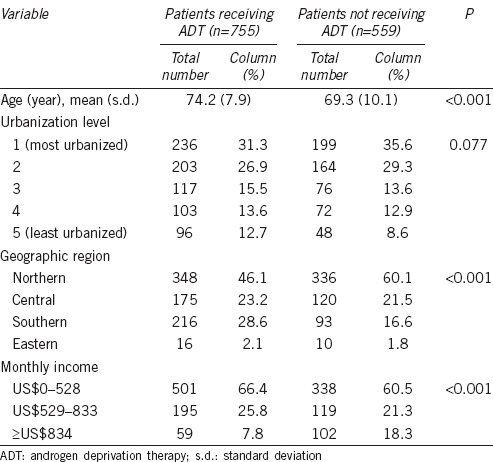
Among 1314 subjects, the incidence rate of dementia per 100 person-years was 2.12 (95% CI: 1.75–2.55) (Table 2). In addition, the incidence rates of dementia per 100 person-years were 2.35 (95% CI: 1.82–2.98) and 1.85 (95% CI: 1.35–2.48), respectively, for PC patients who received ADT and those who did not receive ADT. Table 2 also explores the hazard ratio (HR) for dementia in PC patients who received ADT compared to those who did not receive ADT. After censoring subjects who died during the 5-year follow-up period, Cox proportional hazard regression showed that the crude HR was 0.90 (95% CI: 0.62–1.32, P = 0.589) for PC patients who received ADT compared to those who did not receive ADT. Moreover, the adjusted HR for dementia among PC patients who received ADT was 1.21 (95% CI: 0.82–1.78, P = 0.333) compared to those who did not receive ADT after adjusting for patients’ geographical location, monthly income, urbanization level, and age.
Table 2.
Crude and adjusted HRs for dementia among patients with prostate cancer during a 5-year follow-up period, stratified by whether or not patients received ADT
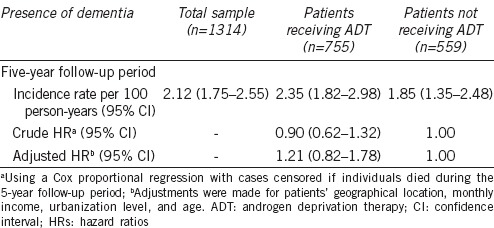
Furthermore, Table 3 shows that the crude HRs of dementia for PC patients receiving ADT with GnRH agonists and without GnRH agonists were 1.13 (95% CI: 0.66–1.93, P = 0.660) and 0.81 (95% CI: 0.54–1.22, P = 0.315), respectively, compared to PC patients not receiving ADT. In addition, the HRs of dementia for PC patients receiving ADT with GnRH agonists and without GnRH agonists were 1.39 (95% CI: 0.80–2.40, P = 0.240) and 1.13 (95% CI: 0.75–1.71, P = 0.564), respectively, compared to PC patients who did not receive ADT after adjusting for geographical location, monthly income, urbanization level, and age.
Table 3.
Crude and adjusted HRs for dementia among patients with prostate cancer during a 5-year follow-up period, stratified by patients receiving ADT with GnRH agonists, patients receiving ADT without GnRH agonists, and patients not receiving ADT
DISCUSSION
This population-based retrospective cohort study found that there was no elevated risk of dementia for PC patients who received ADT compared to PC patients who did not receive ADT. In addition, there was no association between the use of GnRH agonists and subsequent incidence of dementia in PC patients. According to our knowledge, this is the first study that attempted to explore the relationship between ADT and dementia.
To date, increasing neurobiological evidence supports a potential connection between ADT and abnormal brain function.14,15 One study which combined neuropsychological testing with functional magnetic resonance imaging (fMRI) showed a relationship between ADT and decreasing brain activation.15 Another study also found that ADT in PC patients may affect brain structures, such as reduced cerebral gray matter volumes in frontopolar cortex, dorsolateral prefrontal cortex, and primary motor cortex.14 In addition, according to previous literature, the abnormal brain function is considered to be associated with dementia and cognitive impairment.16,17,20 It is plausible that ADT may be associated with the occurrence of dementia because of the change of brain function. Many studies also supposed that ADT might contribute to the cognitive impairment by affecting the brain structures and activation.18,19,20,21,22,23 However, the relevant findings in those studies were conflicting and no prior studies attempted to investigate the relationship between ADT and dementia.
Our study found no increased risk of dementia for PC patients receiving ADT compared to PC patients not receiving ADT. This observation is consistent with some studies which investigated the association between ADT and cognitive impairment. For example, one cross-sectional study in Canada which included 57 patients receiving ADT for nonmetastatic PC and 51 healthy age-matched controls reported that ADT did not affect physical or cognitive functions.22 A study in the United States which recruited 32 subjects receiving ADT also revealed no statistical changes in cognition over time.23 Moreover, a meta-analysis that included 14 articles reported that PC patients receiving ADT did not have worse cognitive function compared to PC patients not receiving ADT.26,27 Furthermore, that meta-analysis provided longitudinal assessments of PC patients from initiation of ADT to a subsequent assessment at least 3 months after ADT initiation, and relevant findings showed that ADT might not increase the risk of cognitive impairment.26
Nevertheless, findings of several studies are inconsistent with our results. A Chinese cross-sectional study found that PC patients receiving ADT had a higher risk of cognitive impairment than PC patients not receiving ADT.20 One meta-analysis also found that PC patients receiving ADT had an elevated risk of cognitive impairment compared to those without PC.26 In addition, a study in the United States also found that the PC patients receiving ADT were more likely to exhibit cognitive changes than a comparison group which involved both PC patients not receiving ADT and patients without PC.19 The inconsistent findings between our study and the above studies could be that cognitive domains previously reported to be affected are not relevant to a diagnosis of dementia. However, the dataset which used in this study does not include the data (including cognitive tests, laboratory tests or brain imaging) which are commonly used to diagnose the types of dementia. Therefore, we could not further investigate the association between ADT and different types of dementia. In addition, the actual relationship between ADT use and cognitive impairment remains controversial, and several methodologic limitations in previous studies were considered to have biased the actual association, including the use of cross-sectional designs, no comparison group, use of noncancer patients as the comparison group, a small sample size, and short follow-up periods.19,26
The specific strength of this study is the use of a large population-based dataset in Taiwan, China. The LHID2005 database provided a sufficient sample size to identify the relationship between ADT and the subsequent occurrence of dementia. In addition, this characteristic of the LHID2005 database could elevate the statistical power, and decrease the selection bias of the results. In addition, the LHID2005 records all relevant diagnoses and medical services for all study subjects since they were included in the NHI system in Taiwan, China. These features of the dataset could avoid the potential effect of a recall bias in the study.
However, there are several limitations to this population-based study. First, the LHID2005 database does not have medical records on cancer staging, such as the TNM Classification of Malignant Tumors (TNM) or relevant pathologic lab data. Second, some lifestyle information and variables relevant to cognition and cognitive reserve were not available, including cigarette smoking, the body-mass index, alcohol consumption, education level and intellectual function which are all possible risk factors for dementia and might affect the connection between ADT and dementia.28,29 Third, the diagnostic records of dementia in LHID2005 might not include all patients with dementia in Taiwan, China. Several patients with mild symptoms of dementia might not look for healthcare services covered by the NHI (Taiwan, China) program because they considered the relevant therapies unnecessary. In addition, demographic differences between cohorts, such as resident region and monthly income might affect patients’ intentions to seek medical service. Finally, most patients recruited in this study were of Chinese ethnicity. Consequently, generalization of the findings to Western societies is not assured.
CONCLUSION
This population-based retrospective cohort study found that there was no difference in the risk of subsequent dementia between PC patients who did and those who did not receive ADT. The findings in this study could provide valuable information for clinicians to assess risks and benefits of ADT. Further epidemiologic studies are still required to confirm the relationship between ADT and subsequent dementia in different races.
AUTHOR CONTRIBUTIONS
LTK participated in the design of the study and drafted the manuscript. HCL performed the statistical analysis and helped to draft the manuscript. SDC and CYH conceived of the study, participated in its design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared no competing interests.
REFERENCES
- 1.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, et al. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 2.Victor LD. Obstructive sleep apnea. Am Fam Physician. 1999;60:2279–86. [PubMed] [Google Scholar]
- 3.Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7(Suppl 5):S37–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadi H, Daneshmand S. Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes. Patient Relat Outcome Meas. 2014;5:63–70. doi: 10.2147/PROM.S52788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keane FK, Chen MH, Zhang D, Moran BJ, Braccioforte MH, et al. Androgen deprivation therapy and the risk of death from prostate cancer among men with favorable or unfavorable intermediate-risk disease. Cancer. 2015;121:2713–9. doi: 10.1002/cncr.29420. [DOI] [PubMed] [Google Scholar]
- 7.Kohutek ZA, Steinberger E, Pei X, Shi W, Zhang Z, et al. Long-term impact of androgen-deprivation therapy on cardiovascular morbidity after radiotherapy for clinically localized prostate cancer. Urology. 2016;87:146–52. doi: 10.1016/j.urology.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 9.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111:543–8. doi: 10.1111/j.1464-410X.2012.11774.x. [DOI] [PubMed] [Google Scholar]
- 11.Kintzel PE, Chase SL, Schultz LM, O’Rourke TJ. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511–22. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- 12.Sharpley CF, Christie DR, Bitsika V. Do hormone treatments for prostate cancer cause anxiety and depression? Int J Clin Oncol. 2014;19:523–30. doi: 10.1007/s10147-013-0569-y. [DOI] [PubMed] [Google Scholar]
- 13.Chung SD, Chen YK, Wu FJ, Lin HC. Hormone therapy for prostate cancer and the risk of stroke: a 5-year follow-up study. BJU Int. 2012;109:1001–5. doi: 10.1111/j.1464-410X.2011.10459.x. [DOI] [PubMed] [Google Scholar]
- 14.Chao HH, Hu S, Ide JS, Uchio E, Zhang S, et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients – An exploratory study. PLoS One. 2013;8:e72032. doi: 10.1371/journal.pone.0072032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao HH, Uchio E, Zhang S, Hu S, Bednarski SR, et al. Effects of androgen deprivation on brain function in prostate cancer patients – A prospective observational cohort analysis. BMC Cancer. 2012;12:371. doi: 10.1186/1471-2407-12-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Du H, Zheng J, Wang J. A voxel-based morphometric analysis of cerebral gray matter in subcortical ischemic vascular dementia patients and normal aged controls. Int J Med Sci. 2011;8:482–6. doi: 10.7150/ijms.8.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Pan P, Song W, Shang HF. Quantitative meta-analysis of gray matter abnormalities in semantic dementia. J Alzheimers Dis. 2012;31:827–33. doi: 10.3233/JAD-2012-120736. [DOI] [PubMed] [Google Scholar]
- 18.Robinson L, Tang E, Taylor JP. Dementia: timely diagnosis and early intervention. BMJ. 2015;350:h3029. doi: 10.1136/bmj.h3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez BD, Jim HS, Booth-Jones M, Small BJ, Sutton SK, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33:2021–7. doi: 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Zhong F, Qiu J, Wang K. Cognitive function in Chinese prostate cancer patients on androgen-deprivation therapy: a cross-sectional study. Asia Pac J Clin Oncol. 2015;11:277–81. doi: 10.1111/ajco.12347. [DOI] [PubMed] [Google Scholar]
- 21.Alibhai SM, Breunis H, Timilshina N, Marzouk S, Stewart D, et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5030–7. doi: 10.1200/JCO.2010.30.8742. [DOI] [PubMed] [Google Scholar]
- 22.Joly F, Alibhai SM, Galica J, Park A, Yi QL, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176:2443–7. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 23.Mohile SG, Lacy M, Rodin M, Bylow K, Dale W, et al. Cognitive effects of androgen deprivation therapy in an older cohort of men with prostate cancer. Crit Rev Oncol Hematol. 2010;75:152–9. doi: 10.1016/j.critrevonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimprich B, Reuter-Lorenz P, Nelson J, Clark PM, Therrien B, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–31. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 25.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12:231–5. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 26.McGinty HL, Phillips KM, Jim HS, Cessna JM, Asvat Y, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22:2271–80. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green HJ, Pakenham KI, Headley BC, Yaxley J, Nicol DL, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90:427–32. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v2–7. doi: 10.1136/jnnp.2005.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


