Abstract
Human spermatozoa encounter an osmotic decrease from 330 to 290 mOsm l−1 when passing through the female reproductive tract. We aimed to evaluate the role of chloride channels in volume regulation and sperm motility from patients with asthenozoospermia. Spermatozoa were purified using Percoll density gradients. Sperm volume was measured as the forward scatter signal using flow cytometry. Sperm motility was analyzed using computer-aided sperm analysis (CASA). When transferred from an isotonic solution (330 mOsm l−1) to a hypotonic solution (290 mOsm l−1), cell volume was not changed in spermatozoa from normozoospermic men; but increased in those from asthenozoospermic samples. The addition of the chloride channel blockers, 4,4′-diisothiocyanatostilbene-2,2′- isulfonic acid (DIDS) or 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) to the hypotonic solution caused the normal spermatozoa to swell but did not increase the volume of those from the asthenozoospermic semen. DIDS and NPPB decreased sperm motility in both sets of semen samples. The inhibitory effect of NPPB on normal sperm motility was much stronger than on spermatozoa from the asthenozoospermic samples. Both sperm types expressed ClC-3 chloride channels, but the expression levels in the asthenozoospermic samples were much lower, especially in the neck and mid-piece areas. Spermatozoa from men with asthenozoospermia demonstrated lower volume regulating capacity, mobility, and ClC-3 expression levels (especially in the neck) than did normal spermatozoa. Thus, chloride channels play important roles in the regulation of sperm volume and motility and are downregulated in cases of asthenozoospermia.
Keywords: asthenozoospermia, chloride channels, ClC-3, sperm motility, volume regulation
INTRODUCTION
Asthenozoospermia, characterized by reduced sperm motility,1 is a common finding among infertile men. Various causes, such as elevated levels of anti-sperm antibodies, varicocele, endocrine abnormalities, inflammation, and structural defects in the sperm flagella, can lead to asthenozoospermia, but the true etiology remains unknown in most cases. Nearly a century ago, it was discovered that many ions (e.g., Ca2+, K+, HCO3−, Cl−) are involved in sperm movement, capacitation, the acrosome reaction, volume regulation, and chemotaxis of human spermatozoa.2 There has been renewed interest in the subject recently as impairment of ion channels in spermatozoa is associated with male subfertility. Chloride, the principal anion, is essential for many functions of human spermatozoa. Several types of chloride channels, such as the cystic fibrosis transmembrane conductance regulator (CFTR),3,4 Ca2+ -dependent Cl− channels (CaCCs),5 ClC channels,6 γ-aminobutyric acid (GABA)-gated and glycine-gated receptors,7,8 have been reported to be involved.
Chloride channel blockers, such as 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), inhibits the zona pellucida (ZP)-induced acrosome reaction in human spermatozoa.5 Incubating spermatozoa in medium containing chloride channel inhibitors leads to capacitation defects.9 Treated sperm display no hyperactivation in capacitating conditions when Cl− is depleted from the medium.10 Inhibition of transmembrane Cl− transport results in failure of sperm penetration and migration through surrogate cervical mucus.11 ClC channels have been found in the bovine testicular and epididymal spermatozoa,12 and recently in healthy human spermatozoa.6 The ClC proteins can bind to protein phosphatase 1 (PP1γ2), which is required for regulating sperm motility.13 These findings are mainly observed in normal spermatozoa. Whether impairment in chloride channels is associated with sperm dysfunction in patients with asthenozoospermia is still unknown.
Human spermatozoa, while passing from the male to the female reproductive tract experience a natural osmotic decrease approximately from 330 mOsm l−1 to 290 mOsm l−1.14 To counter a hypotonicity-induced volume increase, the spermatozoa must undergo a regulatory volume decrease (RVD). It is reported that spermatozoa with defects in RVD mechanisms swell and are unable to migrate to the site of fertilization.15 Spermatozoa from infertile men exhibit lower volume regulating capacity than those from fertile men.16,17 This implies that the inability of spermatozoa to regulate their volume might be one of the causes of infertility. ClC-3 chloride channels have been reported to be involved in RVD of somatic cells and are expressed in the entire flagellum of healthy human spermatozoa with high density, especially in the neck and mid-piece areas,6 the locations of the osmotically sensitive cytoplasmic droplet. The aim of this study was to investigate the volume regulating capacity, the role of chloride channels in volume regulation and sperm motility, and the expression and distribution of ClC-3 chloride channels in spermatozoa from normal and asthenozoospermic men.
MATERIALS AND METHODS
Semen sample collection
The use of semen samples for the study was approved by the Institutional Review Board of the Guangdong Science and Technology Institute of Family Planning and by the Ethics Committee of this institution. Informed consent was obtained from all the donors. This study included 58 asthenozoospermic semen samples and 56 normozoospermic semen samples as the control. Semen samples were obtained by masturbation into specific sterile containers at the institute after 2–5 days of sexual abstinence. After liquefaction of the semen, the sperm parameters (volume, sperm count, the percentage of progressive motility, and motion characteristics) were evaluated by routine procedures according to World Health Organization guidelines1 using computer-aided sperm analysis (CASA). Asthenozoospermia was defined as progressive motility of <32% within 60 min of ejaculation based on three consecutive investigations. Asthenozoospermic semen samples were collected from patients in the Male Reproductive Center, and normozoospermic semen samples were from healthy semen donors in the Human Sperm Bank of the Family Planning Special Hospital of Guangdong. In selecting the semen samples, those patients with varicocele, infections, a history of radiation and/or chemotherapy, abnormal autoimmune symptoms, or endocrine abnormalities were excluded. Once no possible cause for reduced sperm motility could be detected, a conclusive diagnosis of idiopathic asthenozoospermia was reached. The average duration (mean ± standard deviation) of diagnosed infertility for the patients with asthenozoospermia was 3.2 ± 0.8 years.
Sperm preparation and incubation
After semen liquefaction, spermatozoa were washed through a two-step gradient of 40% and 80% of Percoll-saline (330 mOsm l−1). After washing with modified Biggers, Whitten and Whittingham (BWW) solution (330 mOsm l−1), aliquots of the sperm suspension were incubated at 37°C in humidified air with 5% CO2.
Media
The incubation medium was modified BWW medium.18 BWW medium with an osmolarity of 330 mOsm l−1 (similar to that of semen) was named BWW330, and consisted of NaCl 110.00 mmol l−1, KCl 4.80 mmol l−1, CaCl2 1.68 mmol l−1, MgSO4 1.19 mmol l−1, KH2 PO4 1.17 mmol l−1, sodium pyruvate 0.25 mmol l−1, glucose 5.48 mmol l−1, sodium lactate 26.00 mmol l−1, NaHCO3 25.00 mmol l−1, Hepes 20.00 mmol l−1, and was protein free. BWW medium with an osmolarity of 290 mOsm l−1 (similar to that of cervical mucus) was named BWW290, and had the same components as BWW330 except for NaCl, which was adjusted to 85 mmol l−1 from 110 mmol l−1. The BWW solutions were equilibrated in the incubator at 37°C with 5% (v/v) CO2 in the air, and 4 mg ml−1 bovine serum albumin (BSA) was added to the solutions before use. All chemicals used in the study were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chloride channel blockers, DIDS and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) (Sigma-Aldrich, St. Louis, MO, USA), were both prepared as stock solutions of 100 mmol l−1 in dimethylsulfoxide (DMSO) and diluted to a final concentration of 100 μmol l−1 with BWW solutions for the experiments. DMSO controls were included in the experiments. The concentrations of NPPB and DIDS were chosen based on our previous work19,20 and others.6
Measurement of sperm volume by flow cytometry
Changes in sperm cell volume were measured using a flow cytometer (Accuri C6, Becton Dickinson, Franklin Lakes, NJ, USA) as described.21,22 After 30 min incubation in either BWW330 or BWW290 with or without chloride channel blockers, 50 μl aliquots of a sperm suspension (approximately 2 × 106–10 × 106 spermatozoa per ml) were added to 200 μl of the same BSA-free medium containing propidium iodide (PI, 6 μg l−1; Sigma-Aldrich, St. Louis, MO, USA). After gentle agitation to mix the sample, 10 000 cells were analyzed by flow cytometry. PI signals (emission at 605–635 nm) were detected to eliminate signals from nonviable cells (PI positive), and excitation at 488 nm was used to gate out debris for the forward and side scatter window. Forward scatter signals (FSCs), which reflect the relative sperm size, were analyzed after gating for the viable (PI-excluding) spermatozoa. The relative cell size was calculated by dividing the mean forward scatter signal of the treated cells by that of control cells from the same sperm sample. The flow cytometer settings were not changed throughout the study and were calibrated using standard beads before the experiments.
Measurement of sperm motility
Aliquots (5 μl) of the sperm suspensions were taken after 30 min incubation in BWW solutions with or without the chloride channel blockers and viewed on prewarmed (37°C) sperm motility analysis chambers (Makler® Counting Chamber, 10 μm deep; Sefi Medical Instruments, Haifa, Israel) using a negative phase contrast objective (×10). Several fields of view were video recorded over approximately 10 s (with a total magnification of ×100) and analyzed using a CASA system (Sperm Class Analyzer® CASA system, Microptic, Barcelona Spain). At least 200 spermatozoa were tracked for measuring progressive motility (PR), curvilinear velocity (VCL), straight-line velocity (VSL) and average path velocity (VAP). Measurements were made on 50 frames (frame rate, 50 Hz; minimum contrast, 60; minimum size, 3; minimum track points, 25; minimum VAP, 10 μm sec−1).
Immunocytochemical detection of ClC-3, ClC-5 and ClC-7 in spermatozoa
For studies of the localizations of ClC-3, ClC-5 and ClC-7 channels, the sperm suspensions were washed by centrifugation at 200 g for 20 min through a 40%/80% Percoll density gradient. The Percoll-washed sperm suspension was washed again with phosphate-buffered saline (PBS) 3 times, and smeared and air dried on polylysine-coated slides. They were then fixed in 4% paraformaldehyde in PBS (with 0.12 M sucrose) for 30 min at room temperature. They were permeabilized with Triton X-100 (0.5% in PBS) for 5 min and subsequently blocked with 3% BSA in PBS at the room temperature for 45 min. After washing 6 times with PBS (5 min each), the sperm were incubated with primary polyclonal antibodies against ClC-3, ClC-5 and ClC-7 (1:100; Abcam, Cambridge, USA) and incubated with PBS alone as negative controls at 4°C overnight. Stock concentrations of antibodies were kept at 100 μg μl−1 and the final concentration was 1 μg μl−1. The sperm was washed 6 times with PBS and incubated with a secondary antibody (1:100, Alexa Fluor 488-conjugated goat-anti-rabbit IgG; Beyotime Institute of Biotechnology, Haimen, China) in the dark for 1 h at room temperature. Unbound antibodies were removed by washing with PBS 3 times for 5 min each, and then counterstained with the nuclear dye 4’,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of Biotechnology) for 5 min at a final concentration of 5 μg ml−1. Finally, the sperm suspensions were washed with PBS 3 times, mounted with Vectashield anti-fade medium (Vector Laboratories, Burlingame, CA, USA) sealed with colorless nail varnish and observed with a Nikon confocal microscope (Nikon C1Si confocal system, Nikon, Tokyo, Japan; magnification ×1000).
Analysis of ClC-3 expression by flow cytometry
An aliquot of semen containing 10 × 106 spermatozoa was layered on 1/1 ml 40%/80% Percoll made up in BWW330 medium, as described.23 Sperm pellets obtained by centrifugation at 400 g for 20 min were washed in 3 ml BWW330, and centrifuged at 200 g again for 5 min before resuspension in 2 ml BWW330. The dispersed spermatozoa were fixed in 1 ml 4% paraformaldehyde for 30 min at room temperature. A sperm pellet was obtained by centrifugation at 2000 g for 10 min and washed twice with PBS. The fixed spermatozoa were permeabilized with 1 ml 0.5% Triton X-100 for 10 min and blocked in 1 ml PBS containing 3% BSA for 1 h. After pelleting and dispersion in 100 μl PBS, the sperm suspension was divided into two aliquots. A primary antibody against ClC-3 (Abcam; final dilution 1:100) was added to one of the aliquots, and the sample was incubated overnight at 4°C and shaken continuously. The other aliquot was left as a negative control. After washing twice with PBS, the two sperm aliquots were incubated in 30 μl Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Beyotime Institute of Biotechnology; final dilution 1:50) for 1 h in the dark. After washing, the spermatozoa were suspended in 250 μl PBS containing 3 μl PI (final concentration 0.6 μg ml−1) to stain the sperm nuclei and analyzed using flow cytometry (excitation 488 nm; Accuri C6). Cell debris and other cells (such as round cells, germ cells, and leukocytes) were gated out using the forward and side scatter windows, and the spermatozoa were gated in by their high PI fluorescence in the head for the analysis of secondary antibody signals. The aliquot stained with the secondary antibody alone was used to set the threshold fluorescence level. Spermatozoa with fluorescence above the threshold were considered positive for primary antibody staining.
Statistical analysis
Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The data shown in the figures and tables were found to be distributed normally. For statistical analysis, two-tailed Student's t-tests were used. For three or more groups, data were analyzed by one-way analysis of variance (ANOVA); P < 0.05 or P < 0.01 was considered statistically significant.
RESULTS
Donor age and semen characteristics
The donor and semen characteristics of the samples from the normozoospermic men were as follows (presented with median and interquartile range from 25% to 75%): donor age, 31.0 (28.0–33.8) years; semen volume, 3.4 (2.4–4.2) ml; sperm concentration, 69.4 (30.9–126.2) million ml−1; and progressive motility, 41.4% (36.4%–48.9%). Those from patients with asthenozoospermia were as follows: donor age, 31.0 (28.0–36.0) years, the age had no difference between the normozoospermic men and the asthenozoospermic patients; semen volume, 3.2 (2.2–3.9) ml; sperm concentration, 57.3 (32.9–129.7) million ml-1; and progressive motility, 19.2% (12.0%–23.6%).
Volume changes of spermatozoa incubated in BWW290 and BWW330 media
After incubating in either BWW330 or BWW290 for 30 min, the volumes of viable sperm from both the normozoospermic and asthenozoospermic semen were recorded by monitoring the FSC signals using flow cytometry. The results showed that the relative volume (FSC signal) of spermatozoa from the normozoospermic men in BWW330 was not significantly different from that in the BWW290 (n = 25, P = 0.758, t-test;Figure 1a); whereas the volume of spermatozoa from asthenozoospermic patients was increased significantly in BWW290, compared with that in the BWW330 (n = 26, P < 0.01, t-test; Figure 1a).
Figure 1.
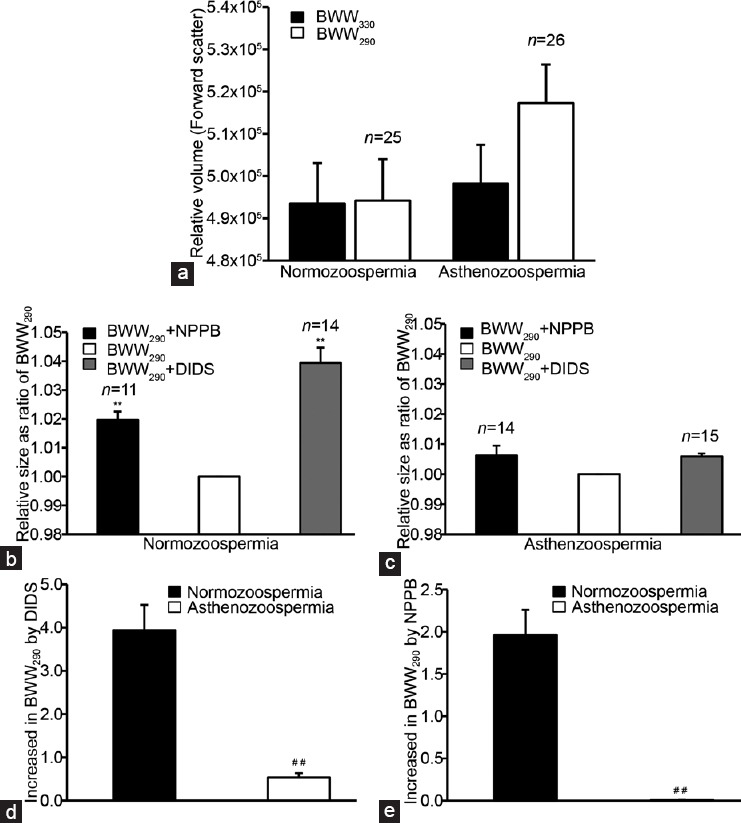
Effects of hypotonic stimulation and the chloride channel blockers NPPB and DIDS on the volumes of spermatozoa from normozoospermic and asthenozoospermic semen samples. Sperm volume was detected by monitoring the forward scatter signals (FSCs) using flow cytometry. (a) Relative sperm volumes expressed as FSC signals of the in the hypotonic (BWW290) and isotonic (BWW330) solutions; (b) and (c) the effects of NPPB and DIDS (both at 100 μmol l−1) on the relative sperm volume (expressed as the FSC signal ratio of blockers/BWW290 control) in the normozoospermic and asthenozoospermic semen samples, respectively; and the percentage sperm volume increases caused by (d) DIDS and (e) NPPB in the hypotonic solution, respectively. **P < 0.01, BWW290 and channel blockers vs BWW330; ##P < 0.01, asthenozoospermia vs normozoospermia. Data are shown as the mean ± s.e. (semen samples, n from 11 to 26). s.e.: standard error; DIDS: 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; NPPB: 5-nitro-2-(3-phenylpropylamino) benzoic acid; BWW: Biggers, Whitten and Whittingham.
Effects of chloride channel blockers on sperm volume
As shown in Figure 1b and 1c, the chloride channel blocker NPPB (100 μmol l−1) increased the relative volume of spermatozoa from the normozoospermic men by about 2% (expressed as the change in the FCS signal ratio of the treatment vs the BWW290 control) in BWW290 (n = 11, P < 0.01, t-test), but did not significantly change the relative size of the spermatozoa from patients with asthenozoospermia in the BWW290 (n = 11, P = 0.196, t-test).
Similar to NPPB, the chloride channel blocker DIDS (100 μmol l−1) increased the relative volume of spermatozoa from the normozoospermic men in BWW290 by about 4% (n = 14, P < 0.01, t-test; Figure 1b and 1c). However, it did not significantly alter the relative size of the spermatozoa from patients with asthenozoospermia in BWW290 (n = 15, P = 0.082, t-test; Figure 1b and 1c).
The efficiencies of DIDS and NPPB, in changing sperm volume are shown in Figure 1d (DIDS) and 1e (NPPB). The change in the sperm volume has been expressed as (FCSblocker − FCS290)/FCS290 × 100%, where FCSblocker is the FCS signal recorded in the presence of channel blockers in BWW290 and FCS290 is the FCS signal obtained in the absence of channel blockers in BWW290. The sperm volume increase induced by the NPPB and DIDS treatments was much higher in spermatozoa from the normozoospermic men than in those from patients with asthenozoospermia (DIDS: P < 0.01 and NPPB: P < 0.01, t-test; Figure 1d and 1e).
The viability of spermatozoa detected by the PI staining assay was not affected by the NPPB and DIDS treatments. In the NPPB treatments, the viability (mean ± standard deviation) in BWW290 was 58.6% ± 12.5% and 47.2% ± 16.6% in spermatozoa from normozoospermic and asthenozoospermic semen samples (n = 14), respectively. In the DIDS treatment groups, the viability was 58.4% ± 10.5% and 50.6% ± 15.8%, respectively (n = 14).
Sperm motility in media with different osmolarities
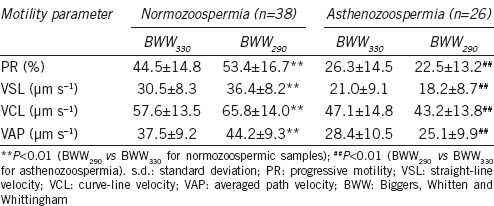
The PR, VCL, VSL, and VAP motility values in BWW290 and BWW330 were analyzed using CASA. All the motility parameters of spermatozoa from the normozoospermic semen samples were increased when incubated in BWW290, compared with those incubated in BWW330 (all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW330; Table 1). However, the motility parameters of the spermatozoa from the asthenozoospermic samples were decreased when exposed to BWW290, compared with those exposed to BWW330 (all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW330; Table 1).
Table 1.
Changes in sperm motility from normozoospermic and asthenozoospermic semen samples in hypotonic (BWW290) and isotonic (BWW330) media (mean±s.d.)
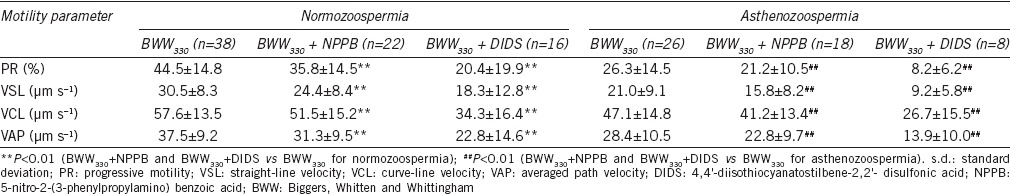
Effects of chloride channel inhibitors on sperm motility in BWW330
Analysis of sperm motility with CASA showed that the chloride channel blocker NPPB (100 μmol l−1) decreased all the motility parameters (PR, VSL, VCL and VAP) in spermatozoa from both the normozoospermic and the asthenozoospermic semen samples in BWW330 (all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW330; Table 2). Under isotonic conditions DIDS also inhibited these motility patterns of spermatozoa from both semen types (all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW330; Table 2).
Table 2.
The inhibitory effects of the chloride channel blockers NPPB and DIDS on the motility of spermatozoa from normozoospermic and the asthenozoospermic semen samples in isotonic solution (BWW330, mean±s.d.)
Effects of chloride channel inhibitors on sperm motility in BWW290
Similar to the effects in BWW330, treatments with NPPB and DIDS also inhibited all four motility patterns of spermatozoa from both semen types in the hypotonic solution BWW290 (NPPB: all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW290; DIDS: all the P < 0.01 for PR, VCL, VSL, and VAP vs the BWW290 for the normozoospermia; P < 0.05 for PR, and P < 0.01 both for VCL, VSL, and VAP vs the BWW290 for the normozoospermia; Table 3). The percentage reductions in PR, VSL, VCL, and VAP, are shown in Figure 2. Further analysis indicated that the inhibitory effects of the chloride channel blockers on sperm motility were stronger in spermatozoa from the normozoospermic samples than in those from the asthenozoospermic samples (Figure 2). The DMSO vehicle control (0.1%, v/v) did not significantly interfere with sperm motility.
Table 3.
The inhibitory effects of the chloride channel blockers NPPB and DIDS on sperm motility from normozoospermic and asthenozoospermic semen samples in hypotonic solution (BWW290, mean±s.d.)
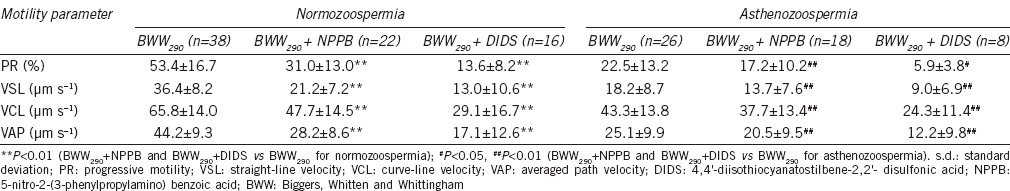
Figure 2.

Inhibitory effects of the chloride channel blockers NPPB and DIDS (both at 100 μmol l−1) on the motility of spermatozoa from the normozoospermic and asthenozoospermic semen samples in hypotonic BWW290. The inhibitory effects of NPPB and DIDS on the (a) progressive motility (PR), (b) straight-line velocity (VSL), (c) curved-line velocity (VCL) and (d) average path velocity (VAP), respectively, when incubated for 30 min. Data are shown as the mean ± s.e. (semen samples, n from 8 to 22). **P < 0.01 asthenozoospermia vs normozoospermia. s.e.: standard error; DIDS: 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid; NPPB: 5-nitro-2-(3-phenylpropylamino) benzoic acid; BWW: Biggers, Whitten and Whittingham.
Localization and expression of ClC-3 channels in spermatozoa from normozoospermic and asthenozoospermic semen samples
As demonstrated by immunocytochemistry, the ClC-3 chloride channel proteins were mainly localized in the neck and mid-piece areas of spermatozoa from the normozoospermic semen samples. However, in those from the asthenozoospermic semen, the ClC-3 immunofluorescence was mainly located in the tail, and the intensity in the neck and mid-piece areas is much weaker than in spermatozoa from the normozoospermic semen (Figure 3a–3d).
Figure 3.

Expression of ClC-3 chloride channels in human spermatozoa. (a) and (b) the distribution of ClC-3 immunofluorescence (green) in spermatozoa from the normozoospermic and asthenozoospermic semen samples, respectively; scale bar = 10 μm. (c) the negative immunostaining control without the primary antibody in normal spermatozoa; the nuclei were stained with DAPI (blue); merge: merged images of the ClC-3 immunostaining and DAPI; scale bar = 10 μm. (d) ClC-3 immunofluorescence intensity in the neck and midpiece of spermatozoa (mean ± s.e., **P < 0.01, asthenozoospermia vs normozoospermia, n = 103 and 126, respectively). (e) ClC-3 expression analyzed by flow cytometry. The Y axes of histograms show cell numbers and the X axes show fluorescence intensity. Black peaks: control spermatozoa (without the primary antibody). Green and red peaks: spermatozoa from the asthenozoospermic (n = 26) and normozoospermic (n = 29) semen samples, respectively. Those spermatozoa with fluorescence intensity greater than the control cells were considered positive for ClC-3 expression. The expression levels of the ClC-3 proteins in spermatozoa from normozoospermic semen were higher than that in those from asthenozoospermic semen. DIC: differential interference contrast images; DAPI: 4’,6-diamidino-2-phenylindole.
Analysis of the ClC-3 protein expression by flow cytometry indicated that the expression levels of the ClC-3 proteins in spermatozoa from normozoospermic semen were higher than that in those from asthenozoospermic semen (Figure 3e). Cells expressing the ClC-3 channels were more prevalent in the normozoospermic semen (91.21% ± 0.72%, n = 29) than in the asthenozoospermic semen (78.14% ±1.48%, n = 26, P < 0.01, t-test, asthenozoospermia vs normozoospermia).
Detection of ClC-5 and ClC-7 in spermatozoa
As shown in Figure 4, ClC-5 and ClC-7 proteins were not detectable by confocal immunofluorescence microscopy in spermatozoa from either of the semen types studied.
Figure 4.
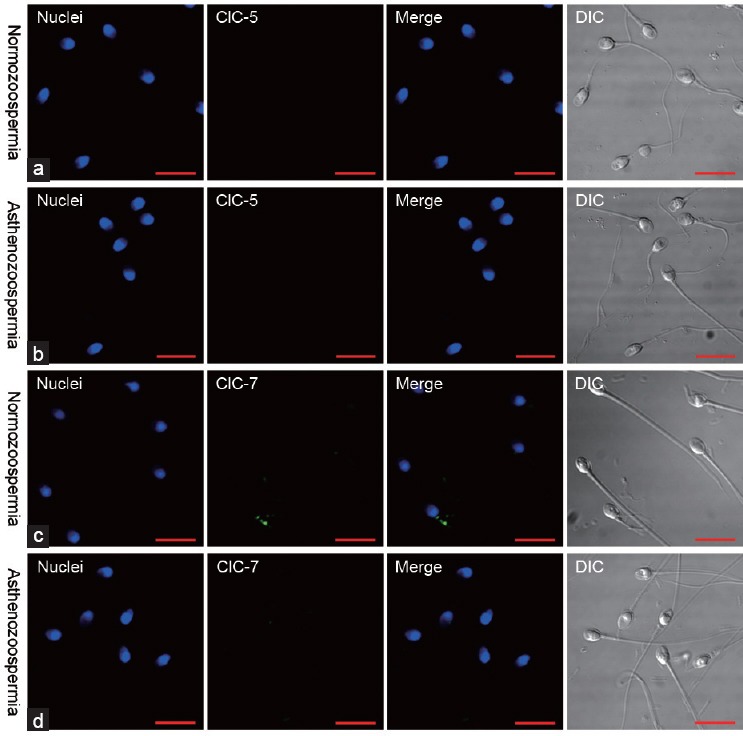
Immunofluorescence of ClC-5 and ClC-7 expression in spermatozoa. ClC-5 was not detectable in spermatozoa from the (a) normozoospermic or (b) asthenozoospermic semen samples. ClC-7 were neither detected in spermatozoa from the (c) normozoospermic nor (d) asthenozoospermic semen samples. Scale bar = 10 μm. DIC: differential interference contrast images.
DISCUSSION
Volume regulation is a fundamental function in spermatozoa, as if disrupted it leads to male infertility. Research has shown that the expression of aquaporins is downregulated, and water fluxes are poor in spermatozoa with low motility.16 The capacity for volume regulation in spermatozoa from infertile men is weaker than that in those from fertile men.17 Thus, there is a close relationship between volume regulation in spermatozoa and male fertility.
There is a drop in osmolarity (from 330 to 290 mOsm l−1), when human spermatozoa pass through the female reproductive tract. Here we found that when incubated in BWW290 at 290 mOsm l−1, mimicking the osmolarity of cervical mucus, spermatozoa from normal semen maintained their volume unchanged, but spermatozoa from the asthenozoospermic samples swelled. This indicates that the volume regulatory mechanism in spermatozoa from asthenozoospermic semen is dysfunctional or disrupted. In contrast to mouse spermatozoa (53–81 μm3), human spermatozoa have a smaller volume (28–34 μm3) and contain less osmotically active fluid.24 Thus, the increase in the human sperm volume and swelling of the flagellum results in more changes than in mouse spermatozoa regarding intracellular ionic strength and the fluid dynamics of axonemal microtubule sliding and flagellar force generation,25 thereby influencing sperm motility. Here, incubation in the hypotonic medium (BWW290) reduced all the detected motility parameters in spermatozoa from asthenozoospermic semen, but the same parameters were increased in those from normozoospermic semen. These results suggest that when the volume regulatory mechanism is impaired in spermatozoa from asthenozoospermic semen it results in even poorer sperm motility.
Chloride channels have been proven to play key roles in volume regulatory mechanisms. Here we investigated the involvement of chloride channels in volume regulation and sperm motility. DIDS and NPPB have been reported to be effective in blocking the volume-sensitive chloride channels in human nasopharyngeal carcinoma CNE-2Z cells.19 The increase in the volume of spermatozoa from the normozoospermic men induced by DIDS and NPPB treatments in the hypotonic medium (BWW290) indicates the involvement of such volume-sensitive chloride channels in sperm volume regulation. However, the two chloride channel blockers could not induce such volume changes in spermatozoa from the asthenozoospermic semen samples in the hypotonic solution, indicating a lower expression of chloride channels. Further investigation demonstrated that DIDS and NPPB reduced the motility parameters in spermatozoa from both types of semen. However, the inhibitory effects were much stronger in spermatozoa from the normozoospermic than in those from the asthenozoospermic samples. These results indicate that chloride channels play important roles in sperm motility, and suggest that the expression or function of the chloride channels is downregulated in spermatozoa from asthenozoospermic samples. Chloride channel blockers could serve as potential targets for male contraception, but this is still far from clinical utilization because of their inhibitory effects on the cell cycle and proliferation.26,27
It has been demonstrated that ClC-3,9,28 ClC-529 and ClC-730 are involved in the migration of somatic cells. The ClC proteins are present in bovine epididymal spermatozoa12 and can bind PP1γ2, a key enzyme involved in regulating sperm maturation and motility. The ClC-3 channels are expressed in healthy human spermatozoa and are involved in sperm volume regulation.6 We found here that ClC-3 proteins were expressed and located in the entire tail in spermatozoa from both types of semen. The presence of the ClC-3 channel proteins in the tail implies that they play an important role in sperm motility. ClC-5 and ClC-7 have been proven to be involved in the movement of somatic cells and are detectable in the epididymal epithelium of mice.31 However, in our immunofluorescence experiments, neither the ClC-5 nor the ClC-7 channel could be detected in spermatozoa from both types of semen.
We studied highly motile spermatozoa from both semen types as selected by Percoll density gradient separation, but the ClC-3 chloride channels were differently expressed and located in the two types. ClC-3 immunofluorescence intensity was high especially in the neck and the midpiece regions, the sites of cytoplasmic droplets (CDs), in spermatozoa from normozoospermic semen, but was low in the same regions in spermatozoa from asthenozoospermic semen. Unlike the excess residual cytoplasm (ERC), an abnormal structure typically observed in spermatozoa from infertile men by clinicians, CDs are considered as a normal component of spermatozoa ejaculated by physiologically and reproductively normal men,17,32 and have been proven to be osmotically sensitive.33,34 ClC-3 is a candidate molecule for volume-sensitive chloride channels and is involved in volume regulation of many types of somatic cells. The potassium channels involved in volume regulation (e.g., TASK2, mink, and Kv1.5) are also found to be expressed in the neck region of human spermatozoa and at the end of the mid-piece of the mouse sperm.35 Evidence supports the notion that regulatory volume decreases are possibly mediated via the membranes of the CDs. Downregulation of the ClC-3 channel in CDs might result in reduced sperm volume-regulating ability and mobility as found in spermatozoa from the asthenozoospermic samples. This work investigated the highly motile spermatozoa from both the semen types but the ClC-3 chloride channels were differently expressed and located in each of them.
There are many causes of poor sperm motility, such as varicoceles, reactive oxygen species, infections, medications, and environmental exposure. Although men with such causes were excluded from this study, it is still difficult to exclude the effects of all the above causes on ClC-3 expression. To study this further, more samples and more detailed and careful categorization of sperm factors should be carried out.
CONCLUSIONS
This study demonstrated that the cell volume-regulating capacity and motility were decreased in spermatozoa from patients with asthenozoospermia. The ClC-3 chloride channels were downregulated in these spermatozoa, especially in the neck and midpiece regions. Chloride channels clearly play important roles in volume regulation and sperm motility. Activation of chloride channels in the sperm neck may be a key factor in the regulation of sperm movement, and reduced ClC-3 expression might serve as a measure for the clinical diagnosis of asthenozoospermia. Moreover, it might prove to be a target for the development of male contraceptive drugs.
AUTHOR CONTRIBUTIONS
SWL and YL were involved in the study design, semen sample collection, molecular and sperm motility experiments, data collection and analysis, and prepared the draft and the final manuscript. LLZ, YTG, and PS carried out the volume regulation experiments and performed data analysis. SP, LXZ, SMD, and LYZ were involved in the sperm motility investigations and data analysis. LWW and LXC played critical roles in the study design, data analysis, and manuscript preparation. All the authors have read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81173064, 81272223, 81273539), the Ministry of Education of China (20124401110009), the Natural Science Foundation of Guangdong Province (S2011010001589) and the Science and Technology Programs of Guangdong (2013B051000059), Guangzhou (2013J500015) and Dongguan (2011108102006).
REFERENCES
- 1.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva, Switzerland: World Health Organization Press; 2010. p. 226. [Google Scholar]
- 2.Lishko PV, Kirichok Y, Ren D, Navarro B, Chung JJ, et al. The control of male fertility by spermatozoan ion channels. Annu Rev Physiol. 2012;74:453–75. doi: 10.1146/annurev-physiol-020911-153258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci U S A. 2007;104:9816–21. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CY, Jiang LY, Chen WY, Li K, Sheng HQ, et al. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum Reprod. 2010;25:317–27. doi: 10.1093/humrep/dep406. [DOI] [PubMed] [Google Scholar]
- 5.Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, et al. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol. 2012;590:2659–75. doi: 10.1113/jphysiol.2011.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung CH, Barfield JP, Cooper TG. Chloride channels in physiological volume regulation of human spermatozoa. Biol Reprod. 2005;73:1057–63. doi: 10.1095/biolreprod.105.044123. [DOI] [PubMed] [Google Scholar]
- 7.Puente MA, Tartaglione CM, Ritta MN. Bull sperm acrosome reaction induced by gamma-aminobutyric acid (GABA) is mediated by GABAergic receptors type A. Anim Reprod Sci. 2011;127:31–7. doi: 10.1016/j.anireprosci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Bray C, Son JH, Kumar P, Harris JD, Meizel S. A role for the human sperm glycine receptor/Cl(-) channel in the acrosome reaction initiated by recombinant ZP3. Biol Reprod. 2002;66:91–7. doi: 10.1095/biolreprod66.1.91. [DOI] [PubMed] [Google Scholar]
- 9.Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, et al. Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR. Biol Reprod. 2009;80:115–23. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- 10.Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, et al. Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem. 2008;283:35539–50. doi: 10.1074/jbc.M804586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung CH, Cooper TG. Effects of the ion-channel blocker quinine on human sperm volume, kinematics and mucus penetration, and the involvement of potassium channels. Mol Hum Reprod. 2001;7:819–28. doi: 10.1093/molehr/7.9.819. [DOI] [PubMed] [Google Scholar]
- 12.Myers K, Somanath PR, Berryman M, Vijayaraghavan S. Identification of chloride intracellular channel proteins in spermatozoa. FEBS Lett. 2004;566:136–40. doi: 10.1016/j.febslet.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, et al. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol Reprod. 1996;54:719–27. doi: 10.1095/biolreprod54.3.719. [DOI] [PubMed] [Google Scholar]
- 14.Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Peng H, Lei L, Zhang Y, Kuang H, et al. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21:922–33. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito K, Kageyama Y, Okada Y, Kawakami S, Kihara K, et al. Localization of aquaporin-7 in human testis and ejaculated sperm: possible involvement in maintenance of sperm quality. J Urol. 2004;172:2073–6. doi: 10.1097/01.ju.0000141499.08650.ab. [DOI] [PubMed] [Google Scholar]
- 17.Fetic S, Yeung CH, Sonntag B, Nieschlag E, Cooper TG. Relationship of cytoplasmic droplets to motility, migration in mucus, and volume regulation of human spermatozoa. J Androl. 2006;27:294–301. doi: 10.2164/jandrol.05122. [DOI] [PubMed] [Google Scholar]
- 18.Biggers JD, Whitten WK, Whittingham DG. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian Embryology. San Francisco: Freeman; 1971. pp. 86–116. [Google Scholar]
- 19.Wang L, Ma W, Zhu L, Ye D, Li Y, et al. ClC-3 is a candidate of the channel proteins mediating acid-activated chloride currents in nasopharyngeal carcinoma cells. Am J physiol Cell Physiol. 2012;303:C14–23. doi: 10.1152/ajpcell.00145.2011. [DOI] [PubMed] [Google Scholar]
- 20.Mao J, Wang L, Fan A, Wang J, Xu B, et al. Blockage of volume-activated chloride channels inhibits migration of nasopharyngeal carcinoma cells. Cell Physiol Biochem. 2007;19:249–58. doi: 10.1159/000100644. [DOI] [PubMed] [Google Scholar]
- 21.Yeung CH, Anapolski M, Cooper TG. Measurement of volume changes in mouse spermatozoa using an electronic sizing analyzer and a flow cytometer: validation and application to an infertile mouse model. J Androl. 2002;23:522–8. [PubMed] [Google Scholar]
- 22.Yeung CH, Anapolski M, Depenbusch M, Zitzmann M, Cooper TG. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum Reprod. 2003;18:1029–36. doi: 10.1093/humrep/deg204. [DOI] [PubMed] [Google Scholar]
- 23.Yeung CH, Cooper TG. Potassium channels involved in human sperm volume regulation-quantitative studies at the protein and mRNA levels. Mol Reprod Dev. 2008;75:659–68. doi: 10.1002/mrd.20812. [DOI] [PubMed] [Google Scholar]
- 24.Gao DY, Liu J, Liu C, McGann LE, Watson PF, et al. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 1995;10:1109–22. doi: 10.1093/oxfordjournals.humrep.a136103. [DOI] [PubMed] [Google Scholar]
- 25.Yeung CH, Barfield JP, Cooper TG. The role of anion channels and Ca 2+ in addition to K+ channels in the physiological volume regulation of murine spermatozoa. Mol Reprod Dev. 2005;71:368–79. doi: 10.1002/mrd.20261. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, Yang H, Zuo W, Yang L, Zhang H, et al. Differential expression and roles of volume-activated chloride channels in control of growth of normal and cancerous nasopharyngeal epithelial cells. Biochem Pharmacol. 2012;83:324–34. doi: 10.1016/j.bcp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zhu L, Jacob TJ, Wang L. Roles of volume-activated Cl- currents and regulatory volume decrease in the cell cycle and proliferation in nasopharyngeal carcinoma cells. Cell Prolif. 2007;40:253–67. doi: 10.1111/j.1365-2184.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganapathi SB, Wei SG, Zaremba A, Lamb FS, Shears SB. Functional regulation of ClC-3 in the migration of vascular smooth muscle cells. Hypertension. 2013;61:174–9. doi: 10.1161/HYPERTENSIONAHA.112.194209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen ML, Schade S, Lyons SA, Amaral MD, Sontheimer H. Expression of voltage-gated chloride channels in human glioma cells. J Neurosci. 2003;23:5572–82. doi: 10.1523/JNEUROSCI.23-13-05572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao L, Zhang XD, Liu X, Chen TY, Zhao M. Chloride channels and transporters in human corneal epithelium. Exp Eye Res. 2010;90:771–9. doi: 10.1016/j.exer.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isnard-Bagnis C, Da Silva N, Beaulieu V, Yu AS, Brown D, et al. Detection of ClC-3 and ClC-5 in epididymal epithelium: immunofluorescence and RT-PCR after LCM. Am J physiol Cell Physiol. 2003;284:C220–32. doi: 10.1152/ajpcell.00374.2001. [DOI] [PubMed] [Google Scholar]
- 32.Abraham-Peskir JV, Chantler E, Uggerhoj E, Fedder J. Response of midpiece vesicles on human sperm to osmotic stress. Hum reprod. 2002;17:375–82. doi: 10.1093/humrep/17.2.375. [DOI] [PubMed] [Google Scholar]
- 33.Chantler E, Abraham-Peskir JV. Significance of midpiece vesicles and functional integrity of the membranes of human spermatozoa after osmotic stress. Andrologia. 2004;36:87–93. doi: 10.1111/j.1439-0272.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 34.Cooper TG, Yeung CH, Fetic S, Sobhani A, Nieschlag E. Cytoplasmic droplets are normal structures of human sperm but are not well preserved by routine procedures for assessing sperm morphology. Hum Reprod. 2004;19:2283–8. doi: 10.1093/humrep/deh410. [DOI] [PubMed] [Google Scholar]
- 35.Cooper TG, Yeung CH. Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des. 2007;13:3222–30. doi: 10.2174/138161207782341240. [DOI] [PubMed] [Google Scholar]


