Abstract
Preoperative and postoperative sperm parameter values from infertile men with varicocele were analyzed by computer-aided sperm analysis (CASA) to assess if sperm characteristics improved after varicocelectomy. Semen samples of men with proven fertility (n = 38) and men with varicocele-related infertility (n = 61) were also analyzed. Conventional semen analysis was performed according to WHO (2010) criteria and a CASA system was employed to assess kinetic parameters and sperm concentration. Seminal parameters values in the fertile group were very far above from those of the patients, either before or after surgery. No significant improvement in the percentage normal sperm morphology (P = 0.10), sperm concentration (P = 0.52), total sperm count (P = 0.76), subjective motility (%) (P = 0.97) nor kinematics (P = 0.30) was observed after varicocelectomy when all groups were compared. Neither was significant improvement found in percentage normal sperm morphology (P = 0.91), sperm concentration (P = 0.10), total sperm count (P = 0.89) or percentage motility (P = 0.77) after varicocelectomy in paired comparisons of preoperative and postoperative data. Analysis of paired samples revealed that the total sperm count (P = 0.01) and most sperm kinetic parameters: curvilinear velocity (P = 0.002), straight-line velocity (P = 0.0004), average path velocity (P = 0.0005), linearity (P = 0.02), and wobble (P = 0.006) improved after surgery. CASA offers the potential for accurate quantitative assessment of each patient's response to varicocelectomy.
Keywords: computer-assisted semen analysis, male infertility, semen analysis, varicocele, varicocelectomy
INTRODUCTION
Varicocele is the most frequent cause of male infertility. This condition can be detected in 19%–41% of patients with primary infertility and 45%–81% of those with secondary infertility.1,2 The impact of varicocele repair on fertility is still one of the more controversial issues, being a subject of debate at every Andrology scientific meeting. The usual sperm parameters used to evaluate male fertility, such as sperm count, motility, and morphology, provide controversial conclusions from different authors or are not sufficiently accurate to define clinical response to surgical treatment.3
On the other hand, a new tool has been introduced in the Andrology laboratory, the CASA system, which allows an objective assessment of sperm kinetics, which in our opinion is underused owing to a lack of documented work to support its clinical value.4 Our aim was to prove the usefulness of this tool in varicocelectomized patients.
MATERIALS AND METHODS
Fertile donors and patients
The fertile group was composed of 38 men with proven fertility (20-41-year-old) by their partner's giving birth in the last 12 months. Men with partner’ s with a history of habitual abortions or a time between stopping contraception and establishing a pregnancy that exceeded 12 months were excluded, as were donors with chronic diseases or andrological disorders, and those who in the last 15 days had suffered fever or received antibiotic treatment. The participants had to undergo an andrological examination.
Sixty-one semen samples from 30 adult men with varicocele were evaluated. All of them were infertile, seeking surgical treatment in our institution (age range: 17–41 years), systemic pathologies were not referred to any case of the recruited patients, all of them having normal hormonal profiles. A paired test could be applied in 10 cases where we had samples assayed before and after treatment (6–9 months after surgery).
The diagnosis of varicocele was made by clinical diagnostics confirmed with color Doppler ultrasound, which identified it as anechoic tubular structures that dilated on the Valsalva maneuver. The presence, grade, and side of varicocele were recorded. Grade I (GI: small) varicoceles were palpable only with the Valsalva maneuver, Grade II (GII: medium) were palpable on examination in a standing position, and Grade III (GII: large) were visible and palpable when the patient was standing. Twenty-one being left-sided varicocele (3 GIII, 10 GII and 8 GI) and 9 bilateral (2 left GIII and right GII; 3 left GII and right GII and 4 left GII and right GI).
All of them underwent sub-inguinal microsurgical varicocelectomy with the Marmar technique.5
The Institutional Review Board of The Clinical Hospital “José de San Martin" approved the study; all the participants received informCtion on the project and gave written informed consent to be included.
Sperm assessment
Conventional semen assays were performed according WHO criteria - 2010.6 Subjective motility was evaluated by expert technicians. The sperm motility was classified as Progressive motility (PR), nonprogressive motility (NP), and immotility (IM).
A Sperm Class Analyzer®(SCA) CASA system (SCA Microptic SL, Barcelona, Spain) was employed to assess kinetic parameters and sperm count. The basic components of the system are: a bright field microscope with phase contrast Ph- (negative phase contrast) microscopy to visualize the sample (Nikon E-200, Japan), a digital camera to capture images, Basler A312 (Basler Inc., Vision-Technology, Ahrensburg, Germany) and a computer with SCA® software (SCA Microptic SL, Barcelona, Spain) installed. Slides with samples were laid on a thermostatic plate at 37°C. A minimum of 400 sperm cell tracks were captured and 25 digitized images per second were analyzed for each sample. The assays were conducted in accordance with instrument's standardization and validation,7 by using 10 μm in depth Leja slides (Leja Products BV®, Nieuw-Vennep, The Netherlands). A qualified operator validated each analyzed image.
Data from individual motile spermatozoa (Sp), defined by eight kinematic parameters (curvilinear velocity [VCL], straight-line velocity [VSL], average path velocity [VAP], linearity [LIN], straightness [STR], mean amplitude of lateral head displacement [ALH], wobble [WOB], a measure of oscillation of the actual path about the average path, and beat cross frequency [BCF]), were assessed and ratios were then calculated relating the different velocities (LIN = VSL/VCL; STR = VSL/VAP, WOB = VAP/VCL). The criteria for detecting hyperactivated spermatozoa was VCL >35 μm s−1, ALH >2.5 μm, STR >85%. This CASA criterion for hyperactivation was established by the SCA manufacturer and corresponds to the “transitional activated pattern.”8
The improved Neubauer hemocytometer was used only in cases of the very low count. Appropriate dilutions were made in Mac Comber fixative (formaldehyde: 1 mg ml−1, NaHCO3: 5 g made up to 100 ml with purified water).
For sperm morphology, once the semen smears had been air-dried, they were fixed in ethanol (96%, v/v) and Papanicolaou-stained.
Statistical analysis
SPSS 14.0 statistical software for Windows (SPSS Inc., Chicago, IL, USA) was employed. All the data were normally distributed. Independent sample t-tests and paired sample t-tests were used according to the variable under study. While analyzing three groups one-way analysis of variance (ANOVA) (with Bonferroni post-hoc test if P < 0.05) was used. P < 0.05 were considered statistically significant.
RESULTS
Table 1 shows data on seminal parameters in all the groups. One-way ANOVA proved statistically significant differences among them. The Bonferroni post-hoc test revealed differences only in VCL, VSL, VAP and progressive motility when pre- and post-surgery semen assays where compared with values from the reference population. As can be clearly seen the results of seminal parameters in the fertile group were far higher those of the patients, even after surgical treatment.
Table 1.
Descriptive statistics for semen parameters values for men from RP, pre-S and post-S, assessed by conventional semen analysis and CASA-SCA
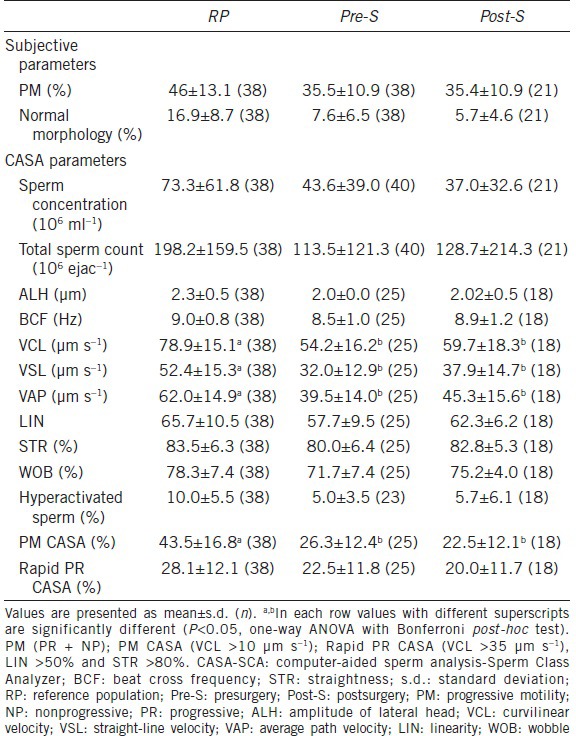
No significant improvement in percentage normal sperm morphology (P = 0.10), concentration (Sp ml−1) (P = 0.52), total count (Sp per ejaction) (P = 0.76) nor subjective motility (% motile) (P = 0.97) was observed after varicocelectomy between the pre- and post-surgery groups.
Table 2 shows data from paired-sample analysis of semen parameters values. No significant improvement was found in percentage normal sperm morphology (P = 0.91), concentration (Sp ml−1) (P = 0.99), total count (Sp per ejaction) (P = 0.89) nor motility (% motile) (P = 0.77) after varicocelectomy by a paired comparison of preoperative and postoperative data. A logarithmic transformation was applied to the total sperm/ejaculate to highlight the difference in this parameter that, although Gaussian but near the limit, having the median: 72, 95% CI 40.1–112.7 for the presurgery group and median: 74, 95% CI 32.7–132.5 for the postsurgery group (P < 0.01). Most sperm kinetic parameters: VCL, VSL, VAP, LIN and WOB improved after varicocele ligation, these differences were statistically significant, although no differences were found between pre- and post-surgery samples of ALH, BCF or hyperactivity.
Table 2.
Analysis of paired-sample semen parameters for 10 men pre-S and post-S assessed by conventional semen analysis and CASA-SCA
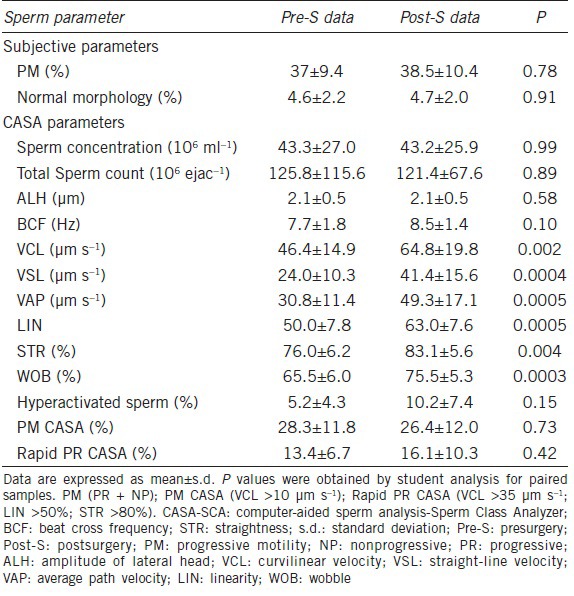
Figure 1 shows the response of each patient's sperm response to treatment. Most men displayed improved the sperm kinematic parameters. We could not identify a particular characteristic in those that did not change their profile.
Figure 1.
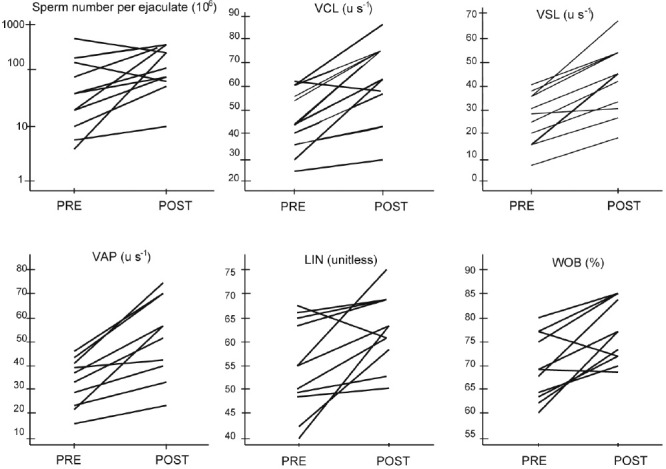
Kinetic parameters before and improved and after surgery in paired samples. Presurgery (PRE), postsurgery (POST).
DISCUSSION
The main goal of the Clinical Laboratory is to offer physicians suitable tools for either diagnosis or prognosis of their patients. Unfortunately, there are still very few medical or surgical options for infertile men besides assisted reproduction. Infections and varicocele are among them, which is why special efforts should be made to evaluate these clinical features accurately. Indeed varicocelectomy for male subfertility is proven effective in men with clinical varicocele and impaired semen quality, thus surgical repair is offered as the first-line treatment.9 This condition can be associated with a spontaneous pregnancy rate of 30%.10 Beyond that, varicocele repair must be proposed in young adult men with impairment of seminal parameters but not yet interested in initiating a pregnancy.
Many attempts have been performed in Andrology to evaluate the patients’ response to varicocelectomy, mainly through assessment of cytological parameters such as sperm count, motility and morphology. Various prognostic factors have been noted in several studies but without a consistent consensus.11,12 As Sponsors of the External Quality Control for the Study of Human Semen in Argentina (Program for External Quality Assessment [EQA-PEEC] of Argentina Biochemistry Foundation) we consider that although a much has been done to unify observational criteria, the subjectivity of sperm parameter assessment makes difficult the expected results, especially in the evaluation of sperm morphology.13,14
In our hands, there was no significant improvement in the overall sperm parameter values studied in the varicocelectomized patients. Our results are partially in accord with those reported by Cakiroglu et al.15 who could only find differences in sperm motility, and completely discrepant with the results reported by Abdel-Meguid et al.2 who showed improvement in sperm count, motility, and morphology.
Computer-assisted sperm analysis (CASA) systems have evolved over approximately 40 years, through advances in devices to capture the image from a microscope, huge increases in computational power concurrent with a reduction in the size of computers, new computer languages, and updated/expanded software algorithms. It allows assessment of the motility of individual spermatozoa, generating huge data sets. When carefully validated, current CASA systems provide important information for quality control and for the understanding of the diversity of sperm responses to changes in their microenvironment. The challenge is now focused on finding consistent biological meanings from this information.
Apparently, CASA results and results from the conventional method are not comparable for sperm concentration and motility analysis. They overestimate sperm concentration and the proportion of rapidly progressive spermatozoa and, consequently, underestimate the percentages of slowly progressive and nonprogressive spermatozoa, compared with the conventional method.16,17 Beyond that, in our laboratory, were able to standardize and validate a CASA system, SCA (Sperm Class Analyzer) for the parameters of sperm concentration and motility according to international standards, establishing that the proposed method meets the requirements for its use in the clinic.6
Data on sperm hyperactivation should especially be discussed as, up to this moment, it is the only kinetic parameter of clear clinical value. In our experience, it predicts a higher recovery rate after a sperm enrichment method such as “swim-up,” being a good tool for basing decisions on a low complexity assisted reproduction technique such as intrauterine insemination. The high standard deviation of this parameter in our study may be interpreted as different interpersonal responses to the intervention. Further studies should be carried out to clarify which, if any, of the kinetic parameters, is critical for male fertility.18,19
Despite the published data by Parikh et al.20 we could not find differences in the overall sperm kinetics after varicocelectomy by using CASA. Neither did Chen et al.21 while comparing the same parameters between varicocelic semen samples with those from a control group, although they reported higher apoptotic index in the former.
Baker et al.22 2013 found that an increase in sperm motility was the only variable associated with postoperative pregnancy regardless of the method by which pregnancy was obtained.22 Surprisingly, when we performed the analysis of paired samples, when both measurements were made on the same subject, and thus the between-subject variability is eliminated from the comparison, the results were different, showing a significant response to treatment. This is the main concept we intend to transmit in this paper. An infertile patient will do everything to achieve fertility, and thus, in the medical office will discuss with the physician all available state-of-the-art science to help him in this goal.
Data on population statistics may be negligible, but the final decision will come from his individual characteristics, and CASA system can provide the real data on varicocelectomy response suggestive of a promising outcome. The clinical value of these kinetic results needs to be proven in clinical practice.
AUTHOR CONTRIBUTIONS
JIA and GRM conceived and designed the study. JIA performed the statistical analysis. MC conducted assessments of patients and varicocelectomies practice. MJF and HER analyzed semen samples using the CASA system. MS evaluated sperm morphology. PC, SMC, and MNP reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research was supported by a clinical Grant from University of Buenos Aires Science and Technology (UBACYT – CB06).
REFERENCES
- 1.Masson P, Brannigan RE. The varicocele. Urol Clin North Am. 2014;41:129–44. doi: 10.1016/j.ucl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol. 2011;59:455–61. doi: 10.1016/j.eururo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Samplaski MK, Yu C, Kattan MW, Lo KC, Grober ED, et al. Nomograms for predicting changes in semen parameters in infertile men after varicocele repair. Fertil Steril. 2014;102:68–74. doi: 10.1016/j.fertnstert.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Samplaski MK, Agarwal A, Sharma R, Sabanegh E. New generation of diagnostic tests for infertility: review of specialized semen tests. Int J Urol. 2010;17:839–47. doi: 10.1111/j.1442-2042.2010.02619.x. [DOI] [PubMed] [Google Scholar]
- 5.Marmar JL, DeBenedictis TJ, Praiss D. The management of varicoceles by microdissection of the spermatic cord at the external inguinal ring. Fertil Steril. 1985;43:583–8. doi: 10.1016/s0015-0282(16)48501-8. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen. 5th ed. Geneva: WHO; 2010. [Google Scholar]
- 7.Chenlo PH, Ariagno JI, Pugliese MN, Repetto H, Sardi LM, et al. Study of human semen: implementation of an objective method. Acta Bioquím Clín Latinoam. 2013;47:61–9. [Google Scholar]
- 8.Suarez S. Hyperactivated motility in sperm. J Androl. 1996;17:331–5. [PubMed] [Google Scholar]
- 9.Ficarra V, Crestani A, Novara G, Mirone V. Varicocele repair for infertility: what is the evidence? Curr Opin Urol. 2012;22:489–94. doi: 10.1097/MOU.0b013e328358e115. [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Lee JY, Kang DH, Lee H, Seo JT, et al. Impact of surgical varicocele repair on pregnancy rate in subfertile men with clinical varicocele and impaired semen quality: a meta-analysis of randomized clinical trials. Korean J Urol. 2013;54:703–9. doi: 10.4111/kju.2013.54.10.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi WS, Kim SW. Current issues in varicocele management: a review. World J Mens Health. 2013;31:12–20. doi: 10.5534/wjmh.2013.31.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Curi S, Ariagno J, Chenlo P, Pugliese N, Sardi M, et al. External quality control sperm analysis. Acta Bioquím Clín Latinoam. 2008;42:183–7. [Google Scholar]
- 14.Ariagno JI, Curi SM, Chenlo P, Repetto HE, Pugliese MN, et al. Our experience in sperm morphology assessment. Asian J Androl. 2011;13:201–2. doi: 10.1038/aja.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cakiroglu B, Sinanoglu O, Gozukucuk R. The effect of varicocelectomy on sperm parameters in subfertile men with clinical varicoceles who have asthenozoospermia or teratozoospermia with normal sperm density. ISRN Urol. 2013;21:698351. doi: 10.1155/2013/698351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curi S, Ariagno J, Repetto H, Chenlo P, Mendeluk G, et al. Laboratory methods for the diagnosis of asthenozoospermia. Arch Androl. 2002;48:177–80. doi: 10.1080/01485010252869252. [DOI] [PubMed] [Google Scholar]
- 17.Vested A, Ramlau-Hansen CH, Bonde JP, Thulstrup AM, Kristensen SL, et al. A comparison of conventional and computer-assisted semen analysis (CRISMAS software) using samples from 166 young Danish men. Asian J Androl. 2011;13:453–8. doi: 10.1038/aja.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay VS, Robertson L. Hyperactivated motility of human spermatozoa a review of physiological function and application in assisted reproduction. Hum Reprod Update. 1998;4:776–86. doi: 10.1093/humupd/4.6.776. [DOI] [PubMed] [Google Scholar]
- 19.Mendeluk GR, Chenlo PH, Sardi-Segovia M, Curi S, Ariagno J, et al. A usefulness of pentoxifylline to improve semen quality. Fertil Steril. 2010;94:e28. doi: 10.1016/j.fertnstert.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Parikh FR, Kamat SA, Kodwaney GG, Balaiah D. Computer-assisted semen analysis parameters in men with varicocele: is surgery helpful? Fertil Steril. 1996;66:440–5. doi: 10.1016/s0015-0282(16)58516-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Lee SS, Chen DC, Chien HH, Chen IC, et al. Apoptosis and kinematics of ejaculated spermatozoa in patients with varicocele. J Androl. 2004;25:348–53. doi: 10.1002/j.1939-4640.2004.tb02799.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker K, McGill J, Sharma R, Agarwal A, Sabanegh E. Pregnancy after varicocelectomy: impact of postoperative motility and DFI. Urology. 2013;81:760–6. doi: 10.1016/j.urology.2012.12.005. [DOI] [PubMed] [Google Scholar]


