Abstract
Prostate cancer is the second most common male cancer, with half of all patients going on to develop metastases. To better identify patients at high risk for prostate cancer progression and reduce prostate cancer-related mortality, improved prognostic factors are required. In this study, we used immunohistochemistry (IHC) to determine the prognostic values of multiple tissue biomarkers in hormone-naοve prostatectomy specimens of prostate cancer. Using 510 prostatectomy specimens collected between 2002 and 2012, IHC analysis was performed for Cerb-2, Cyclin D1, VEGF, EGFR, Rb, PSCA, p53, Bcl-2, Cox-2, PMS2, and Ki-67 on formalin-fixed paraffin-embedded sections. The Cox proportional hazard model was used to determine the predictive risk factors for biochemical recurrence (BCR) of prostate cancer. During a median 44-month follow-up, 128 (25.1%) patients developed BCR. A multivariate regression analysis revealed that Ki-67 (hazard ratio [HR]: 1.60, P = 0.033), PSCA (HR: 0.42, P < 0.001), and Cox-2 (HR: 2.05, P = 0.003) were the only significant prognostic tissue markers of BCR. Resection margin status (HR: 1.67, P = 0.010), pathologic pT0/1/2 stage (vs pT3/4; HR: 0.20, P = 0.002), preoperative PSA levels (HR: 1.03, P < 0.001), biopsied (HR: 1.30, P = 0.022) and pathologic (HR: 1.42, P = 0.005) Gleason scores, and prostate size (HR: 0.97, P = 0.003) were significant clinicopathologic factors. The expression of Ki-67, PSCA, and Cox-2 biomarkers along with other clinicopathologic factors were prognostic factors for BCR in patients with clinically localized prostate cancer following radical prostatectomy.
Keywords: biomarker, immunohistochemistry, prostatectomy, recurrence, tissue microarray
INTRODUCTION
Worldwide, prostate cancer (PCa) is the second most common male malignancy with 1 111 700 new cases in 2012.1 Nearly 50% of patients with localized PCa will develop subsequent metastasis and up to 30% of patients undergoing radical prostatectomy (RP) for clinically localized PC will experience disease progression. To better identify patients at high risk for PCa progression and reduce PC-related mortality, improved prognostic factors are required. The challenge is to identify independent diagnostic markers that are prognostic for the early progressive and life-threatening disease state and distinguish them from those markers prognostic for a less severe form of PCa.
Identification of reliable diagnostic and prognostic markers for PCa would aid clinicians in planning appropriate treatment regimens and in increasing treatment efficacy to prevent cancer-related deaths. Often, a rise in prostate-specific antigen (PSA) levels in PC patients after treatment (biochemical recurrence [BCR]) can represent otherwise undetectable micro-metastatic disease.2 An increase in PSA levels within 2 years of RP indicates a 90% risk of developing metastatic disease.3 Several studies have defined useful prognostic clinicopathologic parameters for clinically localized tumors in men who underwent RP including preoperative serum PSA levels, clinical and pathological staging, and biopsied and pathological Gleason scores.4,5 However, these parameters are uninformative for patients with less severe disease and for the understanding of heterogeneous PCa.
Immunohistochemistry (IHC) is a useful tool for understanding the pathogenesis of disease, differentiating disease characteristics, and determining the tissue of origin of cancers.6 In view of this, many studies have used IHC to identify proteins that are differentially expressed in PCa, resulting in the discovery of conventional tissue biomarkers and multiple promising prognostic tissue biomarkers for PCa. This study aimed at defining the prognostic potential of 11 tissue-specific biomarkers for BCR using IHC in prostatectomy tissue microarrays (TMAs) from a cohort of nonhormone-treated PCa patients who underwent RP.
MATERIALS AND METHODS
Patients and tissue samples
Five-hundred ten consecutive RP samples were prospectively collected at the Center for Prostate Cancer, National Cancer Center, Goyang, Korea, between 2005 and 2012. The samples were retrospectively analyzed after excluding those from patients with missing information including follow-up loss, follow-up duration of <1 year, and prior hormonal or invasive prostate therapy. All samples were reviewed pathologically, blindly by a pathologist (WSP) assisted by a urologist (SHK) according to the guidelines of the 2005 International Society of Urological Pathology (ISUP) consensus conference.7 The enrolled patients’ medical records were obtained from a PCa registry database. Disease progression was defined as BCR after RP; BCR was defined as a postoperative serum PSA elevation of >0.2 ng ml−1 assessed on two different occasions following a decrease to undetectable levels.8 The first measured PSA value of 0.2 ng ml−1 or greater was used to define the time of recurrence. The Institutional Review Board of the Research Institute and Hospital National Cancer Center (IRB No. NCCNCS 05–049) approved this study and written consent was obtained from all study participants before RP.
Immunohistochemistry and assessment of tissue microarray
Thirty-seven TMA blocks were built using representative tumor areas and paired normal control tissue from formalin-fixed paraffin-embedded tumor material.9 Duplicate cores with 2.0 mm in diameter taken from the tumor block were arrayed in recipient blocks to form the prostate cancer TMA. If the tumor showed different Gleason grades, two cores were sampled from different sites. Cores from nontumorous tissue were also arrayed for control analysis. Briefly, suitable areas for tissue retrieval were marked on standard hematoxylin/eosin (H & E)-stained sections, punched out of the paraffin block, and inserted into a recipient block. After H & E staining, all tissues were reviewed to confirm the inclusion of suitable tumor and normal tissues and to ensure consistency in morphological assessment.
Eleven markers such as Cerb-2, Cyclin D1, vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), retinoblastoma (Rb), prostate stem cell antigen (PSCA), Trp53 (p53), B-cell lymphoma 2 (Bcl-2), cyclooxygenase-2 (Cox-2), postmeiotic segregation increased 2 (PMS2), and Ki-67 were used in the IHC staining of TMA blocks using a standard protocol and the Ventana automatic immunostainer (Ventana, Benchmark, AZ, USA). After deparaffinization, heat-induced antigen retrieval was performed using marker-specific solutions. Reactivity was detected using the UltraView detection kit (Ventana, Tucson, AZ, USA). The immunostaining results were analyzed semi-qualitatively.
The percentage of cells stained and the intensity of that staining were assessed within the nuclei and the cytoplasm of malignant cells and compared to that of the paired benign cells. The expression score, defined as the staining intensity multiplied by the percent area positive for tumor, was determined by a single pathologist (WSP) blinded to the clinical outcome. The pathologist used a semi-quantitative composite scoring system as follows:10 (1) the staining intensity was defined as 0 for negative, 1+ for weak, 2+ for moderate, and 3+ for strong and (2) the area positive for tumor was defined as the (10×) fraction of stained tumor cells in the entire tumor. The highest possible expression score was 30.
Statistical analysis
The immunostaining results were statistically analyzed semi-qualitatively for all 13 tumor markers by three statisticians (SIY, BRP, and JJ). To examine the predictive risk factors for BCR of PC, first, the Cox proportional hazard model using a backward variable selection method with an elimination criterion of 0.05 was applied to identify significant predictors of BCR among the 11 previously known clinicopathological variables, which were age, resection margin status, lymphovascular invasion, perineural invasion, seminal vesicle invasion, high-grade intraepithelial neoplasm (HGPIN), pathologic T stage, preoperative PSA levels, prostate size, preoperative biopsied Gleason score (GS), and postoperative pathologic GS. After adjusting for the significant clinicopathological variables, biomarkers with predictive significance among the 11 tissue markers were identified using a backward variable selection method with an elimination criterion of 0.05 in a multivariable Cox proportional hazard model. C-statistics were used to predict the accuracy of the prognostic models encompassing all the significant clinicopathological parameters with each additional significant tissue marker. All results were considered statistically significant when two-sided P < 0.05 using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA) and R software version 3.1.1. (https://cran.r-project.org/bin/windows/base/old/3.1.1/).
RESULTS
During a median 44.0 (12–142) months of follow-up, 128 (25.1%) patients developed BCR with a median duration of 30.5 (12–121) months and with an average PSA value of 0.62 ng ml−1 at the time of recurrence (data not shown). The clinicopathologic characteristics of the patients and the results of IHC staining for each tissue biomarker are summarized in Table 1. To identify independently significant predictive markers of BCR, a multivariate analysis of 11 clinicopathologic characteristics and 11 tissue biomarkers was performed using the Cox proportional hazard regression model (Table 2). This revealed that the resection margin (Hazard ratio [HR]: 1.67, 95% Confidence interval [CI]: 1.13–2.47, P = 0.010), seminal vesicle invasion (HR: 2.25, 95% CI: 1.35–3.76, P = 0.002), pathologic pT0, 1 and 2 stage (vs pT3, 4) (HR: 0.20, 95% CI: 0.07–0.55, P = 0.002), preoperative PSA (HR: 1.03, 95% CI: 1.01–1.04, P < 0.001), preoperative biopsied (HR: 1.30, 95% CI: 1.04–1.62, P = 0.022), and postoperative pathologic GS (HR: 1.42, 95% CI: 1.11–1.82, P = 0.005) were significant clinicopathological characteristics (Table 2). After adjusting those significant clinicopathological variables, Ki-67 (HR: 1.60, 95% CI: 1.04–2.47, P = 0.033), PSCA (HR: 0.42, 95% CI: 0.26–0.65, P < 0.001), and Cox-2 (HR: 2.05, 95% CI: 1.28–3.29, P = 0.003) were left significant among 11 tissue biomarkers (Table 2 and Figure 1).
Table 1.
Summary of clinicopathologic characteristics and immunohistochemical staining (n=510)
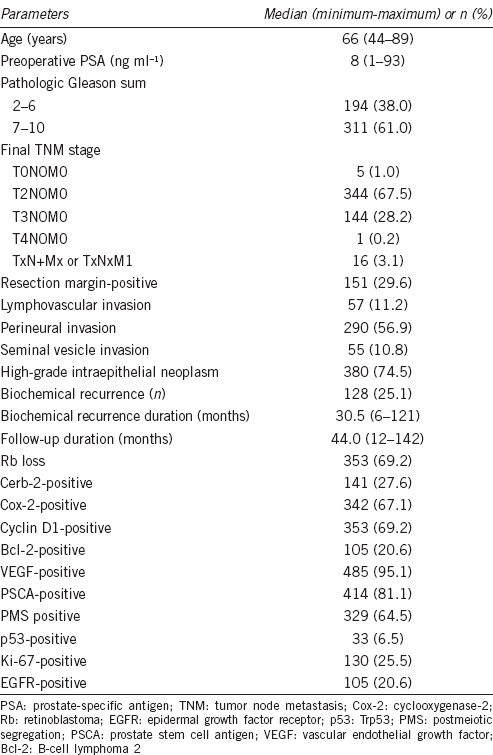
Table 2.
Association of clinicopathologic parameters and the expression of tumor markers with biochemical recurrence-free survival based on cyclooxygenase proportional hazards regression models
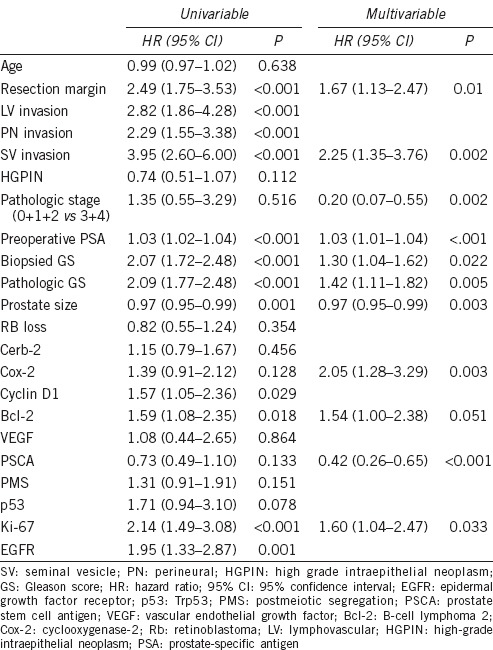
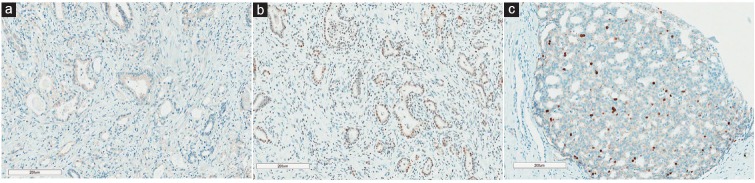
Figure 1.
Immunohistochemical staining images (×100) of Cox-2, PSCA, and Ki-67 markers in prostatectomy specimens. (a) Cox-2. The apical portion of cytoplasm is positive to Cox-2 staining. (b) PSCA. The tumor cells are exhibited immunostaining primarily in the nucleus. (c) Ki-67. The immunoreactivity is noted in the nucleus.
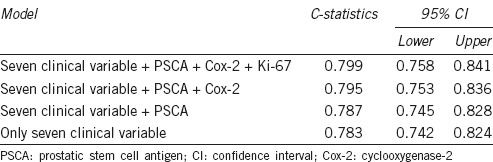
Using C-statistics, a prognostic model incorporating the seven significant clinicopathological parameters was developed and its predictive accuracy for BCR was found to be 0.783 (95% CI: 0.742–0.824) (Table 3). Its predictive accuracy increased stepwise with the addition of each of the three tissue markers and was 0.799 (95% CI: 0.758–0.841) when all three tissue markers were included.
Table 3.
The predictive accuracy of a prognostic model incorporating three tissue markers and all seven significant clinicopathological variables based on C-statistics
DISCUSSION
For several decades, the lack of accurate diagnostic and prognostic biomarkers for PC has provoked researchers to search for new specific markers to accurately predict recurrent and metastatic PCa. Researchers have utilized IHC staining of PCa TMAs to understand the molecular pathways and identify the pathologic and morphologic characteristics of PCa. In addition, IHC analysis has been used to measure prognostic biomarkers of PC to predict clinical behavior and prognosis. This methodology offers substantial practical advantages over methodologies requiring analysis of large cohorts and encompassing wide spectra of tumor presentations and stages for tumor marker analysis.
Similar to previous studies,11,12,13 the current study identified biomarkers Cox-2, Ki-67, and PSCA, along with clinicopathologic parameters including resection margin positivity, pathologic stage, preoperative PSA levels, pathologic Gleason score, and prostate size as independently significant prognostic factors (Table 2) from a relatively homogenous cohort of patients with clinically localized PCa after RP. This observation confirmed the technical utility of using TMAs for determining biomarker expression in archived surgical specimens. Postoperative pathologic evaluation of IHC TMA staining could aid clinicians in deciding which patients require more aggressive follow-up and which patients require immediate BCR treatment.
Ki-67 is a marker of proliferation expressed exclusively in the nuclei of proliferating cells during interphase and is a potential independent prognostic biomarker for PCa after RP.14,15 Ki-67 is expressed in 0%–24% of cells, which is similar to the 25.5% expression detected in PCa cells in this study. Ki-67 expression in prostatectomy samples shown correlation with an adverse prognosis in PC, regardless of the disease stage or treatment,14 provided an accurate estimate of growth capacity, invasiveness, and aggressiveness after RP16 and was useful at identifying patients at high risk for BCR after RP. Finally, Ki-67 expression determined by IHC of TMAs may aid in the decision of whether to administer adjuvant treatment.
Cox-2 or prostaglandin-endoperoxide synthase 2 was significantly associated with BCR in this study (Table 2). Cox-2 is a potent inflammatory mediator in response to inflammatory stimuli and is strongly associated with states of chronic inflammation induced by several inflammatory mediators.17 Cox-2 is also known to mediate production of radical oxygen and nitrogen species, thereby damaging DNA, enhancing cell proliferation, and stimulating angiogenesis.18 This results in hyperproliferation, transformation, tumor growth, invasion, and metastasis, thus potentially increasing the risk for PCa.19 One study reported Cox-2 overexpression not in tumor tissue but rather in proliferative inflammatory atrophy, a putative precursor lesion of PCa with a high correlation with HGPIN.20,21 In addition, a recent review identified E-cadherin, a protein regulated by Cox-2, as a prognostic indicator in PCa with a strong correlation between E-cadherin dysfunction and BCR using TMAs in patients with clinically localized PCa after RP.14
The last significant marker in this study was PSCA, a protein homologous to a group of cell surface proteins involved in the earliest phases of hematopoietic development. PCA expression in PCa ranges from 39.6% to 94%.11,22 Many basic, pathological, and clinical studies have demonstrated a possible role for PSCA in tumorigenesis and progression of PCa through its effects on cell transformation and proliferation based on the expression level of PSCA in benign prostate cells, HGPIN, and PCa. PSCA overexpression was determined to be an adverse predictor for recurrence, clinical progression, and survival and correlated positively with high tumor grade, advanced PCa stage, and androgen-independent progression.11,23 Our previous studies confirmed the role for PSCA as an adverse predictor of BCR with 23.3% of PCa samples expressing the protein.24 Similarly, the current study identified 19.9% of PCa specimens as PSCA-positive using IHC.
Matching these three markers with their corresponding biopsy specimens allowed 123 patients (96.1%) to be identified from among the 128 patients with BCR. Of these, the tumors from 28 patients (21.9%) were positive for all three markers, 65 (50.8%) were positive for two markers, and 30 (23.4%) were positive for a single marker. A predictive model for BCR, incorporating all seven significant clinicopathological parameters, was developed and its accuracy was assessed (Table 3). When the PSCA, Cox-2, and Ki-67 markers were also included in this model, its C-statistic increased from 0.787 to 0.799. A C-statistic of 0 indicates no predictive value whereas C-statistic of 1 indicates 100% predictive accuracy. Thus, incorporating these tissue markers into the model improved its predictive accuracy, indicating that each of them has predictive significance.
As a positive resection margin was observed more frequently in patients with early BCR, a subanalysis was performed to identify predictive risk factors for resection margin positivity among the 11 biomarkers and clinicopathologic factors. This analysis revealed a statistically significant association of Rb (HR: 0.407) and PTEN (HR: 0.285), along with the clinicopathologic factors, lymphovascular invasion (HR: 2.703), tumor volume (HR: 1.034), and HGPIN (HR: 0.477) (data not shown, P < 0.05). In addition, bcl-2 expression might be further evaluated in a study with a longer follow-up period and more patients as this marker almost reached statistical significance as a predictive marker of BCR in multivariate analysis (P = 0.051; Table 2) and there was a significant difference in BCR-free survival between patients with positive and negative bcl-2 tumor expression (P = 0.0183, data not shown). Therefore, these new markers could theoretically aid in the ability to detect altered biomarker expression in tumors with recurrent and progressive disease potential.
This study had several limitations, including its retrospective design and a short follow-up period, which may have prevented the detection of any correlation between tumor marker expression and survival outcome. In addition, the 11 proteins used in this study as potential molecular markers for PCa had been selected based on subjective, rather than scientifically objective criteria, and the expression levels of these proteins were evaluated by IHC staining, which can be subjective in the experimental conditions as well as in the interpretation of outcomes. Therefore, it is possible that other molecules are more closely associated with BCR than those identified in this study.
CONCLUSIONS
The study confirms Ki-67, PSCA, and Cox-2 as being elevated in a significant proportion of PCa and shows their potential importance as prognostic indicators for BCR after RP in patients with clinically localized PCa.
AUTHOR CONTRIBUTIONS
SHK collected the data, performed the statistical analysis, and wrote the manuscript. WSP performed all the pathological reviews. BRP and JJ performed the statistical analysis. JYJ, HKS, JC, and KHL participated in the collection of the RP specimens and helped draft and subsequently review the manuscript.
COMPETING INTERESTS
All the authors had no competing interests in this study.
ACKNOWLEDGMENTS
We would like to thank Ms. Jung Eun KIM and Eun Kyang KIM from the prostate cancer department contributed to the database management. This study was supported by the Korean National Cancer Center Grants (nos. 1510170). This treatise was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20110010731).
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Partin AW, Piantadosi S, Sanda MG, Epstein JI, Marshall FF, et al. Selection of men at high risk for disease recurrence for experimental adjuvant therapy following radical prostatectomy. Urology. 1995;45:831–8. doi: 10.1016/S0090-4295(99)80091-0. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 4.Gleason DF, Mellinger GT Veterans Administration Cooperative Urological Research Group. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging 1974. J Urol. 2002;167(2 Pt 2):953–8. [PubMed] [Google Scholar]
- 5.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 6.Varma M, Jasani B. Diagnostic utility of immunohistochemistry in morphologically difficult prostate cancer: review of current literature. Histopathology. 2005;47:1–16. doi: 10.1111/j.1365-2559.2005.02188.x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 8.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 9.Vogel UF, Bueltmann BD. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am J Clin Pathol. 2006;126:342–8. doi: 10.1309/F2Q38DXN1V1V4GQM. [DOI] [PubMed] [Google Scholar]
- 10.Park WS, Ryu J, Cho KH, Choi MK, Moon SH, et al. Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: use of G1 cycle markers as new prognosticators. Head Neck. 2012;34:1408–17. doi: 10.1002/hed.21939. [DOI] [PubMed] [Google Scholar]
- 11.Taeb J, Asgari M, Abolhasani M, Farajollahi MM, Madjd Z. Expression of prostate stem cell antigen (PSCA) in prostate cancer: a tissue microarray study of Iranian patients. Pathol Res Pract. 2014;210:18–23. doi: 10.1016/j.prp.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Moul JW, Bettencourt MC, Sesterhenn IA, Mostofi FK, McLeod DG, et al. Protein expression of p53, bcl-2, and KI-67 (MIB-1) as prognostic biomarkers in patients with surgically treated, clinically localized prostate cancer. Surgery. 1996;120:159–66. doi: 10.1016/s0039-6060(96)80283-2. [DOI] [PubMed] [Google Scholar]
- 13.Edwards J, Mukherjee R, Munro AF, Wells AC, Almushatat A, et al. HER2 and COX2 expression in human prostate cancer. Eur J Cancer. 2004;40:50–5. doi: 10.1016/j.ejca.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Nariculam J, Freeman A, Bott S, Munson P, Cable N, et al. Utility of tissue microarrays for profiling prognostic biomarkers in clinically localized prostate cancer: the expression of BCL-2, E-cadherin, Ki-67 and p53 as predictors of biochemical failure after radical prostatectomy with nested control for clinical and pathological risk factors. Asian J Androl. 2009;11:109–18. doi: 10.1038/aja.2008.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bubendorf L, Sauter G, Moch H, Schmid HP, Gasser TC, et al. Ki67 labelling index: an independent predictor of progression in prostate cancer treated by radical prostatectomy. J Pathol. 1996;178:437–41. doi: 10.1002/(SICI)1096-9896(199604)178:4<437::AID-PATH484>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Bettencourt MC, Bauer JJ, Sesterhenn IA, Mostofi FK, McLeod DG, et al. Ki-67 expression is a prognostic marker of prostate cancer recurrence after radical prostatectomy. J Urol. 1996;156:1064–8. [PubMed] [Google Scholar]
- 17.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–26, 29. [PubMed] [Google Scholar]
- 18.McArdle PA, Mir K, Almushatat AS, Wallace AM, Underwood MA, et al. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int. 2006;77:127–9. doi: 10.1159/000093905. [DOI] [PubMed] [Google Scholar]
- 19.Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Bergh A, Damber JE. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res. 2005;11:3250–6. doi: 10.1158/1078-0432.CCR-04-2405. [DOI] [PubMed] [Google Scholar]
- 21.Sugie S, Tsukino H, Mukai S, Akioka T, Shibata N, et al. Cyclooxygenase 2 genotypes influence prostate cancer susceptibility in Japanese men. Tumour Biol. 2014;35:2717–21. doi: 10.1007/s13277-013-1358-y. [DOI] [PubMed] [Google Scholar]
- 22.Zhigang Z, Wenlv S. Prostate stem cell antigen (PSCA) expression in human prostate cancer tissues: implications for prostate carcinogenesis and progression of prostate cancer. Jpn J Clin Oncol. 2004;34:414–9. doi: 10.1093/jjco/hyh073. [DOI] [PubMed] [Google Scholar]
- 23.Dubey P, Wu H, Reiter RE, Witte ON. Alternative pathways to prostate carcinoma activate prostate stem cell antigen expression. Cancer Res. 2001;61:3256–61. [PubMed] [Google Scholar]
- 24.Joung JY, Lee YS, Park S, Yoon H, Lee SJ, et al. Haplotype analysis of prostate stem cell antigen and association with prostate cancer risk. J Urol. 2011;185:2112–8. doi: 10.1016/j.juro.2011.01.083. [DOI] [PubMed] [Google Scholar]