Abstract
Prostatic calculi are a common finding on transrectal prostate ultrasound. However, it remains unclear whether they are significantly associated with lower urinary tract symptoms (LUTS). Our objective was to evaluate the association between prostatic calculi and LUTS with a focus on “calculi burden” because no studies have investigated prostatic calculi using “calculi burden” as an indicator. A total of 606 participants who received transrectal prostate ultrasound were divided into two groups according to the presence of prostatic calculi. “Calculi burden” was defined as the sum of the transverse diameters of all visible calculi within the prostate. The International Prostatic Symptom Score (IPSS) and a quality of life (QoL) score were collected. Both groups were compared, and a multivariate analysis was performed to predict moderate/severe LUTS. Linear correlation was evaluated between calculi burden and IPSS in the calculi group. No differences in total IPSS, voiding IPSS, or QoL score were detected between the two groups, but storage IPSS was significantly higher in the calculi group than that of controls. The multivariate analysis showed that the presence of prostatic calculi was not an independent predictor of moderate/severe LUTS. A positive linear correlation was detected between calculi burden and storage IPSS in calculi group (r = 0.148). However, no correlation was found between calculi burden and total IPSS, voiding IPSS, or QoL score. Our results showed that the presence of prostatic calculi was not a significant factor predicting moderate/severe LUTS. However, an increased calculi burden may be associated with aggravating storage symptoms.
Keywords: calculi, lower urinary tract symptoms, prostate, prostatitis
INTRODUCTION
Prostatic calculi are common findings on transrectal prostate ultrasound (TRUS) and computed tomography scan. Prostatic calculi had drawn attention among health-care professionals since they were reported in early 20th century.1,2,3 As calculi within the prostate are formed by acute or chronic inflammation,4,5 urologists must determine whether prostatic calculi independently give rise to lower urinary tract symptoms (LUTS). A few studies have shown that large prostatic calculi are an independent factor for moderate to severe LUTS6,7 whereas others reported no association between prostatic calculi and LUTS.8,9 Thus, it remains unclear whether prostatic calculi are significantly associated with LUTS, and studies on this issue are limited.
The sizes and the distributions of prostatic calculi are diverse, but no standardized classification exists for prostatic calculi. This coulC be why prostatic calculi have been classified obscurely, such as “larger/smaller calculi” in previous studies. “Stone burden” is a useful practical indicator and useful for research on other stone disorders, such as urinary lithiasis or gallbladder stones. No studies have investigated prostatic calculi using “calculi burden” as an indicator. Thus, for our study, we defined “calculi burden” as the sum of the transverse diameters (mm) of all visible calculi within the prostate. In this study, we evaluated the association between prostatic calculi and LUTS and focused on calculi burden as an indicator.
MATERIALS AND METHODS
Research ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board (IRB approval No. AJIRB-MED-MDB-15-051).
Data collection and study design
After receiving approval from the Institutional Review Board, we retrospectively reviewed and analyzed the medical records of 606 participants who received TRUS between October 2013 and March 2015. Informed consent was waived by the board. Participants enrolled for this study were new incoming urological outpatients presenting with LUTS and those examined for health check-up at the Health Promotion Center. TRUS was performed only for those who visited the Health Promotion Center when they requested TRUS voluntarily for prostate check-up. No differences in the prostatic calculi characteristics were detected between the urological outpatients and health check-up group in a previous study.10 Outpatients who received TRUS for follow-up were excluded. Of 606 participants, 65 (10.7%) were taking concurrent urological medications, such as alpha-blockers, 5-alpha reductase inhibitors, and/or antimuscarinics. Those who were taking LUTS medications were not excluded because we set concurrent urological medications as a covariate for multivariate analysis so that we could determine if prostatic calculi act as an independent predictor for LUTS under the influence of the medications.
The participants were divided into two groups according to the presence of prostatic calculi. The International Prostatic Symptom Score (IPSS) and a quality of life (QoL) score were collected for each participant. “Storage IPSS” was defined as the sum of items 2, 4, and 7 and “voiding IPSS” was defined as the sum of items 1, 3, 5, and 6. “Mild LUTS” was defined as IPSS ≤7 and “moderate/severe LUTS” was defined as IPSS ≥8.11 Other relevant variables such as age, body mass index, prostate size measured on TRUS, and prostate-specific antigen level were collected.
Measurement of calculi burden
Calculi burden was measured and calculated instantly at the time when prostatic calculi were detected on TRUS. Examples of calculi burden measurements are shown in Figure 1. A single urologist performed all TRUS procedures and measured calculi burden.
Figure 1.

Examples of measuring calculi burden for a typical round-shaped calculus (a) and linear-shaped calculi (b).
Statistical analysis
We used the independent t-test and Fisher's exact test to compare the distributions of clinical variables with prostatic calculi. A binary logistic regression test was used in the univariate and multivariate analyses to determine independent predictors of moderate/severe LUTS. We set moderate/severe LUTS as the endpoint for the multivariate analysis because guidelines generally recommend starting pharmacological treatment for moderate/severe LUTS.12,13 A step-wise regression technique was used to build multivariate models at a significance level of 0.15 for the covariate to remain in the model. Some covariates with no significance in the univariate analysis were included in the model if they were associated with LUTS. We performed a subgroup analysis to determine whether calculi burden would affect LUTS within the prostatic calculi group. The partial correlation test was conducted to evaluate the linear correlation between calculi burden and each item on IPSS (total IPSS, storage IPSS, voiding IPSS, and QoL score) after adjusting for age, body mass index, concurrent urological medications, and prostate size as confounders. All analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA), and P < 0.05 was considered statistically significant.
RESULTS
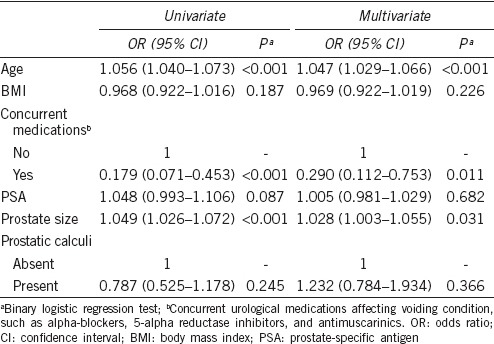
Prostatic calculi of any size were present in 464 (76.6%) of the 606 participants. Age (59.94 vs 52.46 years, P < 0.001), body mass index (24.00 vs 23.16 kg m−2, P = 0.027), prostate size (26.78 vs 24.18 ml, P = 0.001), and storage IPSS (5.52 vs 4.72, P = 0.029) were significantly higher in prostatic calculi group than those in control. No significant differences in total IPSS, voiding IPSS, or QoL scores were observed between the groups (Table 1). A multivariate analysis showed that age (odds ratio [OR]: 1.047, 95% confidence interval [95% CI]: 1.029–1.066; P < 0.001), concurrent urological medications (OR: 0.290, 95% CI: 0.112–0.753; P = 0.011), and prostate size (OR: 1.028, 95% CI: 1.003–1.055; P = 0.031) significantly predicted moderate/severe LUTS. However, the presence of prostatic calculi was not an independent predictor of moderate/severe LUTS (Table 2).
Table 1.
Comparison of clinical variables based on the presence of prostatic calculi
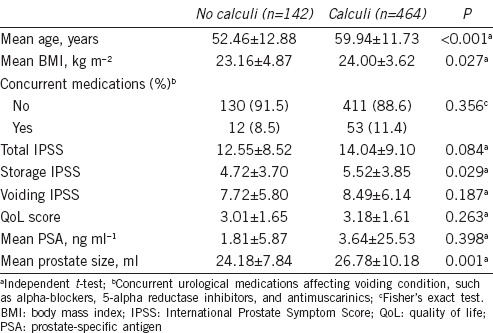
Table 2.
Univariate and multivariate analyses of variables to predict independent factors for moderate/severe lower urinary tract symptoms
The prostatic calculi subgroup analysis revealed a positive linear correlation between calculi burden and storage IPSS (r = 0.148, P = 0.001) (Figure 2). However, no correlations were observed between calculi burden and total IPSS (r = 0.079), voiding IPSS (r = 0.017), or QoL score (r = −0.003).
Figure 2.

The positive linear correlation between calculi burden and the International Prostate Symptom Score for storage symptoms. r*: partial correlation coefficient adjusted for age, body mass index, concurrent urological medications, and prostate size.
DISCUSSION
Our objective was to investigate the impact of prostatic calculi on LUTS with a particular emphasis on calculi burden. The clinical significance of prostatic calculi is a subject of controversy. Our data showed that the presence of calculi in the prostate was not a significant factor affecting moderate/severe LUTS whereas calculi burden was significantly associated with storage symptoms within the prostatic calculi group.
Several studies had evaluated the association between prostatic calculi and LUTS, but they showed contradictory results. Park et al. reported that the presence of prostatic calculi was not a significant factor for severe LUTS even though the calculi group showed a significantly higher IPSS than that of noncalculi group.8 Kim et al. classified prostatic calculi as type A (discrete small reflection), type B (large multi-reflective mass), type M (coexistence of types A and B), and type N (no calculi found). They found no differences in total IPSS, storage IPSS, or voiding IPSS according to calculus type.9 In contrast, Kim et al. classified prostatic calculi as type A (small and discrete calculi) and type B (large and coarse calculi) and revealed that type B was a significant predictor for moderate/severe LUTS.6 Yang et al. classified prostatic calculi as mild calcification (one or multiple small foci without a coarse shadow) and moderate/marked calcification (≥3 hyperechoic foci, ≥3 mm in the largest diameter with coarse shadow) and reported that moderate/marked calcification was an independent risk factor for moderate/severe LUTS.7 These studies are somewhat consistent with our results as they emphasize larger and coarser prostatic calculi as a significant factor for LUTS not the presence of calculi.
Our study showed that more elderly men were present in the calculi group. Studies have found that the development of prostatic calculi is associated with aging. Søndergaard et al. analyzed 300 autopsied prostates and found positive correlation of calculus load and age.14 Percent with calculi ≥1 mm in diameter was 0% (age: 15–39), 50% (age: 40–49), 54% (age: 50–59), 53% (age: 60–69), 63% (age: 70–79), and 73% (age 80 or more). Geramoutsos et al. reported that older patients (>40 years) presented more often with multiple calculi compared to younger patients.15 Although it is still not clear whether prostatic calculi are direct byproduct of age, it is likely that aging is positively associated with the burden of prostatic calculi.
An interesting finding in our study was that calculi burden was positively correlated with storage IPSS. Although the explanation for this finding is unclear, it may be related to inflammation occurring in the prostate. Prostatic calculi are hypothesized to be formed by acute or chronic inflammation,4,5 but they also can obstruct the intraprostatic ducts, which produces further inflammation within the prostate.16,17 One study showed calculi within the prostatic ductuli using an electron microscope.18 Thus, prostatic calculi are highly associated with prostatic inflammation, which seems to be related to storage symptoms. In the REDUCE (REduction by DUtasteride of prostate Cancer Events) trial, chronic prostatic inflammation was associated with higher storage and voiding scores, but the Spearman correlation coefficient was higher for the storage score (rs = 0.056) than the voiding score (rs = 0.046), suggesting that storage symptoms are more relevant to inflammation.19 Geramoutsos et al. evaluated 101 patients with prostatic calculi and reported that the five most common symptoms were pain (18.8%), urgency (12.9%), frequency (10.9%), nocturia (9.9%), and painful ejaculation (2.9%). It is notable that urgency, frequency, and nocturia are identical to storage IPSS whereas pain and painful ejaculation are not IPSS items. Those authors showed that the presence of symptoms and prostatitis were positively correlated with size but not the number or locations of the calculi, which is similar to our results.15 Therefore, it is inferred that prostatic calculi may cause prostatic inflammation and this inflammation is related to storage symptoms.
The association between larger calculi and more severe symptoms is not clear. Histopathology of prostatic calculi reviewed by Klimas et al. showed that larger prostatic calculi cause dilatation of prostatic ducts and acini with loss of their epithelial lining and interacinar fibrosis in addition to a typical inflammatory reaction associated with prostatic calculi in general.20 This may suggest that larger calculi can cause ductal dilatation and subsequent intraprostatic reflux, which may lead to more severe inflammatory reaction and possible more severe symptoms. In an autopsy study by Søndergaard et al., the calculus load was significantly larger in the apical half than in the basal half of the prostate.14 In anatomical perspectives, it is thought that apical prostatic calculi can have an impact on prostatic urethra more likely than basal prostatic calculi. This might be another explanation of the association between larger stones and more severe symptoms.
Our findings may be applicable to LUTS treatment, particularly for patients with prostatic calculi who suffer from storage symptoms refractory to conventional LUTS medications. Antibiotics are an option because several studies have correlated prostatic calculi with bacterial infections.5,21,22,23 Other studies have analyzed the chemical composition of prostatic calculi and uniformly revealed that calcium phosphate stones are the major components of prostatic calculi.4,5,24,25 Thus, another option may be to apply calcium phosphate kidney stone treatments to prostatic calculi, such as potassium citrate or sodium thiosulfate.26,27 Surgical removal of large prostatic calculi is another alternative to reduce calculi burden. Several small series and case reports have been published regarding surgical removal of prostatic calculi.28,29,30 However, well-controlled clinical trials are needed to develop specific prostatic calculi therapy.
Our study had limitations including its retrospective design. However, as calculi burden was measured and calculated immediately at the time of TRUS and prospectively by a single urologist, the possibility of bias may have been reduced. Another limitation is the heterogeneous nature of the participants as we combined urological outpatients with LUTS and those in for health check-up without LUTS, which may have produced selection bias. Particularly, peak urinary flow rate and residual urine volume, two of the most important LUTS parameters, were not included in our study because these tests were performed only for urological outpatients.
CONCLUSIONS
Our results showed that the presence of prostatic calculi was not a significant factor for predicting moderate/severe LUTS whereas prostatic calculi burden significantly correlated with storage symptoms within the prostatic calculi group. Therefore, reducing the calculi burden could be a future target for treating patients with prostatic calculi who suffer from storage symptoms refractory to conventional LUTS medications.
AUTHOR CONTRIBUTIONS
BP performed the data collection and the statistical analysis. BP and SHC drafted the manuscript. BP and SHC designed and conceived of the study.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Thomas BA, Robert JT. Prostatic calculi. J Urol. 1927;18:470–93. [Google Scholar]
- 2.Moore RA. Morphology of prostatic corpora amylacea and calculi. Arch Pathol. 1936;22:22–40. [Google Scholar]
- 3.Huggins C, Bear RS. The course of the prostatic ducts and the anatomy, chemical and x-ray diffraction analysis of prostatic calculi. J Urol. 1944;51:37–47. [Google Scholar]
- 4.Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci U S A. 2009;106:3443–8. doi: 10.1073/pnas.0810473106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessombz A, Meria P, Bazin D, Daudon M. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012;7:e51691. doi: 10.1371/journal.pone.0051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WB, Doo SW, Yang WJ, Song YS. Influence of prostatic calculi on lower urinary tract symptoms in middle-aged men. Urology. 2011;78:447–9. doi: 10.1016/j.urology.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Yang HJ, Huang KH, Wang CW, Chang HC, Yang TK. Prostate calcification worsen lower urinary tract symptoms in middle-aged men. Urology. 2013;81:1320–4. doi: 10.1016/j.urology.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Park SW, Nam JK, Lee SD, Chung MK. Are prostatic calculi independent predictive factors of lower urinary tract symptoms? Asian J Androl. 2010;12:221–6. doi: 10.1038/aja.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Jung KI, Koh JS, Min KO, Cho SY, et al. Lower urinary tract symptoms in benign prostatic hyperplasia patients: orchestrated by chronic prostatic inflammation and prostatic calculi? Urol Int. 2013;90:144–9. doi: 10.1159/000342643. [DOI] [PubMed] [Google Scholar]
- 10.Hong CG, Yoon BI, Choe HS, Ha US, Sohn DW, et al. The prevalence and characteristic differences in prostatic calcification between health promotion center and urology department outpatients. Korean J Urol. 2012;53:330–4. doi: 10.4111/kju.2012.53.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 12.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 13.Gratzke C, Bachmann A, Descazeaud A, Drake MJ, Madersbacher S, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–109. doi: 10.1016/j.eururo.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Søndergaard G, Vetner M, Christensen PO. Prostatic calculi. Acta Pathol Microbiol Immunol Scand A. 1987;95:141–5. doi: 10.1111/j.1699-0463.1987.tb00021_95a.x. [DOI] [PubMed] [Google Scholar]
- 15.Geramoutsos I, Gyftopoulos K, Perimenis P, Thanou V, Liagka D, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004;45:333–7. doi: 10.1016/j.eururo.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Ficarra V. Is chronic prostatic inflammation a new target in the medical therapy of lower urinary tract symptoms (LUTS) due to benign prostate hyperplasia (BPH)? BJU Int. 2013;112:421–2. doi: 10.1111/bju.12177. [DOI] [PubMed] [Google Scholar]
- 17.Ficarra V, Sekulovic S, Zattoni F, Zazzera M, Novara G. Why and how to evaluate chronic prostatic inflammation. Eur Urol Suppl. 2013;12:110–5. [Google Scholar]
- 18.Koseoglu H, Aslan G, Sen BH, Tuna B, Yorukoglu K. Prostatic calculi: silent stones. Actas Urol Esp. 2010;34:555–9. [PubMed] [Google Scholar]
- 19.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, et al. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–84. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klimas R, Bennett B, Gardner WA., Jr Prostatic calculi: a review. Prostate. 1985;7:91–6. doi: 10.1002/pros.2990070110. [DOI] [PubMed] [Google Scholar]
- 21.Finkle AL. The relationship of antecedent genito-urinary infections to the development of prostatic calculi and carcinoma. Bull N Y Acad Med. 1953;29:585–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Meares EM., Jr Infection stones of prostate gland. Laboratory diagnosis and clinical management. Urology. 1974;4:560–6. doi: 10.1016/0090-4295(74)90490-7. [DOI] [PubMed] [Google Scholar]
- 23.Eykyn S, Bultitude MI, Mayo ME, Lloyd-Davies RW. Prostatic calculi as a source of recurrent bacteriuria in the male. Br J Urol. 1974;46:527–32. doi: 10.1111/j.1464-410x.1974.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 24.Sutor DJ, Wooley SE. The crystalline composition of prostatic calculi. Br J Urol. 1974;46:533–5. doi: 10.1111/j.1464-410x.1974.tb03852.x. [DOI] [PubMed] [Google Scholar]
- 25.Torres Ramirez C, Aguilar Ruiz J, Zuluaga Gomez A, Espuela Orgaz R, Del Rio Samper S. A crystallographic study of prostatic calculi. J Urol. 1980;124:840–3. doi: 10.1016/s0022-5347(17)55691-8. [DOI] [PubMed] [Google Scholar]
- 26.Goldfarb DS. A woman with recurrent calcium phosphate kidney stones. Clin J Am Soc Nephrol. 2012;7:1172–8. doi: 10.2215/CJN.00560112. [DOI] [PubMed] [Google Scholar]
- 27.Asplin JR, Donahue SE, Lindeman C, Michalenka A, Strutz KL, et al. Thiosulfate reduces calcium phosphate nephrolithiasis. J Am Soc Nephrol. 2009;20:1246–53. doi: 10.1681/ASN.2008070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal NK, Goel A, Sankhwar S. Transurethral holmium-YAG laser lithotripsy for large symptomatic prostatic calculi: initial experience. Urolithiasis. 2013;41:355–9. doi: 10.1007/s00240-013-0571-x. [DOI] [PubMed] [Google Scholar]
- 29.Bedir S, Kilciler M, Akay O, Erdemir F, Avci A, et al. Endoscopic treatment of multiple prostatic calculi causing urinary retention. Int J Urol. 2005;12:693–5. doi: 10.1111/j.1442-2042.2005.01133.x. [DOI] [PubMed] [Google Scholar]
- 30.Shah SK, Chau MH, Schnepper GD, Lui PD. Open prostatolithotomy for the management of giant prostatic calculi. Urology. 2007;70:1008.e9–10. doi: 10.1016/j.urology.2007.08.018. [DOI] [PubMed] [Google Scholar]


