Abstract
Several studies have evaluated the risk factors influencing biochemical recurrence (BCR) of prostate cancer in patients receiving salvage radiotherapy (SRT) for BCR after radical prostatectomy (RP), but the results remain conflicting. In this study, we performed a meta-analysis to resolve this conflict. We searched the following databases: PubMed, Embase, and Web of Science using the following terms in “All fields”: “salvage radiation therapy,” “salvage IMRT,” “S-IMRT,” “salvage radiotherapy,” “SRT,” “radical prostatectomy,” “RP,” “biochemical recurrence,” “BCR,” “biochemical relapse.” Eleven studies, with a total of 1383 patients, were included in our meta-analysis. Of all the variables, only Gleason score (GS) ≥7 (odds ratio [OR]: 3.82; 95% confidence interval [CI]: 2.60–5.64) and pathological tumor (pT) stage ≥3a (OR: 1.82; 95% CI: 1.36–2.42) were positively correlated with BCR. However, SRT combined with androgen deprivation therapy (ADT) (OR: 0.63; 95% CI: 0.44–0.90) and radiation therapy (RT) dose ≥64 Gy (OR: 0.35; 95% CI: 0.19–0.64) were negatively correlated with BCR. Perineural invasion (OR: 2.64; 95% CI: 1.11–6.26), preoperative prostate-specific antigen (PSA) ≥10 ng ml−1 (OR: 1.36; 95% CI: 0.94–1.96), positive surgical margin (OR: 0.92; 95% CI: 0.7–1.19), and seminal vesicle involvement (SVI) (OR: 1.09; 95% CI: 0.83–1.43) had no effect on BCR. Our meta-analysis indicated that pT stage, GS, RT dose, and SRT combined with ADT may influence BCR, while preoperative PSA, surgical margin, perineural invasion, and SVI have only a weak effect on BCR.
Keywords: biochemical recurrence, prostate cancer, radical prostatectomy, risk factors, salvage radiotherapy
INTRODUCTION
Radical prostatectomy (RP) is one of the first-line treatment options for patients with organ-confined prostate cancer.1,2 However, 40% of men treated with RP will experience biochemical recurrence (BCR) within 5 years.3,4 Salvage radiotherapy (SRT) is currently the recommended option for patients with BCR.5,6,7 Nevertheless, a significant number of patients undergoing SRT for BCR following RP may again experience BCR after SRT.6 Several studies6,7 have found that various factors such as Gleason score (GS), pathological tumor (pT) stage, SRT combined with androgen deprivation therapy (ADT), radiation dose, perineural invasion, preoperative prostate-specific antigen (PSA), surgical margin, and seminal vesicle involvement (SVI) are associated with BCR after SRT. However, there have been conflicting results and the predictive factors for BCR remain to be clearly established. An increasing number of new publications on this controversial subject have emerged in recent years.8,9,10,11
We conducted a systematic review and meta-analysis to evaluate the factors influencing BCR after SRT to build predictive models to help clinicians identify the best candidates who will benefit from SRT.
MATERIALS AND METHODS
Identification of eligible studies
We searched the following electronic databases: PubMed, Embase, and Web of Science from inception to June 15, 2015. The search was performed using the followiCg terms in “All fields”: “salvage radiation therapy,” “salvage IMRT,” “S-IMRT,” “salvage radiotherapy,” “SRT,” “radical prostatectomy,” “RP,” “biochemical recurrence,” “BCR,” “biochemical relapse.” Reference lists of all relevant papers were also searched for further articles.
Inclusion/exclusion criteria
Studies were considered eligible if they met the following criteria: (1) prospective or retrospective studies on patients who had received SRT for BCR following RP and again experienced BCR after SRT; (2) the study should report at least one of the following predictive factors: GS, pT stage, SRT combined with ADT, radiation dose, perineural invasion, preoperative PSA, surgical margin, and SVI; and (3) the study should report the number of patients who had received SRT and the number who had BCR after 5 years of follow-up. The exclusion criteria were as follows: (1) interventions that did not include SRT and (2) studies that contained data that could not be extracted and any attempts to obtain the information from the authors had failed.
Data extraction
Data were extracted independently by two authors (ZWJ and KC) using a standardized form and any disagreement was resolved by consensus between the two authors or consultation with a third reviewer. The data extracted from each paper included first authorship, country of origin, year of publication, age of patients, follow-up duration, BCR rate after 5 years follow-up, study size, and patient demographics, including GS, pT stage, SRT combined with ADT, radiation dose, perineural invasion, preoperative PSA, surgical margin, and SVI.
Statistical methods
Review Manager version 5.2 (Cochrane Collaborative, Oxford, UK) was used to integrate all the individual outcomes. Statistical heterogeneity among studies was evaluated based on the χ2 test and I2 test. Significant heterogeneity was considered when P < 0.10 and I2 was >50%.12 Publication bias was evaluated by the funnel plot and Begg's rank regression test using STATA version 12.0 (Stata Corporation, College Station, TX, USA).12 Publication bias was significant at P < 0.1.
RESULTS
Study inclusion
Figure 1 shows the flowchart of papers retrieved and excluded. We identified 1250 papers from the database search and retrieved the full text for 204 based on abstract reviews. Of these 204 papers, 196 were finally excluded because they did not meet the inclusion criteria. In addition, four papers were identified from the reference lists.13,14,15,16 Finally, a total of 11 papers were eligible for review. Any disagreements at this stage were resolved by consensus.
Figure 1.
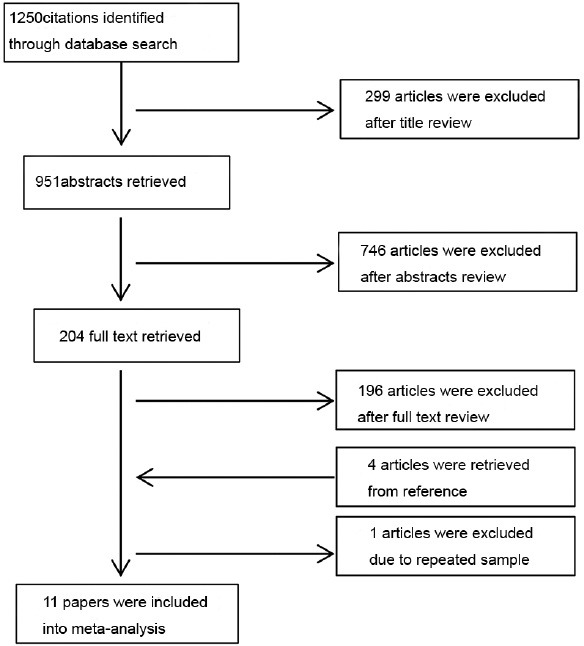
Flow chart of papers retrieved and excluded.
Study characteristics
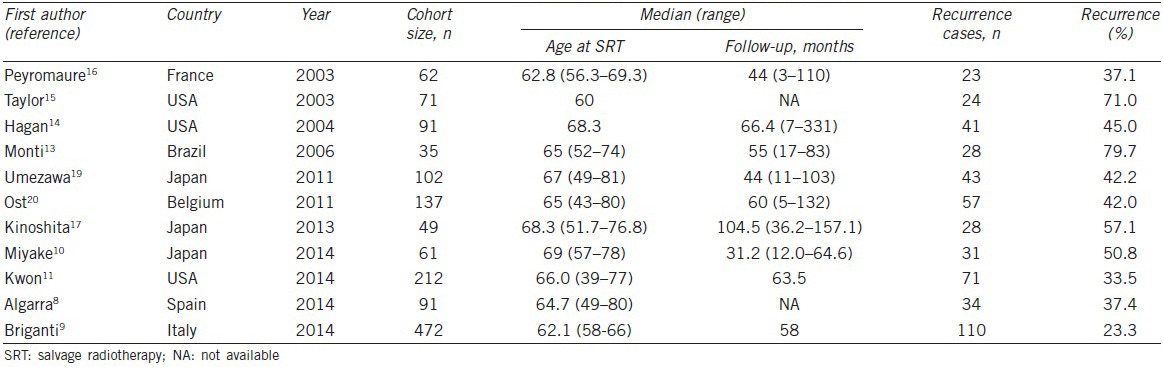
The characteristics of the eligible studies are summarized in Table 1. The 11 studies in this meta-analysis were published from 2003 to 2014, and the study size ranged from 35 to 472 patients. Of the 11 studies, four were conducted in Europe, three in Asia, three in North America, and 1 in Latin America. The patients’ age ranged from 39 to 81 years and the follow-up duration was 3–157.1 months. In addition, the BCR rate after SRT ranged from 23.3% to 79.7% at 5 years.
Table 1.
The characteristics of eligible studies
Main outcomes
The clinical or pathological risk factors influencing BCR of prostate cancer in patients who received SRT for BCR following RP are shown in Figures 2–9. SRT was defined as local radiotherapy to the prostatic bed alone following BCR after RP.10 BCR after RP was defined as PSA level ≥0.2 ng ml−1 with two consecutive increases from a baseline of <0.2 ng ml−1. BCR after SRT was defined as a PSA level that had increased to ≥0.2 ng ml−1 from the post-RT nadir confirmed by one more consecutive result.17,18
Figure 2.
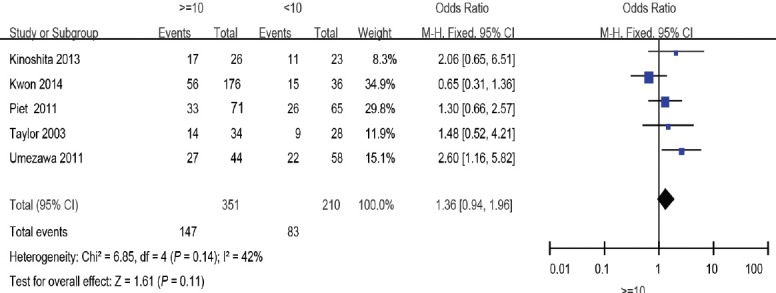
Forest plot of the studies assessing the association between preoperative PSA and BCR. PSA: prostate-specific antigen; BCR: biochemical recurrence.
Figure 9.
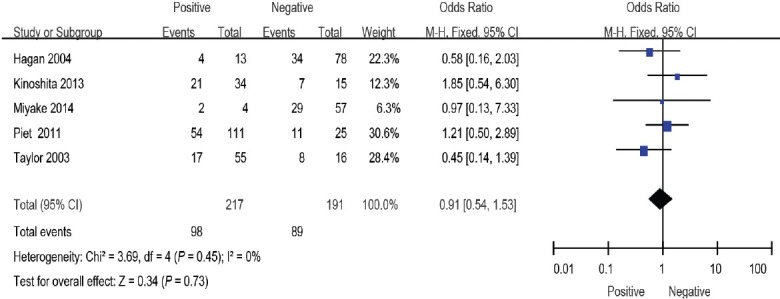
Forest plot of the studies assessing the association between SVI and BCR. SVI: seminal vesicle involvement; BCR: biochemical recurrence.
Preoperative PSA
Five studies11,15,17,19,20 including 561 patients showed a weak association between preoperative PSA ≥10 ng ml−1 group and preoperative PSA <10 ng ml−1 group (odds ratio [OR]: 1.36; 95% confidence interval [CI]: 0.94–1.96). The fixed-effects model was reported here because there was no evidence of heterogeneity (I2 = 29%) (Figure 2).
GS
Four studies8,9,10,13 evaluated GS: 441 patients were ≥7 and 265 were <7. The overall effect of this meta-analysis was against the patients with GS ≥7 (OR: 3.82; 95% CI: 2.60–5.64). The random-effects model was reported here because there was evidence of significant heterogeneity (I2 = 59%) (Figure 3).
Figure 3.
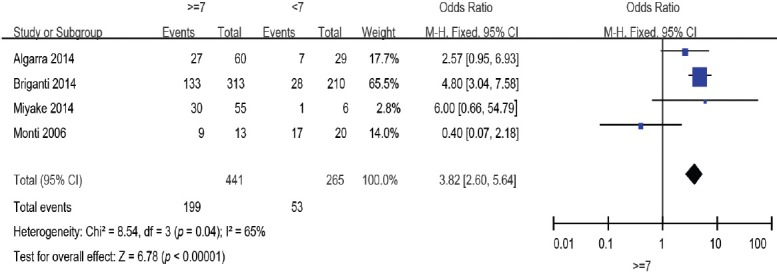
Forest plot of the studies assessing the association between GS and BCR. GS: Gleason score; BCR: biochemical recurrence.
pT stage
Seven studies8,9,10,11,13,16,19 including 1024 patients reported the relationship between pT stage and BCR. When these results were pooled, pT ≥3a was a significant factor predicting BCR after SRT (OR: 1.87; 95% CI: 1.08–3.23). The random-effects model was reported here because there was evidence of significant heterogeneity (I2 = 61%) (Figure 4).
Figure 4.
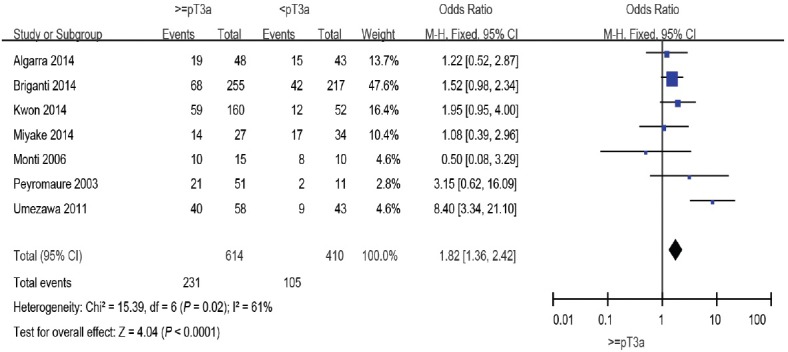
Forest plot of the studies assessing the association between pT stage and BCR. pT: pathological tumor; BCR: biochemical recurrence.
Surgical margin
Positive surgical margins indicate the presence of remnant tumors in the surgical bed, but their impact on cancer progression remains a topic of debate.14 In this meta-analysis, eight studies evaluated the surgical margins9,10,13,14,16,17,19,20 However, the overall effect showed no significant association between surgical margins and BCR (OR: 1.09; 95% CI: 0.83–1.43). The fixed-effects model was reported here because there was no evidence of heterogeneity (I2 = 30%) (Figure 5).
Figure 5.
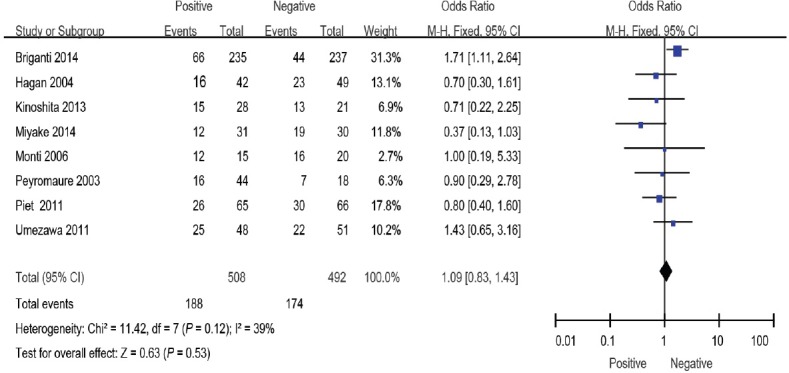
Forest plot of the studies assessing the association between surgical margins and BCR. BCR: biochemical recurrence.
SRT combined with ADT
SRT combined with ADT may be a treatment option for patients with a higher probability of relapse after SRT. However, the effect remains a topic of debate. Five studies11,15,17,19,20 included 292 patients who were given ADT with SRT and 278 who were not given concomitant ADT as a control group. The overall effect of this meta-analysis favored ADT with salvage RT (OR: 0.63; 95% CI: 0.44–0.90). The fixed-effects model was reported here because there was no evidence of heterogeneity (I2 = 4%) (Figure 6).
Figure 6.
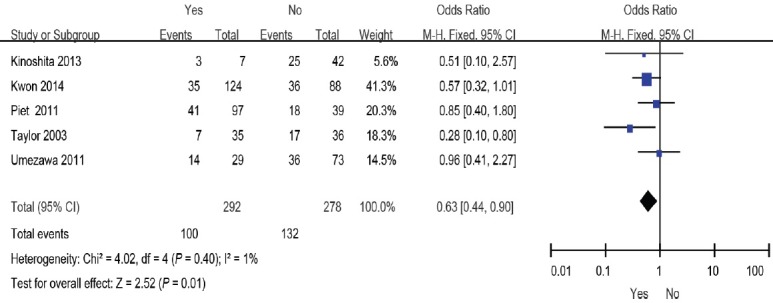
Forest plot of the studies assessing the association between SRT combined with ADT or not and BCR. SRT: salvage radiotherapy; ADT: androgen deprivation therapy; BCR: biochemical recurrence.
Radiation dose
Until now, no prospective data have been published regarding the most efficacious dose of SRT. In this meta-analysis, we evaluated three studies including 264 patients that evaluated radiation dose.14,15,19 Pooling of data from these studies showed a significant effect of radiation dose ≥64 Gy versus the controls (OR: 0.35; 95% CI: 0.19–0.64). The fixed-effects model was used here because there was no evidence of heterogeneity (I2 = 0%) (Figure 7).
Figure 7.
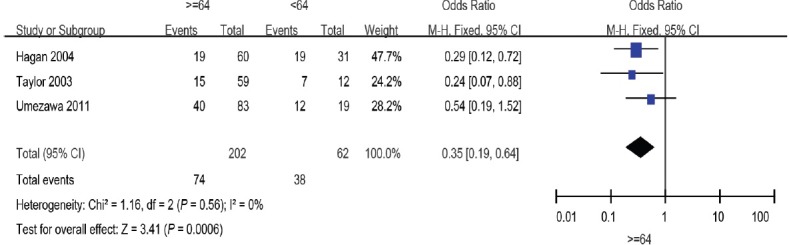
Forest plot of the studies assessing the association between radiation dose and BCR. BCR: biochemical recurrence.
Perineural invasion
Two studies10,20 evaluated perineural invasion, including 106 patients in the positive group and 39 patients in the negative group. The overall effect of this meta-analysis showed no significant association between the two groups (OR: 2.64; 95% CI: 1.11–6.26). The random-effects model was used here because there was evidence of significant heterogeneity (I2 = 70%) (Figure 8).
Figure 8.
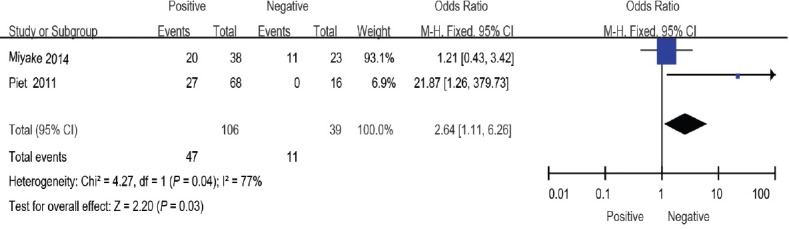
Forest plot of the studies assessing the association between perineural invasion and BCR. BCR: biochemical recurrence.
SVI
Five studies10,14,15,17,20 including a total of 408 patients reported the relationship between SVI and BCR. Pooling of data from these studies showed no significant difference between the positive and negative groups (OR: 0.91; 95% CI: 0.54–1.53). The fixed-effects model was used here because there was no evidence of heterogeneity (I2 = 0%) (Figure 9).
Publication bias and sensitivity analysis
We performed a funnel plot and Egger's test to assess publication bias. The funnel plots were symmetrical among the studies included (Figure 10). Consistent with this, we found no evidence of publication bias by Egger's test (P = 0.453). We performed leave-one-out sensitivity analysis and the results indicated that a single study could not qualitatively change the pooled ORs.
Figure 10.
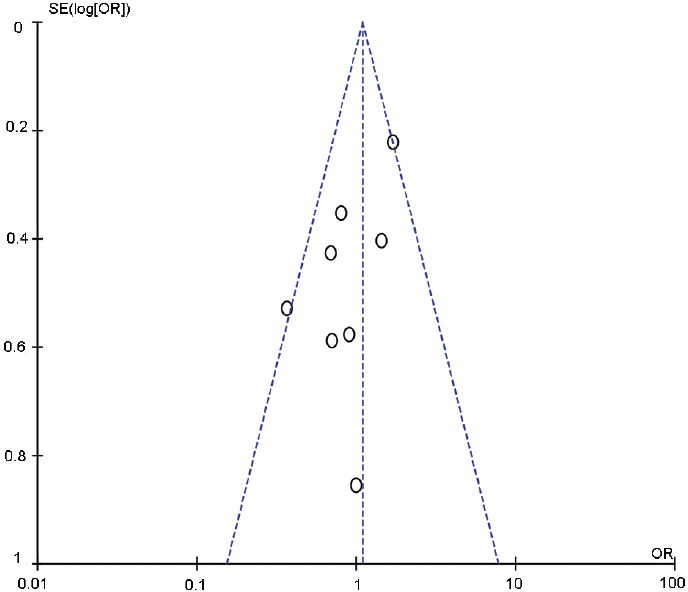
Funnel plot and Egger's test to assess the publication bias.
DISCUSSION
Based on the well-known association between SRT and cancer control outcomes, it is recommended that SRT should be administered to patients with BCR after RP. However, some men still experience BCR, even when SRT is administered early after BCR. Many factors may affect BCR; however, there are conflicting results and the predictive factors for BCR remain to be clearly established. Thus, evaluating the clinical significance of different clinic-pathological parameters in BCR after SRT and identifying patients who may benefit from post-RP radiation are critical for improving outcomes in the salvage setting. For this purpose, BCR-related variables involved in the outcome of patients receiving post-RP RT are of major significance.
This study was the first systematic review and meta-analysis of the risk factors for BCR among patients who have received SRT for BCR following RP. We included 11 studies from 2003 to 2014 with a total of 1383 patients. Our analysis shows that GS, pT, SRT combined with ADT, and radiation dose are associated with the risk of BCR.
Although the predictive value of GS has been reported in several studies, the results remain controversial. One study conducted by Monti et al.13 did not find any association between GS and BCR. However, Peyromaure et al.16 and Stephenson et al.21 found that GS ≥8 was associated with a high risk of BCR. Consistent with our findings, Song et al.22 reported that a GS ≥7 was an accurate predictor of BCR after SRT. In general, we found that GS ≥7 was a risk factor for BCR. Several recent studies have reported that pT stage was a risk factor predicting BCR after SRT. However, the specific stage was not consistent. In our study, pT ≥3a was a significant factor predicting BCR after SRT. For patients with pT ≥3a disease, SRT may not be effective, and postoperative RT may improve biochemical relapse-free survival (bRFS). One study conducted by Jereczek-Fossa et al.23 reported that bRFS in postoperative RT patients was significantly longer than that of patients who had undergone SRT (4-year biochemical control rates: 81.7% vs 60.5%, respectively).
SRT combined with ADT may be an effective treatment option for preventing BCR after SRT. However, the efficacy of this strategy for reducing cancer recurrence remains a topic of debate. Our results indicated that BCR was significantly reduced in patients who received SRT combined with ADT. In contrast, Trock found that SRT combined with ADT did not decrease BCR after SRT,7 while several other studies reported that ADT administered concurrently with SRT was predictive of favorable patient outcomes.11,15,17,20 Notably, Stephenson et al.21 found that ADT administered before and/or during SRT may improve the efficacy of SRT, consistent with our results. SRT was administered at a total dose of 60–70 Gy (median 66 Gy) in the current study, but the optimal dose of SRT remains controversial, and no prospective data are currently available. The Therapeutic Radiology and Oncology (ASTRO) Consensus Panel suggested the highest dose of radiation therapy that can be given without morbidity is justifiable. In this study, we found that the effect of SRT might be improved with a dose of ≥64 Gy. However, the possible side effects should also be taken into consideration when choosing the appropriate dose of SRT. Several studies reported that a dose <64 Gy is rarely associated with severe side effects,24,25,26 while other studies reported that a dose >68 Gy was associated with an increase in late grade 3 urinary toxicity.27
In general, patients have a bad prognosis if their surgical margins are positive. However, several studies showed that patients are more likely to have better prognosis if their surgical margins are positive.11,21,28 Some studies have reported to the contrary. Briganti et al.9 reported that a positive surgical margin may be a risk factor of BCR after SRT. Meanwhile, a study conducted by Umezawa et al.19 showed that the status of the surgical margin did not affect BCR after SRT. This was consistent with our results, which showed that there is no significant association between surgical margins and BCR. In several studies, patients with preoperative PSA >20 ng ml−1 were excluded from SRT,29 and the ideal patient has preoperative PSA <10 ng ml−1. However, our study did not find any obvious association between preoperative PSA and BCR after SRT. Patients with the involvement of SVI are usually excluded from SRT as well but, in our study, the correlation was not obvious. Meantime, we find no matter what the status of perineural, the results may not have statistically significant. It is not clear what caused this phenomenon. Although we found a significant difference, only eight, five, five, and two articles were included to evaluate surgical margin, preoperative PSA, SVI, and perineural invasion, respectively. We look forward to more studies, with large sample sizes, to confirm our findings.
PSA doubling time (PSADT) has recently been used as a parameter to identify appropriate patients for SRT after BCR in clinical practice.30 Trock7 reported that patients with PSADT <6 months may experience significantly improved prostate cancer-specific survival. However, Umezawa et al.19 and Stephenson et al.21 found different results; Umezawa et al.19 showed that patients with PSADT <7 months had poorer survival and a lower 4-year bRFS rate than patients with PSADT ≥7 months, while Stephenson et al.21 found that patients with PSADT ≤10 months had poorer survival, irrespective of GS state and surgical margin. However, there is currently no definitive cut-off value for PSADT to help identify appropriate patients for SRT. In addition, pre-SRT PSA level and PSA nadir after RP might predict the results of SRT after BCR.9,10,11,15,20 The significance of these parameters should be verified in large prospective studies.
Imaging is a valuable targeted tool for predicting patients’ prognosis. Positron emission tomography/computed tomography (PET/CT) using 11C-labeled choline may help to identify patients likely to benefit from SRT, given that 11C-choline PET/CT can detect the site of tumor recurrence earlier than other imaging methods. Castellucci et al.31 found that positive scan results were positively correlated with PSA level, ongoing ADT, and PSAD, while Souvatzoglou et al.32 also found that patients with positive 11C-choline PET/CT results had significantly higher PSA levels. In addition, Rodado-Marina et al.33 revealed that PSA >3 ng ml−1, no early RP, and GS ≥8 were independent risk factors for positive PET/CT in a study of 233 patients. Although the results of the above studies are not identical, they suggest that choline-PET/CT may be a useful predictive imaging tool.
Despite the above positive findings, we remain cautious in our conclusions, as our study was not devoid of some limitations. First, we considered only a single risk factor. However, BCR may be affected by one or more factors. We tried to extract adjusted data from the studies included; however, most of the papers did not report adjusted hazard ratios in multivariate analysis. Our study may only provide some references and we hope that more papers with adjusted data will be published in the future to provide further research. Second, the initial RP was performed by different doctors, even though each operation was carried out according to the standard method. Therefore, the surgical quality may differ and the results may display heterogeneity. Third, we used BCR as the primary endpoint, but did not take comorbidity into account. However, some patients who experience BCR will die from diseases other than prostate cancer.34 The absence of analysis of accurate PSA data after BCR may be another limitation of our study because we could not distinguish the importance of each risk factor. In addition, our meta-analysis only included 11 articles related to the risk factors and the results need verification.
CONCLUSIONS
Our meta-analysis suggests that GS ≥7, pT ≥3a, and SRT not combined with ADT and radiation dose <64 Gy are risk factors for BCR among patients who have received SRT for BCR following RP. However, preoperative PSA, surgical margin, perineural invasion, and SVI have no effect on BCR. Our predictive models might help clinicians to identify the best candidates who will benefit from SRT.
AUTHOR CONTRIBUTIONS
ZWJ and KC drafted the manuscript. BD and DWY supervised and revised the manuscript. YW and YYQ searched and selected the studies. YYK and HLZ collected the clinical data, and YZ and GHS analyzed the data. All authors reviewed and approved the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by a grant from the International Cooperation and Exchange of Science and Technology Commission of Shanghai Municipality (No. 12410709300), a grant from the Guide Project of Science and Technology Commission of Shanghai Municipality (No. 124119a7300), and a grant from the Outstanding Young Talent Training Plan of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013102).
REFERENCES
- 1.Boorjian SA, Eastham JA, Graefen M, Guillonneau B, Karnes RJ, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61:664–75. doi: 10.1016/j.eururo.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Mullins JK, Feng Z, Trock BJ, Epstein JI, Walsh PC, et al. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol. 2012;188:2219–24. doi: 10.1016/j.juro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka N, Fujimoto K, Hirayama A, Nakai Y, Chihara Y, et al. Calculated tumor volume is an independent predictor of biochemical recurrence in patients who underwent retropubic radical prostatectomy. Adv Neurol 2012. 2012:1–7. doi: 10.1155/2012/204215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okajima E, Yoshikawa M, Masuda Y, Shimizu K, Tanaka N, et al. Improvement of the surgical curability of locally confined prostate cancer including non-organ-confined high-risk disease through retropubic radical prostatectomy with intentional wide resection. World J Surg Oncol. 2012;10:249. doi: 10.1186/1477-7819-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65:1034–43. doi: 10.1016/j.eururo.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Karlin JD, Koontz BF, Freedland SJ, Moul JW, Grob BM, et al. Identifying appropriate patients for early salvage radiotherapy after prostatectomy. J Urol. 2013;190:1410–5. doi: 10.1016/j.juro.2013.04.078. [DOI] [PubMed] [Google Scholar]
- 7.Trock BJ. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Algarra R, Tienza A, Hevia M, Zudaire J, Rosell D, et al. Influential factors in the response to salvage radiotherapy after radical prostatectomy. Actas Urol Esp. 2014;38:662–8. doi: 10.1016/j.acuro.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Briganti A, Karnes RJ, Joniau S, Boorjian SA, Cozzarini C, et al. Prediction of outcome following early salvage radiotherapy among patients with biochemical recurrence after radical prostatectomy. Eur Urol. 2014;66:479–86. doi: 10.1016/j.eururo.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Miyake M, Tanaka N, Asakawa I, Morizawa Y, Anai S, et al. Proposed salvage treatment strategy for biochemical failure after radical prostatectomy in patients with prostate cancer: a retrospective study. Radiat Oncol. 2014;9:208. doi: 10.1186/1748-717X-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon O, Kim KB, Lee YI, Byun SS, Kim JS, et al. Salvage radiotherapy after radical prostatectomy: prediction of biochemical outcomes. PLoS One. 2014;9:e103574. doi: 10.1371/journal.pone.0103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller KF, Meerpohl JJ, Briel M, Antes G, von Elm E, et al. Detecting, quantifying and adjusting for publication bias in meta-analyses: protocol of a systematic review on methods. Syst Rev. 2013;2:60. doi: 10.1186/2046-4053-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monti CR, Nakamura RA, Ferrigno R, Rossi AJ, Kawakami NS, et al. Salvage conformal radiotherapy for biochemical recurrent prostate cancer after radical prostatectomy. Int Braz J Urol. 2006;32:416–26. doi: 10.1590/s1677-55382006000400006. 427. [DOI] [PubMed] [Google Scholar]
- 14.Hagan M, Zlotecki R, Medina C, Tercilla O, Rivera I, et al. Comparison of adjuvant versus salvage radiotherapy policies for postprostatectomy radiotherapy. Int J Radiat Oncol. 2004;59:329–40. doi: 10.1016/j.ijrobp.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Taylor N, Kelly JF, Kuban DA, Babaian RJ, Pisters LL, et al. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol. 2003;56:755–63. doi: 10.1016/s0360-3016(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 16.Peyromaure M, Allouch M, Eschwege F, Verpillat P, Debré B, et al. Salvage radiotherapy for biochemical recurrence after radical prostatectomy: a study of 62 patients. Urology. 2003;62:503–7. doi: 10.1016/s0090-4295(03)00468-0. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita H, Shimizu Y, Mizowaki T, Takayama K, Norihisa Y, et al. Risk factors predicting the outcome of salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy. Int J Urol. 2013;20:806–11. doi: 10.1111/iju.12049. [DOI] [PubMed] [Google Scholar]
- 18.Hong SK, Park HZ, Lee WK, Kim DS, Lee JS, et al. Prognostic significance of undetectable ultrasensitive prostate-specific antigen nadir after radical prostatectomy. Urology. 2010;76:723–7. doi: 10.1016/j.urology.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 19.Umezawa R, Ariga H, Ogawa Y, Jingu K, Matsushita H, et al. Impact of pathological tumor stage for salvage radiotherapy after radical prostatectomy in patients with prostate-specific antigen < 1.0 ng/ml. Radiat Oncol. 2011;6:150. doi: 10.1186/1748-717X-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ost P, Lumen N, Goessaert AS, Fonteyne V, De Troyer B, et al. High-dose salvage intensity-modulated radiotherapy with or without androgen deprivation after radical prostatectomy for rising or persisting prostate-specific antigen: 5-year results. Eur Urol. 2011;60:842–9. doi: 10.1016/j.eururo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song DY, Thompson TL, Ramakrishnan V, Harrison R, Bhavsar N, et al. Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology. 2002;60:281–7. doi: 10.1016/s0090-4295(02)01709-0. [DOI] [PubMed] [Google Scholar]
- 23.Jereczek-Fossa BA, Zerini D, Vavassori A, Fodor C, Santoro L, et al. Sooner or later? Outcome analysis of 431 prostate cancer patients treated with postoperative or salvage radiotherapy. Int J Radiat Oncol. 2009;74:115–25. doi: 10.1016/j.ijrobp.2008.07.057. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JL, Buskirk SJ, Heckman MG, Crook JE, Ko SJ, et al. Late toxicity after postprostatectomy salvage radiation therapy. Radiother Oncol. 2009;93:203–6. doi: 10.1016/j.radonc.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Pearse M, Choo R, Danjoux C, Gardner S, Morton G, et al. Prospective assessment of gastrointestinal and genitourinary toxicity of salvage radiotherapy for patients with prostate-specific antigen relapse or local recurrence after radical prostatectomy. Int J Radiat Oncol. 2008;72:792–8. doi: 10.1016/j.ijrobp.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Feng M, Hanlon AL, Pisansky TM, Kuban D, Catton CN, et al. Predictive factors for late genitourinary and gastrointestinal toxicity in patients with prostate cancer treated with adjuvant or salvage radiotherapy. Int J Radiat Oncol. 2007;68:1417–23. doi: 10.1016/j.ijrobp.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Cozzarini C, Fiorino C, Da Pozzo LF, Alongi F, Berardi G, et al. Clinical factors predicting late severe urinary toxicity after postoperative radiotherapy for prostate carcinoma: a single-institute analysis of 742 patients. Int J Radiat Oncol. 2012;82:191–9. doi: 10.1016/j.ijrobp.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Chade DC, Shariat SF, Cronin AM, Savage CJ, Karnes RJ, et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: a multi-institutional collaboration. Eur Urol. 2011;60:205–10. doi: 10.1016/j.eururo.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee AK, D’Amico AV. Utility of prostate-specific antigen kinetics in addition to clinical factors in the selection of patients for salvage local therapy. J Clin Oncol. 2005;23:8192–7. doi: 10.1200/JCO.2005.03.0007. [DOI] [PubMed] [Google Scholar]
- 30.Milecki P, Antczak A, Martenka P, Kwias Z. What is the possible role of PSA doubling time (PSADT) and PSA velocity (PSAV) in the decision-making process to initiate salvage radiotherapy following radical prostatectomy in patients with prostate cancer? Cent European J Urol. 2011;64:67. doi: 10.5173/ceju.2011.02.art3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424–9. doi: 10.2967/jnumed.114.138313. [DOI] [PubMed] [Google Scholar]
- 32.Souvatzoglou M, Krause BJ, Pürschel A, Thamm R, Schuster T, et al. Influence of 11C-choline PET/CT on the treatment planning for salvage radiation therapy in patients with biochemical recurrence of prostate cancer. Radiother Oncol. 2011;99:193–200. doi: 10.1016/j.radonc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Rodado-Marina S, Coronado-Poggio M, Garcia-Vicente AM, Garcia-Garzon JR, Alonso-Farto JC, et al. Clinical utility of (18) F-fluorocholine positron-emission tomography/computed tomography (PET/CT) in biochemical relapse of prostate cancer after radical treatment: results of a multicentre study. BJU Int. 2015;115:874–83. doi: 10.1111/bju.12953. [DOI] [PubMed] [Google Scholar]
- 34.Abdollah F, Boorjian S, Cozzarini C, Suardi N, Sun M, et al. Survival following biochemical recurrence after radical prostatectomy and adjuvant radiotherapy in patients with prostate cancer: the impact of competing causes of mortality and patient stratification. Eur Urol. 2013;64:557–64. doi: 10.1016/j.eururo.2013.03.006. [DOI] [PubMed] [Google Scholar]


