Abstract
Sildenafil and tadalafil are efficacious and well tolerated in Chinese men with erectile dysfunction (ED). Recent study results indicate that men with ED in China who were naïve to phosphodiesterase inhibitor type 5 (PDE5) therapy prefer tadalafil 20-mg (on-demand) versus sildenafil 100-mg (on-demand). Differences in psychosocial outcomes may help to explain treatment preference in favor of tadalafil. This open-label, randomized, crossover study compared psychosocial outcomes and drug attribute choices between tadalafil and sildenafil in Chinese men with ED naïve to PDE5 inhibitor therapy. Eligible patients were randomized to sequential 20-mg tadalafil/100-mg sildenafil (n = 190) or 100-mg sildenafil/20-mg tadalafil (n = 193) for 8 weeks each and were asked which treatment they preferred to take for the 8-week extension phase. Psychosocial outcomes were assessed using the Psychological and Interpersonal Relationship Scale (PAIRS), Drug Attributes Questionnaire (DRAQ), and Sexual Life Quality Questionnaire (SLQQ). When taking tadalafil versus sildenafil, men had a higher mean endpoint score on the PAIRS Spontaneity Domain (tadalafil = 2.86 vs sildenafil = 2.72; P < 0.001), and a lower mean endpoint score on the Time Concerns Domain (tadalafil = 2.41 vs sildenafil = 2.55; P < 0.001). A numerical increase in the Sexual Self-Confidence Domain was observed when taking tadalafil versus sildenafil (tadalafil = 2.76 vs sildenafil = 2.72; P = 0.102). The most frequently chosen drug attributes explaining treatment preference were able to get an erection long after having drug, and ability to get an erection every time. SLQQ results were comparable between treatment groups. These psychosocial outcomes may explain why more Chinese men preferred tadalafil versus sildenafil for the treatment of ED in this clinical trial.
Keywords: drug attributes, erectile dysfunction, phosphodiesterase type 5 inhibitor, psychological assessment of sexual dysfunction
INTRODUCTION
Phosphodiesterase type 5 (PDE5) inhibitors are the first line of treatment choice for men with erectile dysfunction (ED) in Western society.1 Traditional Chinese medicine has been widely used in China for the treatment of impotence; however, more men are switching to PDE5 inhibitors for ED treatment.2 Currently, there are three PDE5 inhibitors offered in China: sildenafil citrate, tadalafil, and vardenafil.
Previous studies have shown both sildenafil3 and tadalafil4,5 to be efficacious and well tolerated in Chinese men with ED, and it is expected that treatment with tadalafil may address some of the psychosocial concerns with sexual performance in men with ED. Some preference studies have shown patients6,7,8 and partners8,9 prefer tadalafil over sildenafil in the treatment of ED; however, until recently, the treatment preference patterns of men and their partners with ED in China was unknown.
The preference, efficacy, and safety results from this clinical trial were previously reported.10 Results from this study indicated that both tadalafil and sildenafil significantly improved erectile function as assessed by the IIEF and SEP diary, and both drugs were well tolerated. The preference results showed that 69.1% of patients preferred tadalafil 20 mg taken on-demand versus 30.9% of patients who preferred sildenafil 100 mg on-demand (P < 0.001). As the efficacy and tolerability were comparable between tadalafil and sildenafil, differences in psychosocial outcomes may help to explain treatment preference in favor of tadalafil.
Psychosocial outcomes have been shown to improve significantly following treatment adherence with tadalafil, compared to sildenafil, when administered on-demand.11 As such, the current analysis compared the psychosocial outcomes between tadalafil and sildenafil for the treatment of ED in Chinese men naïve to PDE5 inhibitor therapy.
MATERIALS AND METHODS
This was an open-label, randomized, multicenter, crossover study to compare treatment preference, efficacy, tolerability, psychosocial outcomes, and drug attribute choices between tadalafil and sildenafil in Chinese men with ED naïve to PDE5 inhibitor therapy. The preference, efficacy, and tolerability data have been reported previously (ClinicalTrials.gov Identifier: NCT01352507).10 This study was conducted from June 2011 to July 2012 at 15 centers in China and was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice. Written informed consent was obtained from all patients and Local Institutional Review Committees approved the study.
Patients
The study included Chinese men who were at least 18 years of age (and <65 years), who were in a stable relationship with a female partner, and who had a history of ED of any severity (mild, moderate, or severe) or etiology (psychogenic, organic, or mixed) for at least 3 months but were naïve to any treatment with a PDE5 inhibitor. Eligible patients were required to make at least 4 sexual intercourse attempts during the 4-week run-in period and the final 4 weeks of each 8-week treatment period.
Patients were excluded if their ED was caused by another primary sexual disorder or if they had a penile implant, clinically significant penile deformity, history of radical prostatectomy, evidence of clinically significant renal insufficiency, active symptomatic hepatobiliary disease, or hemoglobin A1c >11.0% at Visit 1 (Screening). Patients were excluded if they had a history of the following conditions in the 90 days preceding enrollment: chronic stable angina treated with long- or short-acting nitrates, myocardial infarction or coronary artery bypass graft surgery, or percutaneous coronary intervention. Finally, patients were excluded if they had a history of the following conditions in the 6 months before enrollment: angina occurring during sexual intercourse, unstable angina, or evidence of congestive heart failure.
Study design
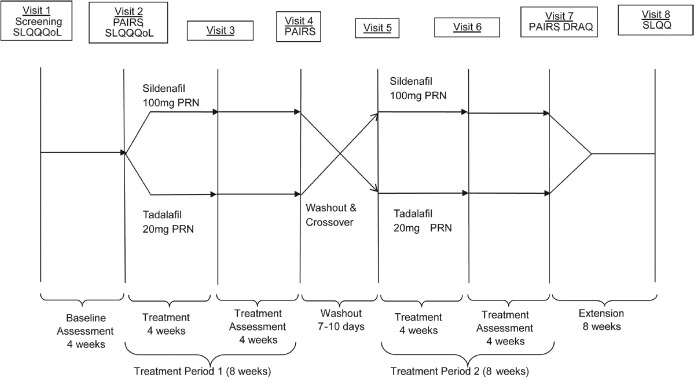
The study consisted of a 4-week baseline period, two 8-week treatment periods separated by a 7–10 days washout period, and an 8-week extension period (Figure 1). The details of the study design have been previously reported.10 Eligible patients were randomized to sequential 20 mg tadalafil/100 mg sildenafil or 100 mg sildenafil/20 mg tadalafil for 8 weeks each. Patients were then asked which treatment they preferred to take for the 8-week extension phase. Both tadalafil and sildenafil were administered as needed before sexual activity but were not administered more than 1 dose per day. The doses of tadalafil and sildenafil used in this study represent the approved maximum labeled doses in China, thus minimizing the influence of efficacy (resulting from dose) on psychosocial outcomes. Additionally, down-titration was not permitted. Patient compliance was assessed by direct questioning or examination of diary cards at each visit. Patients who were noncompliant were discontinued from the study. The use of any other ED treatment was prohibited for the duration of the study.
Figure 1.
Study design. Wash-out period is 7–10 days; up to 10 days have been included in the timeline (i.e., 1.5 weeks). The number of weeks has been rounded up to the nearest integer. DRAQ: Drug Attributes Questionnaire; PAIRS: Psychological and Interpersonal Relationship Scale; PRN: pro re nata (on demand); QoL: quality of life; SLQQ: Sexual Life Quality Questionnaire.
Study objectives
The aim of this analysis was to determine whether psychosocial outcomes differed when men with ED received tadalafil compared with sildenafil. Objectives included comparing psychosocial outcomes using the Psychological and Interpersonal Relationship Scale (PAIRS) between both treatments, determining a patient's preferred drug attributes using the Drug Attributes Questionnaire (DRAQ) at the end of both treatments, and determining sexual quality of life using the Sexual Life Quality Questionnaire (SLQQ) in patients following treatment. Finally, this study evaluated the safety and tolerability of both tadalafil and sildenafil treatment.
Psychological and Interpersonal Relationship Scales
The PAIRS is a self-administered, 29-item scale containing four domains – Sexual Self-Confidence, Spontaneity, Time Concerns, and Sexual Miscommunication, which are related to the broader psychosocial and interpersonal outcomes associated with ED and its treatment.12 Only three domains (Sexual Self-Confidence, Spontaneity, and Time Concerns) were assessed in this study. Patients rated each item in PAIRS on a 4-point scale (1 = strongly disagree; 2 = disagree; 3 = agree; 4 = strongly agree). Domain scores were calculated for each patient as the mean of all items in the domain with a lowest possible score for each domain of 1 and a highest possible score of 4. Higher scores on the Sexual Self-Confidence and Spontaneity Domains represent higher sexual self-confidence and spontaneity, whereas a lower score on the Time Concerns Domain represents fewer time concerns before and during sexual encounters. The PAIRS was administered at baseline (Visit 2) and at the end of both treatment periods (Visits 4 and 7). A change from baseline to endpoint in each PAIRS Domain score was measured in this study.
Drug Attribute Questionnaire
The DRAQ was administered at the end of the second treatment period (Visit 7) to assess why a patient preferred either sildenafil or tadalafil. Patients chose the best and second best statements to explain their treatment preference. The responses to the DRAQ were conditional upon the patient's treatment preference.
Sexual Life Quality Questionnaire
The SLQQ is a validated, multidimensional instrument that consists of two domains – Sexual quality of life (QoL) (10 questions) and Treatment Satisfaction (6 questions).13 The SLQQ-QoL Domain compared the patient's current sexual experience with their experience prior to the onset of the patient's ED. The SLQQ-QoL was answered by the patient at screening (Visit 1), baseline (Visit 2), and at the end of the extension period (Visit 8) with a 4-week recall period. The SLQQ Treatment Satisfaction Domain was completed at Visit 8.
Statistical analysis
A sample size of 370 patients (185 patients per sequence group) was estimated to achieve 90% power to detect an increased preference for tadalafil over sildenafil citrate of 10% (60% vs 50%) using a two-sided Chi-square test with a significance level of 0.05 assuming 30% of Chinese patients have a missing treatment preference. The preference analysis includes all randomized subjects who completed both treatment periods (until Visit 7). Baseline was defined as the randomization visit (Visit 2). Change from baseline to end of each treatment period was defined as the value at the end each treatment period minus the baseline value.
PAIRS Domains were analyzed using a mixed effect analysis of covariance (ANCOVA) model for crossover designs for the change from baseline to end of each treatment period. The model included treatment, period, sequence, and pooled site as fixed effects, centered baseline value of the efficacy measure (defined as the baseline value for a patient minus the overall baseline mean value) as a covariate, patient within sequence as a random effect, and centered baseline-by-treatment interaction as a fixed effect. Since the wash-out period was planned for more than 7 days (7–10 days), no carryover effect was included in the mixed model.
For patients and their partners, baseline (Visit 2), endpoint (Visit 8), and changes from baseline to endpoint in the SLQQ-QoL Domain score were summarized descriptively by treatment, respectively. The SLQQ Treatment Satisfaction Domain score at the end of extension phase (Visit 8) was also summarized descriptively by treatment. Frequency tables using counts and percentages were generated for DRAQ.
RESULTS
Patient disposition and baseline demographics
A total of 418 patients signed consent to participate in the study, and 383 patients completed the baseline phase and were randomized to a treatment sequence. One hundred and ninety patients were assigned to receive tadalafil followed by sildenafil, with 164 patients completing the sequence. One hundred and ninety-three patients were assigned to receive sildenafil followed by tadalafil, with 173 patients completing this sequence. Eighty-eight percent of randomized patients completed the study through the extension phase.
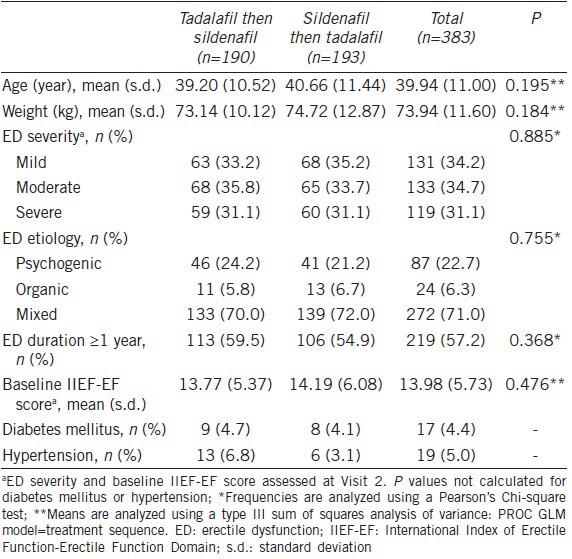
Baseline characteristics are shown in Table 1. Patients had a mean age of 39.9 years (range of 21.4–65.2). The majority of patients (57.2%) had an ED duration of ≥1 year. The overall baseline characteristics were comparable between the two sequence groups.
Table 1.
Baseline demographics and characteristics
Psychological and Interpersonal Relationship Scales
Mean PAIRS results are shown in Figure 2. In the Sexual Self-Confidence Domain, the baseline scores were 2.01 for both sildenafil and tadalafil. The mean change from baseline in the tadalafil group was 0.75 (vs 0.71 in the sildenafil group) to a score of 2.76 at endpoint (vs 2.72 in the sildenafil group) (P = 0.102).
Figure 2.
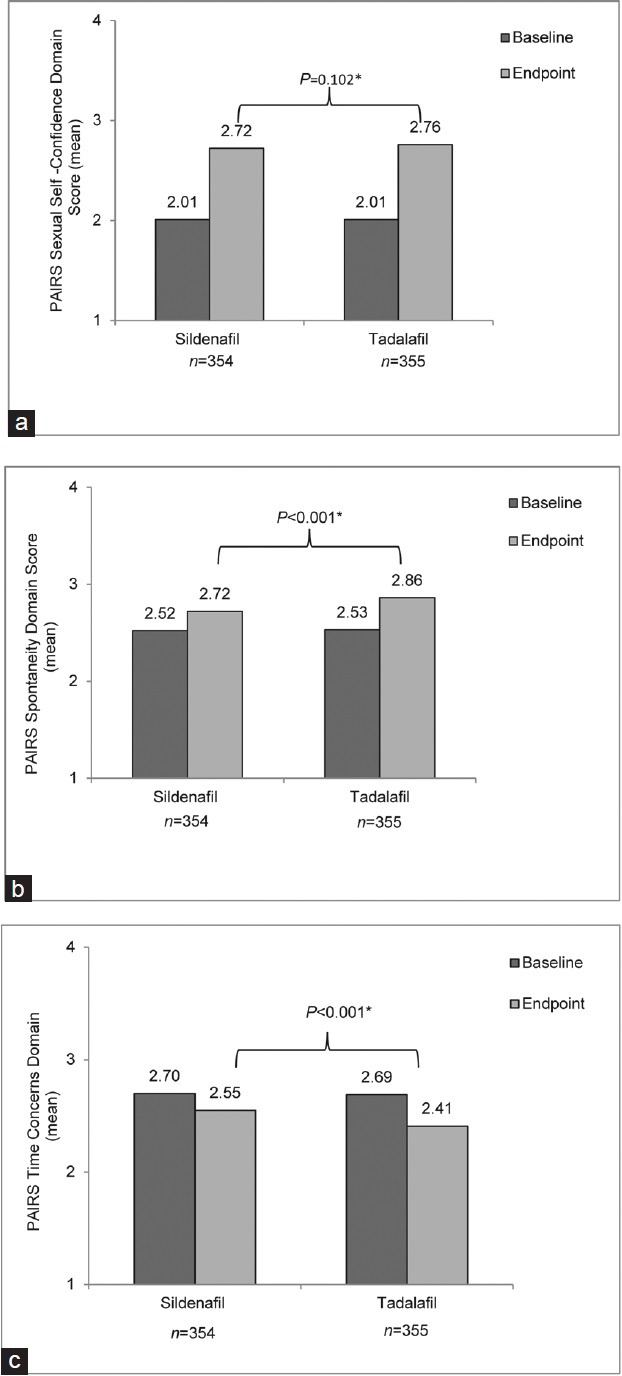
Mean Psychological and Interpersonal Relationship Scales (PAIRS) results. (a) Sexual Self-Confidence Domain, (b) Spontaneity Domain, (c) Time Concerns Domain. *From the crossover mixed effect model for the comparison in change from baseline values (tadalafil vs sildenafil).
In the Spontaneity Domain, the baseline scores were 2.52 and 2.53 for sildenafil and tadalafil respectively. The mean change from baseline in the tadalafil group was 0.33 (vs 0.20 in the sildenafil group) to a score of 2.86 at endpoint (vs 2.72 in the sildenafil group) (P < 0.001). In the Time Concerns Domain, the baseline scores were 2.70 and 2.69 for sildenafil and tadalafil respectively. The mean change from baseline in the tadalafil group was −0.28 (vs −0.15 in the sildenafil group) to a score of 2.41 at baseline (vs 2.55 in the sildenafil group) (P < 0.001). The lower Time Concerns Domain score indicates that men felt less time pressure and less sense of urgency before and during sexual encounters when taking tadalafil compared with sildenafil.
Drug Attribute Questionnaire
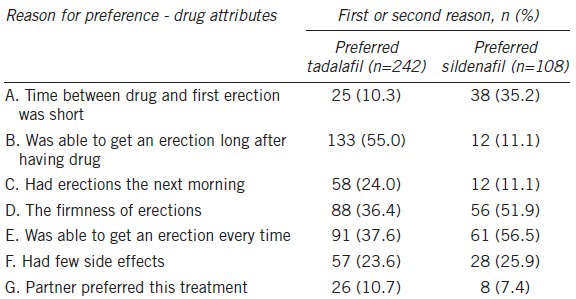
Table 2 shows the 1st and 2nd best reasons chosen by patients to explain why they preferred either tadalafil or sildenafil. Among the answers to DRAQ, “Was able to get an erection long after having drug” was the main reason that patients chose tadalafil, with 133 out of 242 (55.0%) patients indicating this response as the 1st or 2nd reason of preference while only 12/108 (11.1%) patients who preferred sildenafil indicated the same reason. Of the 108 patients who preferred sildenafil, 61 (56.5%) identified, “Was able to get an erection every time” as the 1st or 2nd reason for their preference.
Table 2.
Drug Attribute Questionnaire results
Sexual Life Quality Questionnaire
Two hundred and thirty-one patients who preferred tadalafil and 105 patients who preferred sildenafil treatment completed the SLQQ-QoL and SLQQ-Treatment Satisfaction Domains at the end of the extension phase. Mean scores of change from baseline in both the QoL and Treatment Satisfaction Domains were comparable among patients who preferred either tadalafil or sildenafil treatment (Figure 3).
Figure 3.
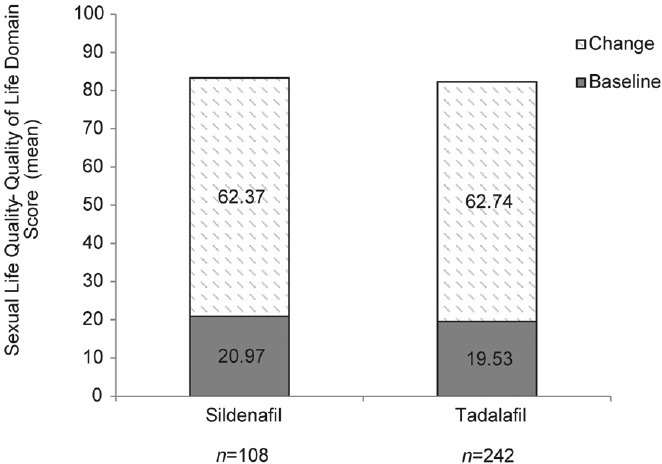
Sexual Life Quality Questionnaire-Quality of Life (SLQQ-QoL) domain.
Safety
Safety results have been previously reported.10 The overall safety profiles of 20 mg tadalafil and 100 mg sildenafil treatment were comparable. The incidence of treatment emergent adverse events (TEAEs) were low for tadalafil and sildenafil treatment in the preextension phase (tadalafil: 8.3%; sildenafil: 7.2%) and extension phase (tadalafil: 3.0%; sildenafil: 1.9%). No severe TEAEs were reported during tadalafil treatment, and no serious adverse events (SAEs) were reported throughout the study. The most frequently reported TEAEs were: headache (2.8% on tadalafil; 1.4% on sildenafil), dizziness (1.7% on tadalafil; 0.8% on sildenafil), and flushing (0.6% on tadalafil; 1.7% on sildenafil).
DISCUSSION
Results reported previously from this study indicated that 20 mg tadalafil and 100 mg sildenafil were effective and safe treatments for ED in Chinese patients naïve to PDE5 inhibitor treatment; results also demonstrated that more patients preferred treatment with tadalafil over sildenafil. Previous studies indicate that patient preference is of paramount importance in managing patients with ED,14 and psychosocial factors are thought to impact this preference. Current ED guidelines also emphasizes that the assessment of ED treatment must consider the effects on patients and partner satisfaction, which is also related to psychosocial outcomes as well as efficacy and safety.15 As such, this analysis evaluated the psychosocial outcomes and drug attributes of 20 mg tadalafil versus 100 mg sildenafil; allowing the identification of factors that may impact patient preference.
In this study, significant improvements in the PAIRS Time Concerns and Spontaneity Domains were observed after baseline, and the improvement in the tadalafil group was superior to that in the sildenafil group, which indicates that men felt less time pressure, less sense of urgency, and less planning before and during sexual encounters when taking tadalafil compared with sildenafil. While a significant improvement in the Sexual Self-Confidence Domain was not observed with tadalafil versus sildenafil, the mean score change from baseline after tadalafil treatment (0.75) was numerically greater than that after sildenafil treatment (0.71).
The significant improvement in both the Time Concerns and Spontaneity Domains are consistent with the randomized, open-label, crossover study conducted by Dean et al. in which men with ED had improved spontaneity and less time concerns related to sexual encounters when treated with tadalafil on-demand (at doses of 10 or 20 mg) compared to sildenafil on-demand (at doses of 25, 50, or 100 mg).11 Additionally, similar improvements in the Time Concerns and Spontaneity Domains were observed in a study directly comparing the psychosocial outcomes between once-daily tadalafil and on-demand tadalafil or sildenafil.16 It is suspected that the differences in Time Concerns and Spontaneity Domains between tadalafil and sildenafil may be due to the differences in their duration of action as tadalafil has a duration of action of up to 36 h17 versus 4–5 h for sildenafil.18 The differences between the tadalafil and sildenafil pharmacokinetic profiles allows patients greater freedom and less need to plan ahead, treatment with tadalafil may address some of the psychosocial concerns with sexual performance in Chinese men with ED.
In this study, the drug attributes most frequently chosen by men preferring tadalafil were “ability to get an erection long after having drug” and “ability to get an erection every time.” For men preferring silCenafil, the drug attributes most frequently chosen were “ability to get an erection every time” and “firmness of erections.” The DRAQ was administered after a patient had identified his preferred medication and does not allow patients to compare attributes of one drug to the other. However, it is expected that DRAQ responses provide an indication of why the chosen medication was preferred. Regardless of preferred treatment, however, the most common drug attribute choices selected by men who preferred sildenafil and men who preferred tadalafil suggest that men prefer ED medications that enable a return to the erectile function and partner interaction they experienced prior to having ED.11
While the mean change in SLQQ-QoL scores from baseline was comparable between both the tadalafil and sildenafil treatment groups, the mean SLQQ-QoL scores at the end of the extension phase were increased among both tadalafil and sildenafil-treated patients, suggesting that both treatments resulted in an improvement in the way patients evaluated their sexual quality of life.
A possible limitation of this study was the open-label design, which could increase conscious and unconscious bias in the conduct and interpretation of the trial. However, the use of a centralized interactive voice-response system (IVRS) as well as the crossover design with a 7–10 days washout period was expected to minimize this potential for bias. The study analyzed both the intention-to-treat (ITT) population and the population of patients who completed both treatment periods in order to minimize the potential for interpretation bias. Finally, some of the authors are employed by the pharmaceutical company responsible for the study design, execution, monitoring, data analysis and verification of this study.
CONCLUSION
Results from this study suggest that psychosocial and relationship factors, in addition to efficacy and tolerability, play a role in the preference treatment of men with ED in China. As measured with PAIRS, men with ED had higher spontaneity and less time concerns related to sexual encounters when treated with tadalafil compared with sildenafil. These psychosocial outcomes and the ability to dissociate the intake of the pill with achieving an erection, a unique benefit to a long-acting PDE5 inhibitor such as tadalafil, may help explain why more men preferred tadalafil for the treatment of ED in this clinical trial.
AUTHOR CONTRIBUTIONS
WJB, HJL, XFW contributions: data collection, drafting and critical revision of the manuscript. JJJ, WPX contributions: drafting and critical revision of the manuscript. SS contributions: study design and conception, drafting and critical revision of the manuscript.
All authors read and approved the final manuscript.
COMPETING INTERESTS
WJB, HJL and XFW have no competing interests to declare. WPX and JJJ are employees of Lilly Suzhou Pharmaceutical Co., Ltd., Sorsaburu Sebastian is an employee of Eli Lilly and Company.
ACKNOWLEDGMENTS
This study was supported by Eli Lilly and Company, who was responsible for the study design, execution and monitoring, and also for the data analysis and verification. The identifier label for this study was H6D-CR-LVIZ. The authors would like to thank the investigators who participated in this study. The authors acknowledge Dr. Ji Chen for his statistical support of this manuscript and Stephanie Brillhart of inVentiv Health Clinical for writing and editorial support for this manuscript.
REFERENCES
- 1.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524–40. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang EY. Switching between traditional Chinese medicine and viagra: cosmopolitanism and medical pluralism today. Med Anthropol. 2007;26:53–96. doi: 10.1080/01459740601184448. [DOI] [PubMed] [Google Scholar]
- 3.Jiann BP, Yu CC, Tsai JY, Wu TT, Lee YH, et al. What to learn about sildenafil in the treatment of erectile dysfunction from 3-year clinical experience. Int J Impot Res. 2003;15:412–7. doi: 10.1038/sj.ijir.3901047. [DOI] [PubMed] [Google Scholar]
- 4.Guo YL, Zhu JC, Pan TM, Ding Q, Wang YX, et al. Efficacy and safety of on-demand tadalafil for the treatment of erectile dysfunction in South-East Asian men. Int J Urol. 2006;13:721–7. doi: 10.1111/j.1442-2042.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- 5.Yip WC, Chiang HS, Mendoza JB, Tan HM, Li MK, et al. Efficacy and safety of on demand tadalafil in the treatment of East and Southeast Asian men with erectile dysfunction: a randomized double-blind, parallel, placebo-controlled clinical study. Asian J Androl. 2006;8:685–92. doi: 10.1111/j.1745-7262.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 6.Brock G, Chan J, Carrier S, Chan M, Salgado L, et al. The treatment of erectile dysfunction study: focus on treatment satisfaction of patients and partners. BJU Int. 2007;99:376–82. doi: 10.1111/j.1464-410X.2006.06586.x. [DOI] [PubMed] [Google Scholar]
- 7.Tolra JR, Campana JM, Ciutat LF, Miranda EF. Prospective, randomized, open-label, fixed-dose, crossover study to establish preference of patients with erectile dysfunction after taking the three PDE-5 inhibitors. J Sex Med. 2006;3:901–9. doi: 10.1111/j.1743-6109.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Pommerville P, Brock G, Gagnon R, Mehta P, et al. Physician-rated patient preference and patient- and partner-rated preference for tadalafil or sildenafil citrate: results from the Canadian “TreaCment of Erectile Dysfunction” observational study. BJU Int. 2006;98:623–9. doi: 10.1111/j.1464-410X.2006.06384.x. [DOI] [PubMed] [Google Scholar]
- 9.Conaglen HM, Conaglen JV. Investigating women's preference for sildenafil or tadalafil use by their partners with erectile dysfunction: the partners’ preference study. J Sex Med. 2008;5:1198–207. doi: 10.1111/j.1743-6109.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 10.Bai WJ, Li HJ, Dai YT, He XY, Huang YR, et al. An open-label, multicenter, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in Chinese men naïve to phosphodiesterase 5 inhibitor therapy. Asian J Androl. 2015;17:61–7. doi: 10.4103/1008-682X.143244. [Doi: 10.4103/1008-682X.143244] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean J, Hackett GI, Gentile V, Pirozzi-Farina F, Rosen RC, et al. Psychosocial outcomes and drug attributes affecting treatment choice in men receiving sildenafil citrate and tadalafil for the treatment of erectile dysfunction: results of a multicenter, randomized, open-label, crossover study. J Sex Med. 2006;3:650–61. doi: 10.1111/j.1743-6109.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 12.Swindle RW, Cameron AE, Lockhart DC, Rosen RC. The psychological and interpersonal relationship scales: assessing psychological and relationship outcomes associated with erectile dysfunction and its treatment. Arch Sex Behav. 2004;33:19–30. doi: 10.1023/B:ASEB.0000007459.48511.31. [DOI] [PubMed] [Google Scholar]
- 13.Woodward JM, Hass SL, Woodward PJ. Reliability and validity of the sexual life quality questionnaire (SLQQ) Qual Life Res. 2002;11:365–77. doi: 10.1023/a:1015513228469. [DOI] [PubMed] [Google Scholar]
- 14.Mulhall JP. Understanding erectile dysfunction medication preference studies. Curr Opin Urol. 2004;14:367–73. doi: 10.1097/00042307-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Wespes E, Eardley I, Giuliano F, Hatzichristou D, Hatzimouratidis K, et al. Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Aurioles E, Porst H, Kim ED, Montorsi F, Hackett G, et al. A randomized open-label trial with a crossover comparison of sexual self-confidence and other treatment outcomes following tadalafil once a day vs tadalafil or sildenafil on-demand in men with erectile dysfunction. J Sex Med. 2012;9:1418–29. doi: 10.1111/j.1743-6109.2012.02667.x. [DOI] [PubMed] [Google Scholar]
- 17.Young JM, Feldman RA, Auerbach SM, Kaufman JM, Garcia CS, et al. Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J Androl. 2005;26:310–8. doi: 10.2164/jandrol.04126. [DOI] [PubMed] [Google Scholar]
- 18.Viagra (Sildenafil Citrate) Package Label. Pfizer Laboratories. 2013. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=652 .