Abstract
Objective
To determine the role of opioid, β-adrenergic, and metabotropic glutamate 5 receptors in sacral neuromodulation of bladder overactivity.
Material and Methods
In α-chloralose anesthetized cats, intravesical infusion of 0.5% acetic acid (AA) irritated the bladder and induced bladder overactivity. Electric stimulation (5 Hz, 0.2 ms, 0.16–0.7V) of S1 or S2 sacral dorsal roots inhibited the bladder overactivity. Naloxone, propranolol, or MTEP were given intravenously (i.v.) to determine different neurotransmitter mechanisms.
Results
AA significantly (p <0.05) reduced bladder capacity to 7.7 ± 3.3 mL from 12.0 ± 5.0 mL measured during saline infusion. S1 or S2 stimulation at motor threshold intensity significantly (p <0.05) increased bladder capacity to 179.4 ± 20.0% or 219.1 ± 23.0% of AA control, respectively. Naloxone (1 mg/kg) significantly (p <0.001) reduced the control capacity to 38.3 ± 7.3% and the bladder capacity measured during S1 stimulation to 106.2 ± 20.8% of AA control, but did not significantly change the bladder capacity measured during S2 stimulation. Propranolol (3 mg/kg) significantly (p <0.01) reduced bladder capacity from 251.8 ± 32.2% to 210.9 ± 33.3% during S2 stimulation, but had no effect during S1 stimulation. A similar propranolol effect also was observed in naloxone-pretreated cats. In propranolol-pretreated cats during S1 or S2 stimulation, MTEP (3 mg/kg) significantly (p <0.05) reduced bladder capacity and naloxone (1 mg/kg) following MTEP treatment further reduced bladder capacity. However, a significant inhibition could still be induced by S1 or S2 stimulation after all three drugs were administered.
Conclusions
Neurotransmitter mechanisms in addition to those activating opioid, β-adrenergic, and metabotropic glutamate 5 receptors also are involved in sacral neuromodulation.
Keywords: Bladder, cat, neuromodulation, neurotransmitter
INTRODUCTION
Overactive bladder (OAB) is characterized by urinary urgency with or without urge incontinence, which affects about 13% of women and 11% of men worldwide (1). OAB symptoms typically present concomitantly with urinary frequency and nocturia. Sacral neuromodulation has been Food and Drug Administration approved as an effective OAB treatment since the 1990s (2–4). However, currently the mechanisms underlying sacral neuromodulation of bladder function are not fully understood. Since electric stimulation of the pudendal or tibial nerves also is clinically effective in OAB treatment (5–8), it has been assumed that sacral neuromodulation might stimulate the afferent nerve fibers in the S3 spinal root that originate from pudendal and/or tibial nerves. Therefore, sacral neuromodulation may share the same mechanisms as pudendal/tibial neuromodulation.
Our previous studies in cats have revealed that multiple neurotransmitter mechanisms are involved in pudendal or tibial neuromodulation of bladder overactivity (3,9–13). The opioid receptors play a major role in tibial inhibition of bladder overactivity (12,13), while metabotropic glutamate receptor 5 (mGluR5) is involved in pudendal inhibition (3,10). Our recent study in cats further shows that the β-adrenergic receptors also are involved in pudendal inhibition of bladder overactivity (9,11). Therefore, it is logical to hypothesize that these neurotransmitters may play a role in sacral neuromodulation of bladder overactivity in cats.
In this study, the possible role of opioid, mGluR5, and β-adrenergic receptors in sacral neuromodulation of bladder overactivity was examined in α-chloralose anesthetized cats. Dilute (0.5%) acetic acid (AA) was used to irritate the bladder, activate bladder nociceptive C-fiber afferents, and induce bladder overactivity. The S1 or S2 sacral dorsal roots were stimulated to inhibit the irritation-induced bladder overactivity and mimic sacral neuromodulation. The S1/S2 roots in cats are equivalent to the S3 root in human due to the spinal segmental differences of the different species (14,15). Naloxone (an opioid receptor antagonist), MTEP (3-((2-Methyl-4-thiazolyl)ethynyl)pyridine, an mGluR5 receptor antagonist), and propranolol (a β-adrenergic receptor antagonist) were used individually or in combination to determine the involvement of these receptors in the inhibition of bladder overactivity induced by S1/S2 dorsal root stimulation. This study not only reveals the possible neurotransmitter mechanisms underlying sacral neuromodulation but also clarifies the possible relationship between sacral neuromodulation and pudendal/tibial neuromodulation.
MATERIALS AND METHODS
The Animal Care and Use Committee at the University of Pittsburgh approved all protocols involving the experimental use of animals in this study.
Surgical Procedures
The experiments were conducted on a total of 24 cats (13 male and 11 female, 2.8–4.2 kg, Liberty Research, Waverly, NY, USA) under isoflurane (2–5% in O2) anesthesia during surgery followed by α-chloralose anesthesia (65 mg/kg i.v. and supplemented as needed) during data collection. Systemic blood pressure was monitored throughout the experiment via a catheter inserted into the right carotid artery. Heart rate and blood oxygen level were monitored by a pulse oximeter (9847V, NONIN Medical, Plymouth, MN, USA) with the sensor attached to the ear or tongue. A tracheostomy was performed and an endotracheal tube was inserted to ensure airway patency. A catheter for intravenous infusion of fluid and drugs was inserted into the left cephalic vein. A laparotomy was performed and the ureters were isolated, cut, and drained externally. The urethra was then exposed and a double lumen catheter was inserted through an urethrotomy into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pressure transducer to monitor the bladder pressure and the other was connected to a pump to infuse (1–3 mL/min) the bladder with saline or 0.5% AA. All incisions were closed with suture.
The spinal cord and cauda equina were exposed between the L4 and S3 vertebrae via a dorsal laminectomy. The spinal dura was cut and the S1–S2 sacral dorsal roots on the right side were separated for electric stimulation. A bipolar stainless steel hook electrode was used during the experiment to stimulate individual S1/S2 dorsal roots by delivering electric pulses that were generated by an electric stimulator (S88, Grass Medical Instruments, Quincy, MA, USA). The animal was mounted in a modified Narishige “Eccles” spinal cord frame in which the hip was supported by metal pins, and the spinous process at the rostral end of the laminectomy was secured with a clamp. The skin, cut mid-sagittally from L4 to S3, was tied along each margin to form a pool that was filled with warmed (35–37°C) mineral oil. The temperature of the animal was maintained at 36–38°C using a heating pad during the experiments.
Experimental Protocol
Our previous studies in cats (16,17) showed that reflex bladder activity could be inhibited by electric stimulation (5 Hz frequency and 0.2 ms pulse width) of S1 or S2 dorsal roots at threshold (T) intensity for inducing reflex twitching of the anal sphincter or toe, while stimulation of S3 dorsal root or S1–S3 ventral roots was not effective. Therefore, stimulation (5 Hz, 0.2 ms) of S1 or S2 dorsal roots at motor threshold intensity was used in this study to inhibit reflex bladder activity.
At the beginning of each experiment, multiple cystometrograms (CMGs) were performed with saline infusion (1–3 mL/min) to determine the bladder capacity that was defined as the bladder volume threshold to induce a bladder contraction of large amplitude (>30 cm H2O) and long duration (>20 sec). Then, 0.5% AA was infused into the bladder to irritate the bladder and induce bladder overactivity. Once the control bladder capacity stabilized during repeated AA CMGs, the inhibitory effect of sacral dorsal root stimulation was determined by additional four AA CMGs: 1) control CMG without stimulation; 2) CMG during S1 dorsal root stimulation; 3) CMG during S2 dorsal root stimulation; 4) control CMG again to examine any poststimulation effect. Then, the animals were divided into two experimental groups.
In the first group (N = 11 cats), the animals received 1 mg/kg (i.v.) naloxone (12) followed by 3 mg/kg (i.v.) propranolol (9,11). In the second group (N = 13 cats), the animals received 3 mg/kg (i.v.) propranolol followed by 3 mg/kg (i.v.) MTEP (3,10) and then 1 mg/kg (i.v.) naloxone. The different drugs were given at about 50–60 min intervals. The specific dosages of the different drugs have been shown in our previous cat studies to be effective for several hours in suppressing tibial or pudendal inhibition of bladder overactivity (3,9–12). The effective duration of the large doses of drugs are long enough for testing the repeated CMGs and the drug interactions in this study. After each drug treatment, the four CMGs (control, S1 stimulation, S2 stimulation, and control) were repeated to determine the drug effects. A 10-min waiting period for propranolol/MTEP and 5 min for naloxone were used for the drugs to take effect. A waiting period of 5 min also was used between CMGs for the bladder reflex to recover, which was shown to be long enough in our previous studies (3,9–12).
Data Analysis
The bladder capacity was measured from each CMG and normalized to the capacity measured during the first control CMG for different drug treatments. Repeated measurements (2–3 CMGs) of control bladder capacity in the same animal under the same conditions (saline or AA) were averaged. The normalized data from different animals are presented as mean ± standard error. Statistical significance (p <0.05) was determined by repeated-measures ANOVA followed by Bonferroni (two-way) post hoc multiple comparison to determine the effects of each drug on reflex bladder activity and sacral S1/S2 inhibition or by Dunnett (one-way) post hoc multiple comparison to determine the effects of S1/S2 dorsal root stimulation on reflex bladder activity in the same treatment conditions. Student’s t-test was used to detect the significant (p <0.05) change of reflex bladder activity induced by AA irritation. The statistical methods used for analyzing data from each experiment also are indicated in the figure legends.
RESULTS
S1/S2 Dorsal Root Stimulation Inhibited Bladder Overactivity
Intravesical infusion of dilute (0.5%) AA irritated the bladder, induced bladder overactivity, and significantly (p <0.05) reduced bladder capacity to 7.7 ± 3.3 mL from 12.0 ± 5.0 mL measured during saline CMGs (N = 24 cats). Stimulation of S1 or S2 dorsal roots at motor threshold intensity (0.16–0.7V) inhibited AA-induced bladder overactivity and significantly (p <0.05) increased bladder capacity to 12.8 ± 4.7 or 16.7 ± 6.7 mL, respectively (N = 24 cats). Typical CMG traces are shown on the first row in Figures 1a and 2a. After dorsal root stimulation, the following AA control CMG showed that the bladder capacity returned to the prestimulation level, i.e., no poststimulation effect. A poststimulation effect also was not observed after the drug treatments described in the following sections.
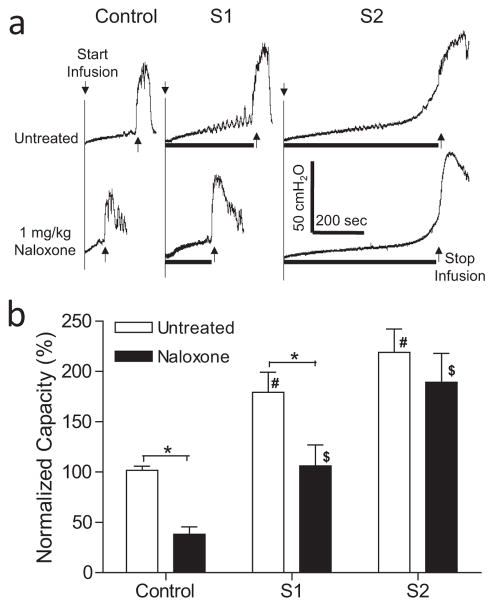
Figure 1.
Effect of naloxone on inhibition of bladder overactivtiy induced by S1 or S2 dorsal root stimulation at motor threshold (T) intensity. a. Typical CMG traces from a cat. The black bar under the pressure trace indicates the stimulation duration. Stimulation: 5 Hz, 0.2 ms, T = 0.3V for S1, T = 0.16V for S2. Infusion rate = 2 mL/min. b. Normalized bladder capacity measured during repeated CMGs before and after naloxone treatment (N = 11 cats). * indicates a significant (p <0.001, two-way ANOVA) difference before and after naloxone treatment. # or $ indicates significantly (p <0.05, one-way ANOVA) different from control in the same treatment group.
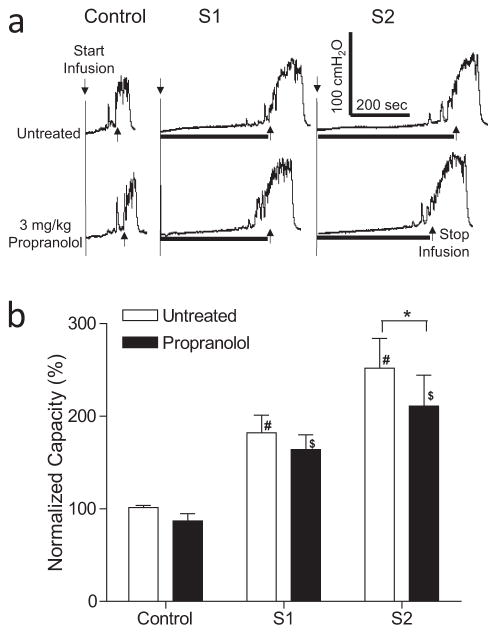
Figure 2.
Effect of propranolol on inhibition of bladder overactivity induced by S1 or S2 dorsal root stimulation at motor threshold (T) intensity. a. Typical CMG traces from a cat. The black bar under the pressure trace indicates stimulation duration. Stimulation: 5 Hz, 0.2 ms, T = 0.24V for S1, T = 0.2V for S2. Infusion rate = 2 mL/min. b. Normalized bladder capacity measured during repeated CMGs before and after propranolol treatment (N = 13 cats). * indicates a significant (p <0.01, two-way ANOVA) difference before and after propranolol treatment. # or $ indicates significantly (p <0.01, one-way ANOVA) different from control in the same treatment group.
Effect of Naloxone on Sacral Inhibition of Bladder Overactivity
In the untreated animals during AA infusion, S1 or S2 dorsal root stimulation significantly increased bladder capacity to 179.4 ± 20.0% (p <0.01) or 219.1 ± 23.0% (p <0.05), respectively, of AA control capacity (N = 11 cats, Fig. 1). Naloxone (1 mg/kg, i.v.) significantly (p <0.001) reduced the control bladder capacity to 38.3 ± 7.3% and the bladder capacity measured during S1 dorsal root stimulation to 106.2 ± 20.8% of AA control (Fig. 1). However, naloxone did not significantly change the bladder capacity (189.3 ± 28.8%) measured during S2 dorsal root stimulation (Fig. 1). After naloxone treatment both S1 and S2 dorsal stimulation still significantly (p <0.05) increased bladder capacity (Fig. 1).
Effect of Propranolol on Sacral Inhibition of Bladder Overactivity
In the untreated animals during AA infusion, S1 or S2 dorsal root stimulation significantly (p <0.01) increased bladder capacity to 182.0 ± 19.1% or 251.8 ± 32.2%, respectively, of AA control capacity (N = 13 cats, Fig. 2). Propranolol (3 mg/kg, i.v.) significantly (p <0.01) suppressed the increase in bladder capacity during S2 dorsal root stimulation to 210.9 ± 33.3%, but did not significantly change the increase in bladder capacity (163.9 ± 16.1%) during S1 dorsal root stimulation (Fig. 2). Propranolol did not change AA control bladder capacity (Fig. 2). After propranolol treatment, both S1 and S2 dorsal root stimulation still significantly (p <0.05) increased bladder capacity (Fig. 2).
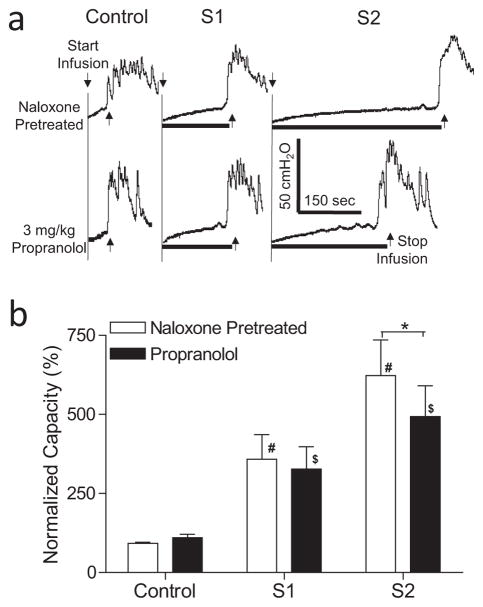
In the animals pretreated with naloxone (1 mg/kg, i.v.), S1 or S2 dorsal root stimulation significantly increased bladder capacity to 358.7 ± 77.5% (p <0.05) or 622.9 ± 112.6% (p <0.01), respectively, of naloxone control capacity (N = 11 cats, Fig. 3). Propranolol (3 mg/kg, i.v.), which did not change naloxone control bladder capacity (Fig. 3), significantly (p <0.001) suppressed the increase in bladder capacity during S2 dorsal root stimulation to 493.5 ± 97.1%, but did not significantly reduce the increase in bladder capacity (327.3 ± 70.4%) during S1 dorsal root stimulation (Fig. 3). After propranolol and naloxone treatments both S1 and S2 dorsal root stimulation still significantly (p <0.05) increased bladder capacity (Fig. 3).
Figure 3.
Effect of propranolol on inhibition of bladder overactivity induced by S1 or S2 dorsal root stimulation at motor threshold (T) intensity in naloxone-pretreated cats. a. Typical CMG traces from a cat. The black bar under the pressure trace indicates stimulation duration. Stimulation: 5 Hz, 0.2 ms, T = 0.3V for S1, T = 0.26V for S2. Infusion rate = 3 mL/min. b. Normalized bladder capacity measured during repeated CMGs before and after propranolol treatment in naloxone-pretreated cats (N = 11). * indicates a significant (p <0.001, two-way ANOVA) difference before and after propranolol treatment. # or $ indicates significantly (p <0.05, one-way ANOVA) different from control in the same treatment group.
Effect of MTEP on Sacral Inhibition of Bladder Overactivity
In the animals pretreated with propranolol (3 mg/kg, i.v.), S1 or S2 dorsal root stimulation significantly (p <0.01) increased bladder capacity to 219.6 ± 39.9% or 264.9 ± 49.5%, respectively, of propranolol control capacity (N = 13 cats, Fig. 4). MTEP (3 mg/kg, i.v.), which did not change propranolol control bladder capacity (Fig. 4), significantly (p <0.05) reduced the increases in bladder capacity elicited by S1 or S2 dorsal root stimulation to 174.8 ± 30.6% or 222.8 ± 46.9% of control, respectively (Fig. 4). After MTEP treatment, only S2 dorsal root stimulation still significantly (p <0.05) increased bladder capacity (Fig. 4). In the animals pretreated with both propranolol and MTEP, naloxone (1 mg/kg, i.v.) significantly (p <0.001) reduced the control bladder capacity and the bladder capacities measured during both S1 and S2 dorsal root stimulation (N = 12 cats, Fig. 5). However, after the combined propranolol, MTEP, and naloxone treatments both S1 and S2 dorsal root stimulation still significantly (p <0.05) increased bladder capacity (Fig. 5).
Figure 4.
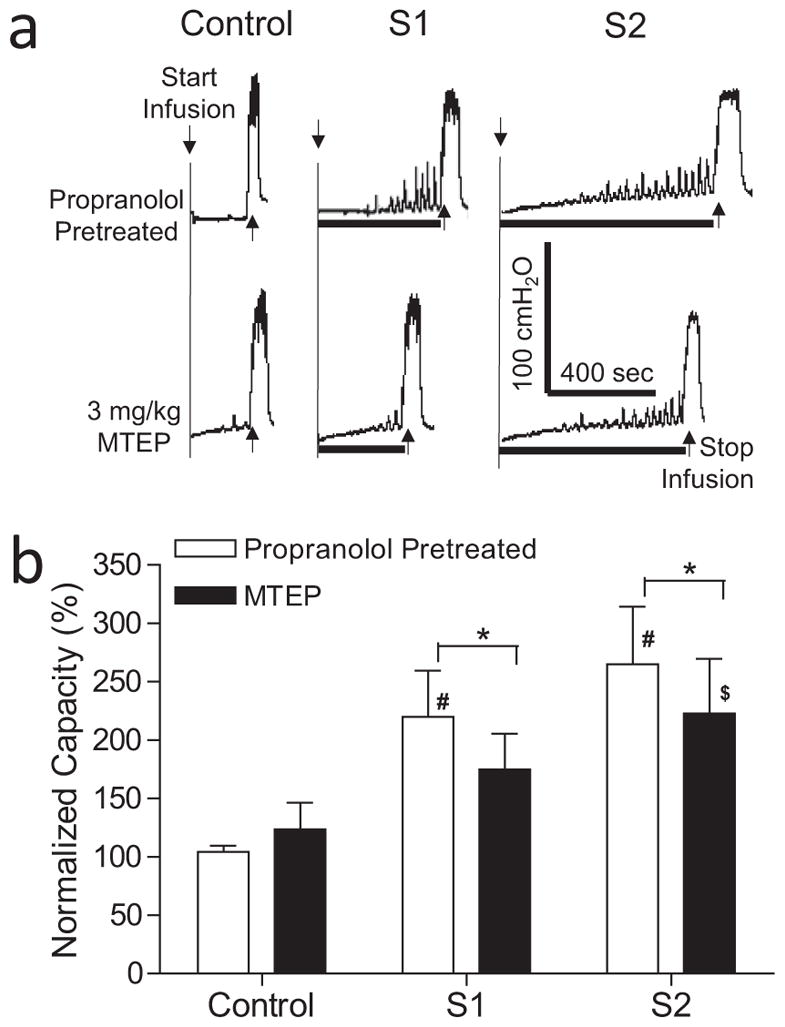
Effect of MTEP on inhibition of bladder overactivtiy induced by S1 or S2 dorsal root stimulation at motor threshold (T) intensity in propranolol-pretreated cats. a. Typical CMG traces from a cat. The black bar under the pressure trace indicates stimulation duration. Stimulation: 5 Hz, 0.2 ms, T = 0.7V for S1, T = 0.5V for S2. Infusion rate = 1 mL/min. b. Normalized bladder capacity measured during repeated CMGs before and after MTEP treatment in propranolol-pretreated cats (N = 13). * indicates a significant (p <0.05, two-way ANOVA) difference before and after MTEP treatment. # or $ indicates significantly (p <0.01, one-way ANOVA) different from control in the same treatment group.
Figure 5.
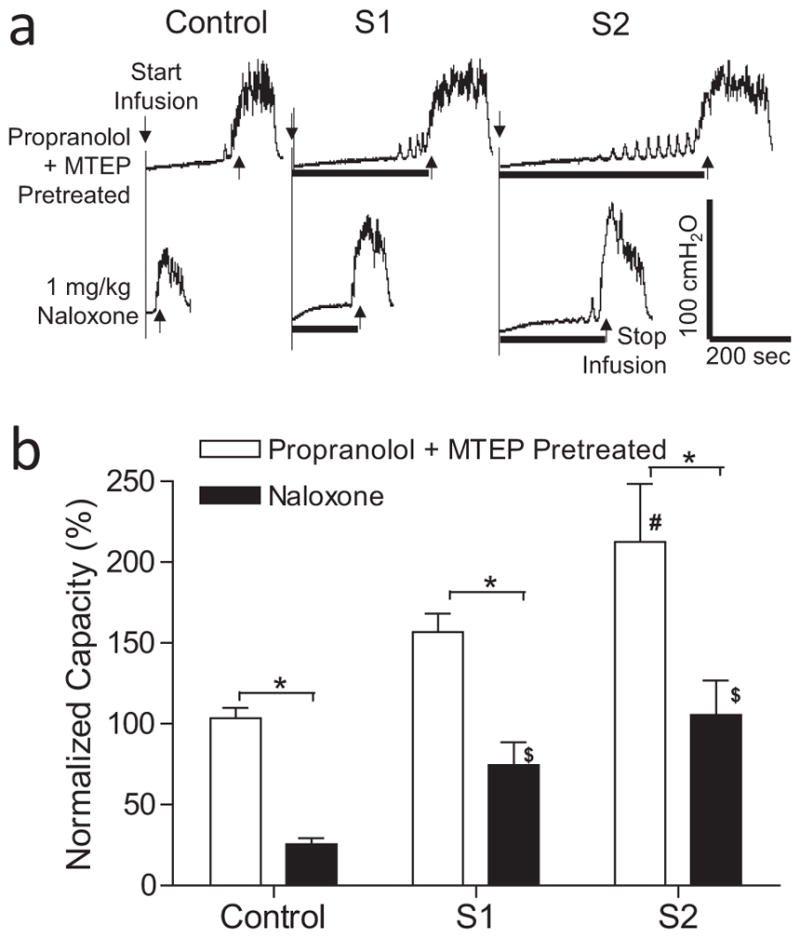
Effect of naloxone on inhibition of bladder overactivity induced by S1 or S2 dorsal root stimulation at motor threshold (T) intensity in propranolol and MTEP-pretreated cats. a. Typical CMG traces from a cat. The black bar under the pressure trace indicates stimulation duration. Stimulation: 5 Hz, 0.2 ms, T = 0.4V for S1, T = 0.3V for S2. Infusion rate = 3 mL/min. b. Normalized bladder capacity measured during repeated CMGs before and after naloxone treatment in propranolol and MTEP-pretreated cats (N = 12). * indicates a significant (p <0.001, two-way ANOVA) difference before and after naloxone treatment. # or $ indicates significantly (p <0.05, one-way ANOVA) different from control in the same treatment group.
DISCUSSION
This study revealed that multiple neurotransmitter mechanisms contribute to sacral neuromodulation of reflex bladder overactivity in anesthetized cats and that different mechanisms can be involved in the neuromodulation elicited by stimulation of adjacent sacral (S1 and S2) dorsal roots. Blocking opioid receptors with naloxone significantly reduced bladder capacity during S1 stimulation, but did not change bladder capacity during S2 stimulation (Fig. 1). In contrast, blocking β-adrenergic receptors with propranolol significantly reduced the inhibition induced by S2 but not by S1 stimulation (Figs. 2 and 3). After propranolol treatment, blocking mGluR5 receptors by MTEP significantly reduced the inhibition induced by either S1 or S2 stimulation (Fig. 4) and unmasked the sensitivity of S2 stimulation to naloxone treatment (Fig. 5). However, after treatment with a combination of propranolol, MTEP, and naloxone, stimulation of either S1 or S2 still produced a significant inhibition (Fig. 5) indicating that additional mechanisms contribute to sacral neuromodulation.
The contribution of multiple neurotransmitters to sacral neuromodulation was not completely unexpected because our previous studies (3,9,12) showed that electric stimulation of peripheral nerves (pudendal and tibial) that send afferent axons through the sacral dorsal roots inhibits reflex bladder activity by release of multiple neurotransmitters. For example, opioid receptors play a major role in the inhibition of bladder overactivity induced by tibial nerve stimulation (12,13), while these receptors are not involved in the inhibition induced by pudendal nerve stimulation (18). Since S1 but not S2 dorsal root stimulation is sensitive to naloxone treatment (Fig. 1), it is reasonable to speculate that S1 dorsal root stimulation activates some of the afferent nerve fibers carried by the tibial nerve, thereby contributing to the naloxone-sensitive inhibition; while there are fewer tibial afferent fibers passing through the S2 dorsal root (14) accounting for the naloxone resistance.
On the other hand, our previous studies also showed that β-adrenergic receptors are involved in the inhibition of bladder overactivity induced by pudendal nerve stimulation (9,11). Because S2 but not S1 dorsal root stimulation is sensitive to propranolol treatment (Figs. 2 and 3) and the majority of pudendal afferent fibers travel through the S2 dorsal root into the spinal cord in cats (15), it is reasonable to speculate that the sensitivity to propranolol is due in part to S2 dorsal root stimulation activating a population of pudendal afferent nerve fibers. Additional studies are warranted to further determine the contributions of tibial and pudendal afferent nerves to the inhibition induced by sacral dorsal root stimulation.
After propranolol treatment, administration of the mGluR5 receptor antagonist, MTEP, further reduced the inhibition induced by S2 dorsal root stimulation (Fig. 4). This also is consistent with activation of pudendal afferent nerves because our previous studies showed that pudendal afferent input can activate mGluR5 receptors in the central nervous system to inhibit bladder reflex activity (3,10,18). The MTEP effect on S1 dorsal root stimulation (Fig. 4) may reflect the minor population of pudendal afferents in this root, as well as the presence of tibial nerve afferents. While MTEP has not yet been tested on tibial neuromodulation, an antagonist for another type of metabotropic glutamate receptor (mGluR2/3) has been shown to reduce tibial inhibition of bladder overactivity (19). This raises the possibility that the mGluR5 receptor might also have a role in tibial neuromodulation, because it is known that multiple subtypes of mGluRs can be expressed in neurons and at synapses (20). This will be examined in future experiments.
After both propranolol and MTEP treatments, S2 dorsal root stimulation becomes sensitive to naloxone (Fig. 5). Although this result is difficult to interpret due to the complicated multiple drug treatments and the direct effect of naloxone to decrease bladder capacity, it shows that an opioid mechanism that is not activated by S2 dorsal root stimulation originally (Fig. 1) can be unmasked after blocking other neurotransmitter mechanisms (β-adrenergic and mGluR5). The direct excitatory effect of naloxone to reduce bladder capacity is attributable to blocking tonically active opioid inhibitory mechanisms. This effect was more prominent in the present experiments than in previous experiments in cats with irritated bladders (12,13). The reason for this difference is uncertain.
However, after the multiple treatments, both S1 and S2 dorsal root stimulation still significantly inhibited bladder overactivity (Fig. 5), indicating that neurotransmitter receptors other than β-adrenergic, mGluR5, and opioid must also participate in the inhibition. Our previous studies in cats have shown that GABAA, glycine, and 5HT receptors are involved in pudendal inhibition (21–23). Therefore, additional studies are needed to determine the possible involvement of these neurotransmitter mechanisms in sacral neuromodulation of bladder overactivity. The significant amount of residual inhibition (Fig. 5) could also be due to activation of afferent nerves other than tibial/pudendal nerves, because our previous study in cats has shown that stimulation of posterior femoral cutaneous nerve can also inhibit reflex bladder activity (24). In addition, many other somatic afferent nerves from the leg/foot also travel in S1 and S2 dorsal roots (14). The effects of stimulating different somatic afferent nerves from the leg/foot on reflex bladder activity and the neurotransmitters involved need to be explored.
It is worth noting that naloxone and propranolol were tested individually in independent groups of cats, but MTEP was only tested in a complicated three-drug combination study consisting of propranolol, followed by MTEP and finally naloxone. This study was designed to sequentially remove three neurotransmitter mechanisms. It showed that norepinephrine, glutamate, and opioids are all involved but the three-drug combination did not completely eliminate sacral inhibition. Thus, other transmitters (e.g., GABA, glycine, or 5HT) must also have a role. Clearly more studies are needed to examine the effect of MTEP alone, MTEP in combination with naloxone and the role of other putative transmitters.
Single doses of drugs were tested in the present experiments instead of conducting a dose response study, because the effective dose for each drug was determined in our previous experiments of tibial or pudendal neuromodulation in cats (3,10,12). Naloxone (1 mg/kg, i.v.) completely removed tibial inhibition of bladder overactivity (12), and MTEP (3 mg/kg, i.v.) maximally reduced pudendal inhibition (3). Propranolol (3 mg/kg, i.v.) combined with MTEP (3 mg/kg, i.v.) completely removed pudendal inhibition of spinal reflex bladder activity mediated by nociceptive C-fiber afferents (10). Therefore, the drug dosages used in this study are large enough to fully remove the inhibition produced by stimulation of tibial/pudendal afferent nerves passing through the S1 and S2 dorsal roots. This reduces the possibility that the significant inhibition remaining after administration of the three drugs (Fig. 5) is due to low doses of drugs.
The cat model of bladder overactivity induced by AA irritation has been used in many previous studies in which drugs and neuromodulation were tested (3,12,13,19,21,22,25,26). It has been shown that the control bladder capacity is stable during repeated CMGs lasting for 5–6 hours (25). In addition, the effect of pudendal neuromodulation also is stable during vehicle control tests that used the repeated CMG protocol similar to the one used in this study and tested seven dosages of the vehicle (saline) for 4–5 hours (22). Therefore, in the present study in which only 2–3 dosages of drugs were tested, it is unlikely that the drug effects are due to a change in baseline bladder capacity during the experiments. Furthermore, naloxone only reduced the inhibition induced by S1 but not by S2 dorsal root stimulation (Fig. 1), while propranolol only reduced the inhibition induced by S2 but not S1 dorsal root stimulation (Figs. 2 and 3). These selective effects are not likely due to a spontaneous change over time that alters baseline CMG parameters or the response to dorsal root stimulation. However, the present experiments are complicated due to the variations in the pharmacokinetics and dynamics of the different drugs and the testing of multiple stimulations during multiple CMGs. Thus, further studies using a simpler experimental protocol to determine the time course of the drug effects on sacral neuromodulation are certainly warranted.
Currently, the pathology of OAB symptoms in human are still unknown. Therefore, it is not possible to develop an animal model of OAB. However, it is well known that bladder afferent fibers consist of non-nociceptive Aδ-fibers and nociceptive C-fibers. Saline distention of bladder activates the non-nociceptive afferent Aδ-fibers that trigger a supraspinal micturition reflex, while AA irritation of bladder activates the nociceptive afferent C-fibers that trigger a spinal bladder reflex. Since OAB symptoms could be caused by overactivity of the Aδ-fiber afferent pathway or activation of the C-fiber afferent pathway, it is critical to understand neuromodulation of both afferent pathways. Our previous studies in cats have revealed that pudendal/tibial neuromodulation of non-nociceptive or nociceptive reflex bladder activity involves different neurotransmitter mechanisms (12,23). Therefore, it is possible that sacral neuromodulation of these different bladder afferent pathways might also involve different neurotransmitter mechanisms. The current study only investigated the neurotransmitters involved in sacral neuromodulation of nociceptive bladder activity induced by AA irritation but not the non-nociceptive bladder activity induced by saline distention. However, a completely different experimental design will be needed in order to study the sacral neuromodulation of saline distention-induced bladder activity, because our previous studies have revealed that a long-lasting poststimulation inhibition can be induced by sacral neuromodulation under non-nociceptive bladder conditions but not under nociceptive bladder conditions (16,17).
In this study, the S1 or S2 dorsal roots were stimulated at 5 Hz and at the motor threshold intensity to mimic clinical sacral neuromodulation. Our previous studies in cats (16,17) have shown that stimulation of S1 or S2 dorsal roots at 5 Hz is effective in inhibiting bladder activity while 15 or 30 Hz is not effective. Other studies in cats (27,28) also showed that 7–10 Hz was the optimal frequency range for S1 or S2 spinal root stimulation to inhibit reflex bladder activity. Although clinical sacral neuromodulation often uses 10 or 15 Hz, a recent study in human (29) indicates that 5.2 Hz can produce the same efficacy as higher frequencies (10, 21, or 40 Hz). Therefore, 5 Hz seems to be the optimal frequency to mimic clinical sacral neuromodulation in acute cat experiments. Our previous studies in cats (16,17) have also shown that stimulation of S1 or S2 ventral roots over a range of frequencies is not effective in increasing bladder capacity during CMG. In addition, clinical studies also show that the therapeutic effects of sacral neuromodulation are due to stimulation of afferent nerve fibers instead of efferent nerve fibers in the spinal roots (30). Therefore, S1 or S2 dorsal root stimulation becomes the optimal choice to test sacral neuromodulation in cats. Finally, motor threshold intensity was used in this study to mimic clinical application of sacral neuromodulation that uses stimulation intensity slightly below motor threshold (29).
Sacral neuromodulation is a very effective treatment for OAB. It can produce greater than 50% improvement on OAB symptoms on about 75% of patients (2,4,31). This high efficacy is in dramatic contrast to pharmacotherapy that can only reduce 1–2 voids per day for OAB patients (8,32). Our current study indicates that sacral neuromodulation can activate opioid, mGluR5, and β-adrenergic receptors in addition to other neurotransmitter mechanisms to achieve the therapeutic effect. Therefore, sacral neuromodulation can be considered as a combination therapy targeting multiple neurotransmitter mechanisms. This information raises the question of whether pharmacotherapy that targets a single neurotransmitter mechanism (33) would be able to match the efficacy of sacral neuromodulation in the treatment of OAB.
In summary, this study in anesthetized cats indicates that sacral neuromodulation may target multiple neurotransmitter receptors to treat OAB including opioid, mGluR5, and β-adrenergic receptors. Afferent axons passing from both the tibial and pudendal afferent nerves through the sacral dorsal roots could be activated by sacral neuromodulation to achieve the therapeutic effect. Understanding the neurotransmitter mechanisms underlying sacral neuromodulation may further improve this effective OAB therapy or discover new molecular targets for developing new therapies for OAB.
Acknowledgments
Source(s) of financial support: This study is supported by the National Institutes of Diabetes, Digestive and Kidney Diseases under Grants DK-094905, DK-102427, and DK-091253.
Footnotes
Conflict of Interest: The authors reported no conflict of interest.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Authorship Statements
All authors designed and conducted the study. All authors approved the final manuscript.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Hassouna MM, Siegel SW, Nyeholt AA, et al. Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol. 2000;163:1849–1854. [PubMed] [Google Scholar]
- 3.Larson JA, Ogagan PD, Chen G, et al. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol. 2011;589:5833–5843. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Kerrebroeck PEV, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438–1443. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 7.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn. 2010;29:1267–1271. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 8.Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–1061. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 9.Kadow BT, Lyon TD, Zhang Z, et al. Sympathetic β-adrenergic mechanism in pudendal inhibition of nociceptive and non-nociceptive reflex bladder activity. Am J Physiol Renal Physiol. 2016;311:F78–F84. doi: 10.1152/ajprenal.00180.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese JN, Rogers MJ, Xiao Z, et al. Role of spinal metabotropic glutamate receptor 5 in pudendal inhibition of the nociceptive bladder reflex in cats. Am J Physiol Renal Physiol. 2015;308:F832–F838. doi: 10.1152/ajprenal.00623.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers MJ, Xiao Z, Shen B, et al. Propranolol, but not naloxone, enhances spinal reflex bladder activity and reduces pudendal inhibition in cats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R42–R49. doi: 10.1152/ajpregu.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai C, Larson JA, Ogagan PD, et al. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090–F1097. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Slater RC, Ferroni MC, et al. Role of μ, κ, and δ opioid receptors in tibial inhibition of bladder overactivity in Cats. J Pharmacol Exp Ther. 2015;355:228–234. doi: 10.1124/jpet.115.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- 15.Thor KB, Morgan C, Nadelhaft I, Houston M, De Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Bandari J, Bansal U, et al. Sacral neuromodulation of nociceptive bladder overactivity in cats. Neurourol Urodyn. 2016 doi: 10.1002/nau.23105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F, Zhao S, Shen B, et al. Neural pathways involved in sacral neuromodulation of reflex bladder activity in cats. Am J Physiol Renal Physiol. 2013;304:F710–F717. doi: 10.1152/ajprenal.00334.2012. [DOI] [PubMed] [Google Scholar]
- 18.Mally AD, Matsuta Y, Zhang F, et al. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol. 2013;189:1574–1579. doi: 10.1016/j.juro.2012.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuta Y, Mally AD, Zhang F, et al. Contribution of opioid and metabotropic glutamate receptor mechanisms to inhibition of bladder overactivity by tibial nerve stimulation. Am J Physiol Regul Integr Comp Physiol. 2013;305:R126–R133. doi: 10.1152/ajpregu.00572.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gereau RW, 4th, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers MJ, Shen B, Reese JN, et al. Role of glycine in nociceptive and non-nociceptive bladder reflexes and pudendal afferent inhibition of these reflexes in cats. Neurourol Urodyn. 2016;35:798–804. doi: 10.1002/nau.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwen Z, Matsuta Y, Shen B, et al. Involvement of 5-HT3 receptors in pudendal inhibition of bladder overactivity in cats. Am J Physiol Renal Physiol. 2013;305:F663–F671. doi: 10.1152/ajprenal.00105.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Z, Reese J, Schwen Z, et al. Role of spinal GABAA receptors in pudendal inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2014;306:F781–F789. doi: 10.1152/ajprenal.00679.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai C, Shen B, Mally AD, et al. Inhibition of micturition reflex by activation of somatic afferents in posterior femoral cutaneous nerve. J Physiol. 2012;590:4945–4955. doi: 10.1113/jphysiol.2012.239475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullmann FA, Wells GI, Langdale CL, Zheng J, Thor KB. Stability of the acetic acid-induced bladder irritation model in alpha chloralose-anesthetized female cats. PLoS One. 2013;8:e73771. doi: 10.1371/journal.pone.0073771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- 27.Schultz-Lampel D, Jiang C, Lindstrom S, Thuroff JW. Experimental results on mechanisms of action of electrical neuromodulation in chronic urinary retention. World J Urol. 1998;16:301–304. doi: 10.1007/s003450050071. [DOI] [PubMed] [Google Scholar]
- 28.Snellings AE, Grill WM. Effects of stimulation site and stimulation parameters on bladder inhibition by electrical nerve stimulation. BJU Int. 2012;110:136–143. doi: 10.1111/j.1464-410X.2011.10789.x. [DOI] [PubMed] [Google Scholar]
- 29.Marcelissen TA, Leong RK, Nieman FH, de Bie RA, van Kerrebroeck PE, de Wachter SG. The effect of pulse rate changes on the clinical outcome of sacral neuromodulation. J Urol 2011. 2011;185:1781–1785. doi: 10.1016/j.juro.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 30.Kessler TM, La Framboise D, Trelle S, et al. Sacral neuromodulation for neurogenic lower urinary tract dysfunction: systematic review and meta-analysis. Eur Urol. 2010;58:865–874. doi: 10.1016/j.eururo.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt RA, Jonas U, Oleson KA, et al. Sacral nerve stimulation for treatment of refractory urinary urge incontinence. J Urol. 1999;162:352–357. [PubMed] [Google Scholar]
- 32.Chapple CR, Khullar V, Gabriel Z, Muston D, Bitoun CE, Weinstein D. The effects of antimuscarinic treatment in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol. 2008;54:543–562. doi: 10.1016/j.eururo.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 33.Andersson KE, Chapple CR, Cardozo L, et al. Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Cur Opin Urol. 2009;19:380–394. doi: 10.1097/MOU.0b013e32832ce8a4. [DOI] [PubMed] [Google Scholar]